Introduction
Urothelial carcinoma (UC) is a common genitourinary
disease, which represents the fourth most common malignancy
worldwide. It originates exclusively from the urothelium and may
occur in the lower urinary tract (bladder and urethra) or the upper
urinary tract (renal pelvis and ureter) (1,2).
Bladder cancer (BC) is the most common type of UC, whereas upper
urinary tract urothelial carcinoma (UTUC) is relatively uncommon
and accounts for only 5–10% of all UCs (3). To date, the pathogenesis of UC has
not been fully elucidated. Known risk factors include cigarette
smoking, exposure to workplace-related chemicals and intake of
drugs, such as phenacetine, chlornaphrazine and cyclophosphamide
(4,5).
Sulfotransferases (SULT), a family of
multifunctional enzymes, transfer the sulfo group from the cofactor
5′-phosphoadenosine-3′-phosphosulfate to the nucleophilic sites of
their substrates (6). It was
previously demonstrated that SULTs are key contributors to the
conjugation and removal of several phenolic xenobiotics and a
variety of endogenous compounds, including neurotransmitters and
steroid hormones (7). At least 11
different SULT enzymes were recently identified in humans. Among
these, sulfotransferase isoform 1A1 (SULT1A1) appears to be a key
phenol SULT, as it has been detected in a wide range of human
tissues, including liver, lung and kidney (8).
The SULT1A1 gene, located on chromosome
16p12.1-p11.2, contains several genetic polymorphisms that possibly
affect individual susceptibility to cancer (9). A functional polymorphism in exon 7 of
the SULT1A1 gene, with a G→A substitution, results in a change in
the amino acid sequence from arginine to histidine, leading to a
decrease in enzymatic activity and variable rates of activation or
detoxification of procarcinogens (10,11).
Carcinogenic aromatic amines, such as 4-aminobiphenyl, which is
contained in tobacco smoke, are one of the causal factors of UC.
Furthermore, SULT1A1 has been shown to efficiently catalyze the
metabolic activation of an N-hydroxyderivative of 4-aminobiphenyl
(12). Therefore, it is
hypothesized that the Arg213His polymorphism in the SULT1A1 gene
may be closely associated with high susceptibility to UC.
To the best of our knowledge, several molecular
epidemiological studies have investigated the association between
the Arg213His polymorphism and UC risk in diverse populations.
However, the results of those studies were inconsistent or even
contradictory and, to date, no study has conducted a quantitative
assessment to confirm this association. Therefore, we performed a
meta-analysis of all eligible studies to derive a more precise
estimation of the association between the Arg213His polymorphism
and UC risk.
Materials and methods
Identification of eligible
studies
A comprehensive literature search was performed
through the PubMed, Medline, Embase and Web of Science databases
for relevant published articles (the last search update was January
31, 2014) using the key words ‘SULT1A1’, ‘polymorphism’,
‘variation’, or ‘mutation’ and ‘bladder’, or ‘urothelial’ in
combination with ‘cancer’, ‘tumor’ or ‘carcinoma’. The search was
limited to human studies and English language publications.
Additional studies were identified by hand, searching the reference
lists of original and review articles.
Inclusion and exclusion criteria
The included studies were required to meet the
following criteria: The studies i) used a case-control design, ii)
evaluated the SULT1A1 Arg213His polymorphism and the risk of UC and
iii) the genotype distribution of the polymorphism in cases and
controls was described in detail and the results were expressed as
odds ratio (OR) and corresponding 95% confidence interval (CI). The
major exclusion criteria were as follows: i) Study not
investigating cancer, ii) review articles, iii) only case
population and iv) duplicate of previous publication.
Data extraction
Information was carefully extracted from all
eligible studies independently by two investigators according to
the inclusion criteria listed above. In case of conflicting
evaluations, a consensus was reached through discussion. The
following information was collected from each study: first author's
name, year of publication, ethnicity, country of origin, cancer
type, genotyping method, source of control groups (population- or
hospital-based) and deviation from the Hardy-Weinberg equilibrium
(HWE) of the control group. Different ethnic descents were
categorized as Asian, Caucasian, or mixed (composed of different
ethnic groups). Cancer types were divided into BC, UTUC or mixed
(including different types of UC) due to anatomic differences. The
corresponding and first authors of the published studies were
contacted via e-mail in case original data were not provided.
Statistical analysis
Crude ORs with their corresponding 95% CIs were used
to assess the strength of the association between the SULT1A1
Arg213His polymorphism and UC risk. The pooled ORs were estimated
for the homozygote (A/A vs. G/G), heterozygote (G/A vs. G/G),
dominant (G/A+A/A vs. G/G) and recessive (A/A vs. G/G+G/A) models.
Between-study heterogeneity was assessed with the Chi-square-based
Q test and heterogeneity was considered statistically significant
when P<0.10 (13). The pooled
OR estimate of each study was calculated with the fixed-effects
[the Mantel-Haenszel method (14)]
or the random-effects models [the DerSimonian and Laird method
(15)]. The fixed-effects model
was adopted when the studies were found to be homogeneous (Q test
P>0.10). Otherwise, the random-effects model was applied. To
further investigate possible sources of between-study
heterogeneity, a meta-regression analysis was performed and a
Galbraith plot was created. In addition to the comparison among all
subjects, we also conducted stratification analyses by ethnicity,
cancer type and source of controls. Sensitivity analyses were
performed to assess the stability of the results, namely, a single
study in the meta-analysis was deleted each time to reflect the
effect of the individual data set on the pooled OR. Begg's funnel
plot and Egger's linear regression test were used to assess
publication bias. Moreover, departure from HWE in controls was
assessed by the Chi-square test for goodness of fit and a P<0.05
was considered as a significant disequilibrium. All the statistical
analyses were performed with Stata software, version 12.1
(StataCorp LP, College Station, TX, USA), using two-sided
P-values.
Results
Study characteristics
According to the abovementioned inclusion criteria,
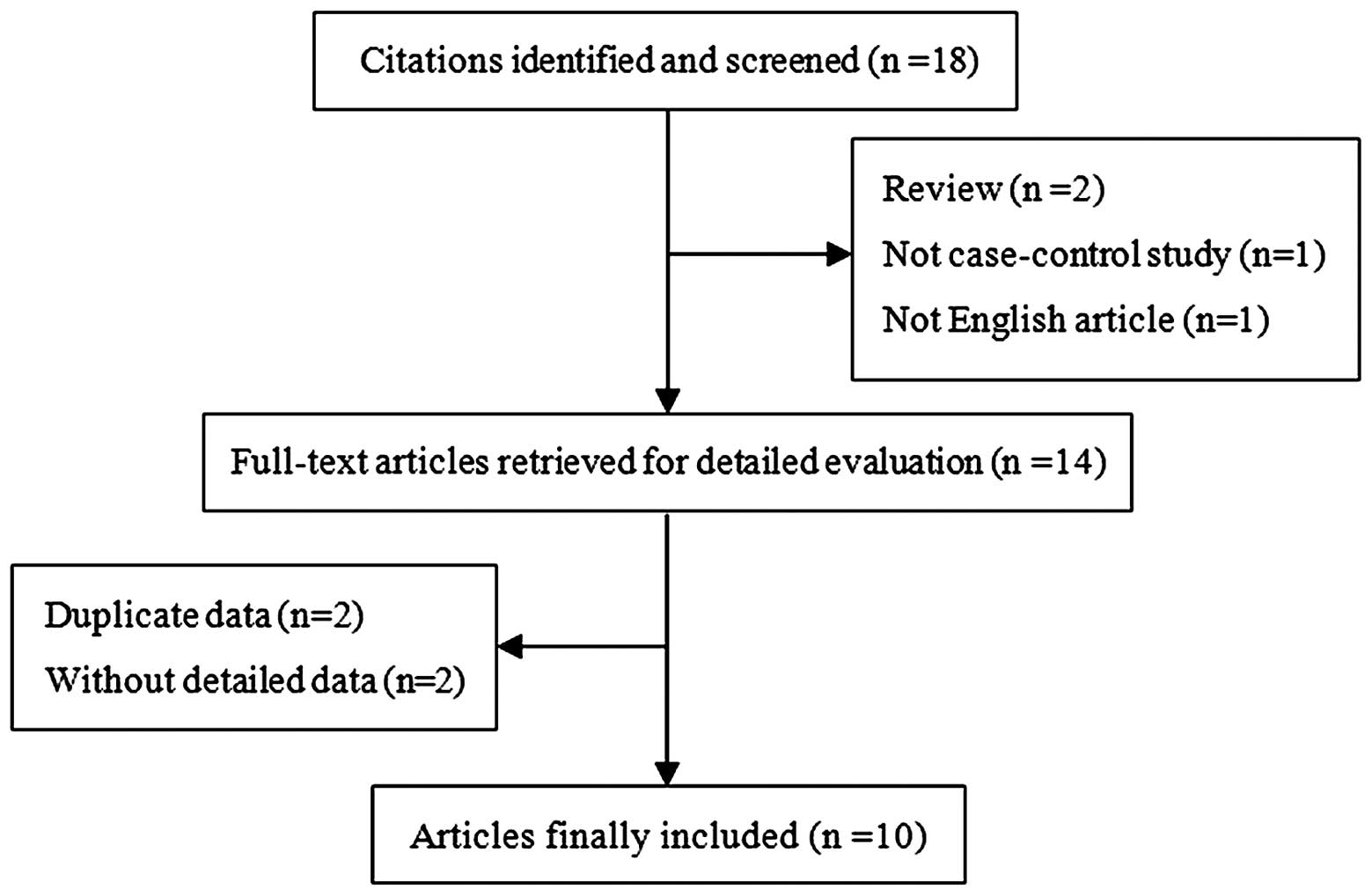
we identified 10 independent studies (1,8,16–23),
including a total of 2,495 cases and 2,905 controls (Fig. 1). These 10 independent studies
consisted of 5 Asian, 4 Caucasian and 1 mixed population. The
controls (8 hospital- and 2 population-based) were mainly matched
by gender and age. The cancers were histologically or
pathologically confirmed in all 10 studies. There were 5 studies on
BC, 4 on mixed cancers (including different types of UC, although
the majority of cases were BC) and 1 on UTUC. In addition, the
distribution of genotypes in the controls in all the studies was
consistent with HWE, except for 2 studies. The main characteristics
of all the eligible studies are summarized in Table I.
 | Table ICharacteristics of the studies
included in the meta-analysis. |
Table I
Characteristics of the studies
included in the meta-analysis.
| | | | | | Sample size | Cases | Controls |
|---|
| | | | | |
|
|
| | |
|---|
| Author (year) | Country | Ethnicity | Cancer type | Genotyping | Control source | Cases | Controls | G/G | G/A | A/A | G/G | G/A | G/A | HWE | (Refs.) |
|---|
| Cui et al
(2013) | Japan | Asian | BC | PCR-RFLP | HB | 282 | 257 | 218 | 59 | 5 | 201 | 52 | 4 | Yes | (20) |
| Chung et al
(2013) | China | Asian | Mixed | PCR-RFLP | HB | 191 | 364 | 168 | 19 | 4 | 315 | 44 | 5 | No | (1) |
| Wang et al
(2008) | China | Asian | Mixed | PCR-RFLP | HB | 300 | 300 | 261 | 37 | 2 | 240 | 54 | 6 | Yes | (8) |
| Covolo et al
(2008) | Italy | Caucasian | BC | PCR-RFLP | HB | 197 | 211 | 120 | 69 | 8 | 114 | 87 | 10 | Yes | (17) |
| Kellen et al
(2007) | Belgium | Caucasian | BC | PCR-RFLP | PB | 200 | 385 | 101 | 79 | 20 | 190 | 149 | 46 | No | (18) |
| Roupret et
al (2007) | UK | Caucasian | UTUC | TaqMan | HB | 268 | 268 | 119 | 99 | 50 | 140 | 101 | 27 | Yes | (16) |
| Tsukino et
al (2004) | Japan | Asian | Mixed | PCR-RFLP | HB | 306 | 306 | 238 | 62 | 6 | 242 | 60 | 4 | Yes | (15) |
| Hung et al
(2004) | France | Caucasian | BC | PCR-RFLP | HB | 201 | 214 | 121 | 72 | 8 | 116 | 88 | 10 | Yes | (19) |
| Zheng et al
(2003) | USA | Mixed | BC | PCR-RFLP | PB | 384 | 386 | 196 | 155 | 33 | 164 | 174 | 48 | Yes | (21) |
| Ozawa et al
(2002) | Japan | Asian | Mixed | PCR-RFLP | HB | 166 | 214 | 128 | 32 | 6 | 154 | 53 | 7 | Yes | (14) |
Meta-analysis
The associations between the Arg213His polymorphism
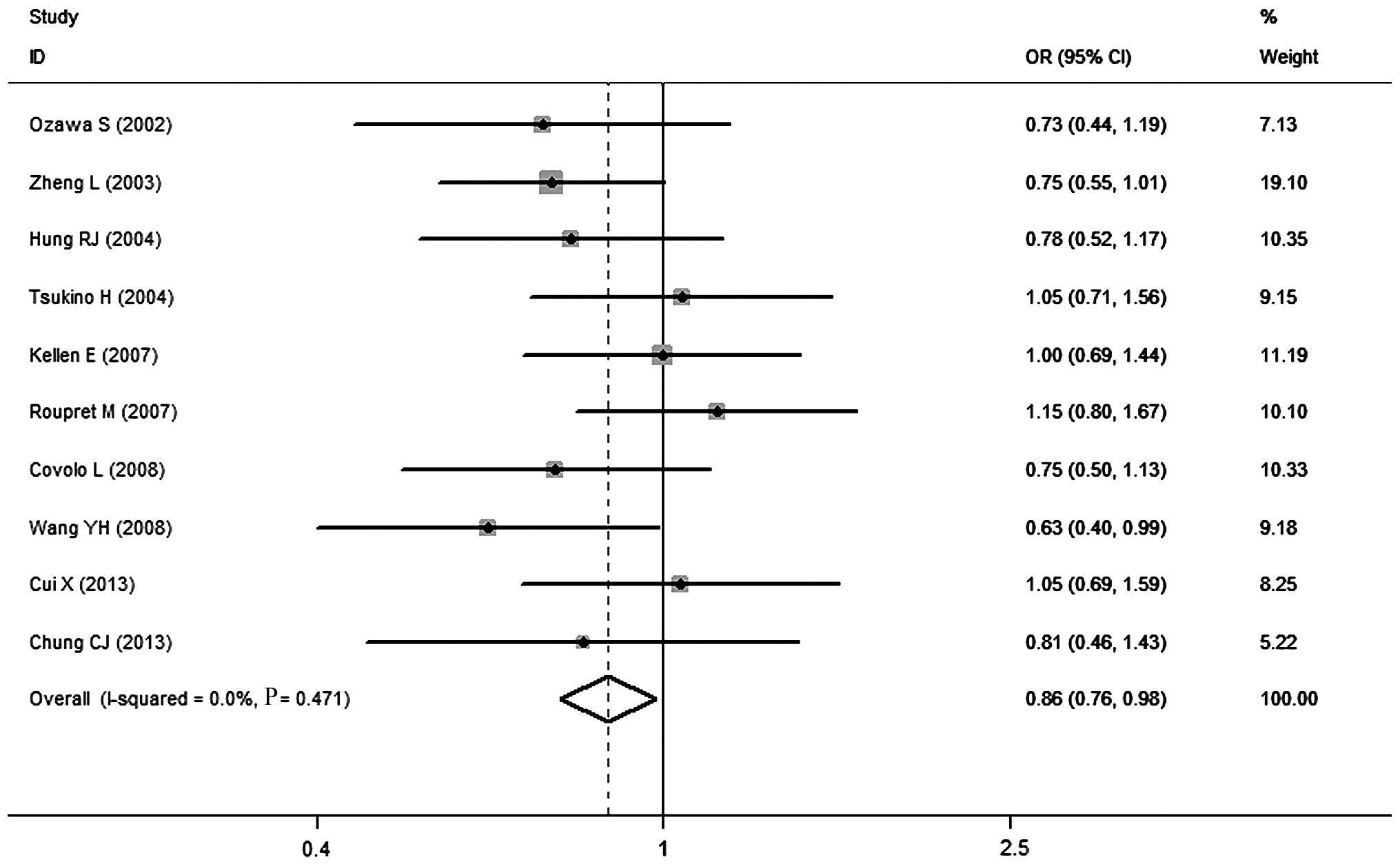
in SULT1A1 and the risk of UC are summarized in Table II. Overall, a significant
association was observed between this polymorphism and decreased UC
risk in the heterozygote model (OR=0.86, 95% CI: 0.76–0.98;
Fig. 2), while no obvious
association was found in the homozygote, recessive and dominant
models. However, there was significant between-study heterogeneity;
therefore, we performed subgroup analyses. Through stratified
analyses, the heterogeneity of the subgroup was significantly
reduced. In the subgroup analysis by ethnicity, no significant
association was observed, except for the mixed population in the
homozygote (OR=0.58, 95% CI: 0.35–0.94) and the dominant (OR=0.71,
95% CI: 0.53–0.94) models. A stratified analysis was also performed
by source of controls. We demonstrated that the SULT1A1 Arg213His
polymorphism was significantly associated with a decreased UC risk
in population-based controls under the dominant model (OR=0.67, 95%
CI: 0.46–0.97). When restricting the analysis to cancer type, the
results indicated that individuals with the G/G genotype had a
significantly higher BC risk in the heterozygote (OR=0.84, 95% CI:
0.71–0.99), the homozygote (OR=0.71, 95% CI: 0.52–0.97) and the
dominant (OR=0.82, 95% CI: 0.70–0.96) models. Of note, a
conflicting association was found in the UTUC subgroup under the
homozygote (OR=2.18, 95% CI: 1.28–3.69) and the recessive (OR=2.05,
95% CI: 1.24–3.38) models. Moreover, the association between the
SULT1A1 Arg213His polymorphism and UC risk was evaluated according
to different smoking status. The stratification by smoking status
revealed that the Arg213His polymorphism was associated with a
decreased risk of UC in non-smokers (OR=0.70, 95% CI: 0.53–0.92)
but not in smokers (OR=0.85, 95% CI: 0.70–1.03) under the dominant
model (Table III).
 | Table IISummary OR of the SULT1A1 Arg213His
polymorphism and urothelial carcinoma risk. |
Table II
Summary OR of the SULT1A1 Arg213His
polymorphism and urothelial carcinoma risk.
| Variables | No.a | Cases | Ctrls | GA vs. GG | Pb | AA vs. GG | Pb | GA/AA vs. GG | Pb | AA vs. GA/GG | Pb |
|---|
| Total | 10 | 2,495 | 2,905 | 0.86
(0.76–0.98) | 0.471 | 0.96
(0.65–1.41) | 0.046 | 0.88
(0.75–1.03) | 0.089 | 1.01
(0.80–1.28) | 0.115 |
| Ethnicity | |
|
Asian | 5 | 1,245 | 1,441 | 0.86
(0.70–1.05) | 0.392 | 1.05
(0.58–1.88) | 0.577 | 0.87
(0.69–1.09) | 0.270 | 1.04
(0.59–1.84) | 0.617 |
|
Caucasian | 4 | 866 | 1,078 | 0.92
(0.76–1.12) | 0.369 | 1.08
(0.59–1.96) | 0.041 | 0.95
(0.72–1.25) | 0.082 | 1.22
(0.89–1.67) | 0.066 |
|
Mixed | 1 | 384 | 386 | 0.75
(0.55–1.01) | – | 0.58
(0.35–0.94) | – | 0.71
(0.5–30.94) | – | 0.66
(0.41–1.06) | – |
| Cancer types | |
| BC | 5 | 1,264 | 1,453 | 0.84
(0.71–0.99) | 0.574 | 0.71
(0.52–0.97) | 0.828 | 0.82
(0.70–0.96) | 0.483 | 0.77
(0.56–1.04) | 0.929 |
|
Mixed | 4 | 268 | 268 | 0.81
(0.64–1.02) | 0.390 | 1.02
(0.53–1.96) | 0.413 | 0.82
(0.63–1.07) | 0.250 | 1.02
(0.55–1.92) | 0.449 |
|
UTUC | 1 | 963 | 1,184 | 1.15
(0.80–1.67) | – | 2.18
(1.2–83.69) | – | 1.37
(0.97–1.92) | – | 2.05
(1.24–3.38) | – |
| SOC | |
| HB | 8 | 1,911 | 2,134 | 0.87
(0.75–1.02) | 0.420 | 1.17
(0.77–1.80) | 0.224 | 0.90
(0.74–1.09) | 0.090 | 1.33
(0.96–1.84) | 0.361 |
| PB | 2 | 584 | 771 | 0.84
(0.67–1.06) | 0.226 | 0.67
(0.46–0.97) | 0.362 | 0.81
(0.61–1.08) | 0.188 | 0.72
(0.51–1.04) | 0.566 |
 | Table IIISULT1A1 Arg213His polymorphism and
urothelial carcinoma risk in smokers and nonsmokers. |
Table III
SULT1A1 Arg213His polymorphism and
urothelial carcinoma risk in smokers and nonsmokers.
| Smoking status | No.a | Cases | Controls | GA/AA vs. GG | Pb |
|---|
| Smokers | 6 | 1,123 | 981 | 0.85
(0.70–1.03) | 0.855 |
| Nonsmokers | 6 | 495 | 671 | 0.70
(0.53–0.92) | 0.247 |
BC is the most common type of UC. We also identified
the association between the Arg213His polymorphism and BC risk. In
two Japanese studies (14,15), the majority of cases were BC
patients. Herein, these two case groups may be considered as two
groups of BC patients by approximation. Namely, we considered that
these two case groups may represent the characteristics of BC
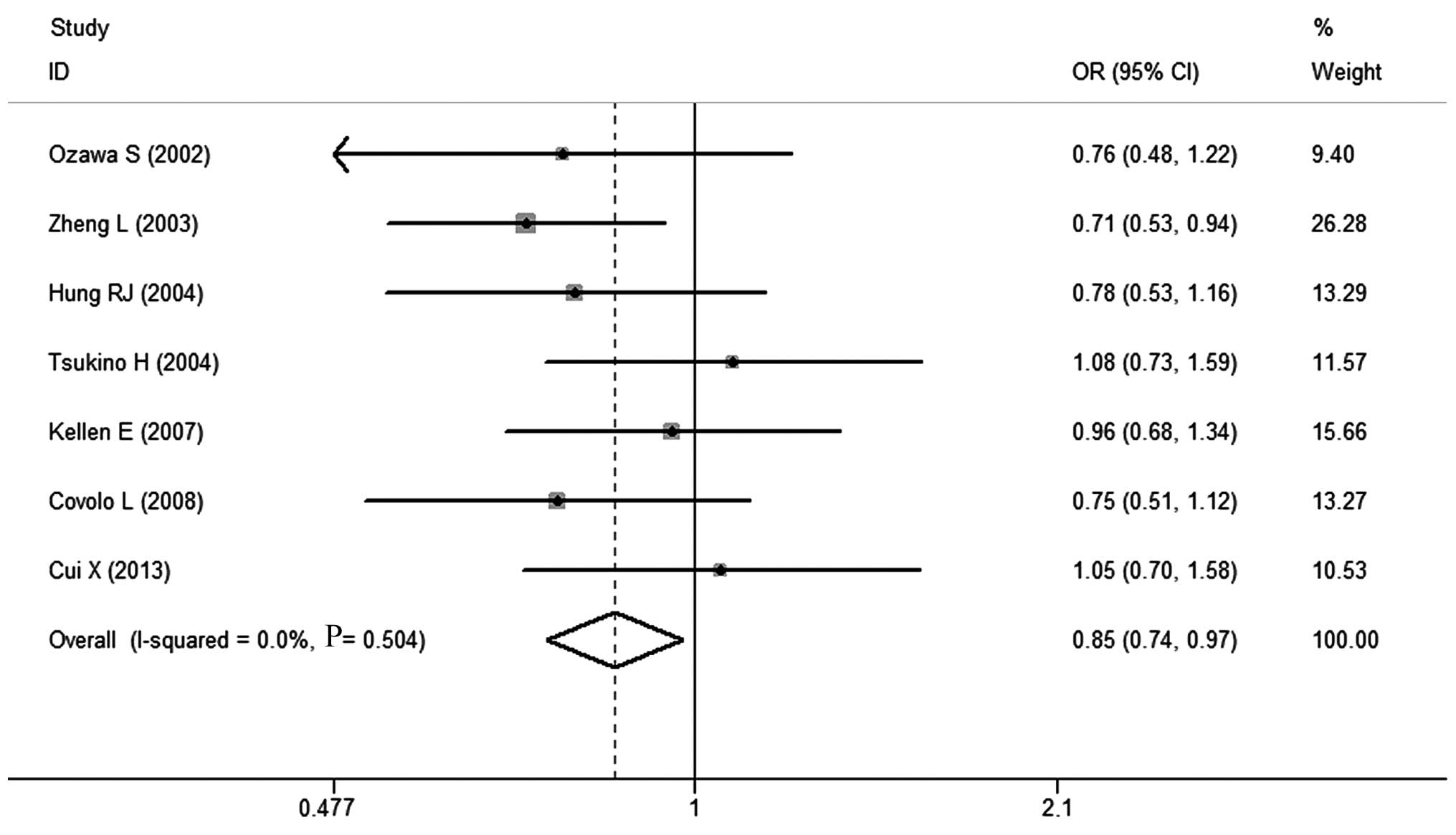
patients. As shown in Table IV, a
significantly decreased risk of BC was associated with the SULT1A1
G/G polymorphism for the heterozygote (OR=0.88, 95% CI: 0.74–0.99)
and the dominant (OR=0.85, 95% CI: 0.74–0.97) models (Fig. 3). In the subgroup analysis by
ethnicity, we did not observe any significant association between
this polymorphism and BC risk among Asians and Caucasians in any of
the genetic models. In the stratified analysis by source of
controls, a significant association was found with population-based
source in the homozygote model (OR=0.67, 95% CI: 0.46–0.97). These
results were similar to those obtained when restricting the
analysis to cancer type.
 | Table IVSummary OR of the SULT1A1 Arg213His
polymorphism and bladder cancer risk. |
Table IV
Summary OR of the SULT1A1 Arg213His
polymorphism and bladder cancer risk.
| Variables | No.a | Cases | Ctrls | GA vs. GG | Pb | AA vs. GG | Pb | GA/AA vs. GG | Pb | AA vs. GA/GG | Pb |
|---|
| Total | 7 | 1,736 | 1,973 | 0.88
(0.74–0.99) | 0.626 | 0.76
(0.56–1.02) | 0.795 | 0.85
(0.74–0.97) | 0.504 | 0.81
(0.61–1.08) | 0.899 |
| Ethnicity | |
|
Asian | 3 | 754 | 777 | 0.95
(0.74–1.22) | 0.457 | 1.20
(0.59–2.44) | 0.900 | 0.98
(0.77–1.24) | 0.479 | 1.23
(0.61–2.49) | 0.930 |
|
Caucasian | 3 | 598 | 810 | 0.85
(0.68–1.06) | 0.540 | 0.79
(0.51–1.24) | 0.989 | 0.84
(0.68–1.04) | 0.620 | 0.83
(0.54–1.27) | 0.997 |
|
Mixed | 1 | 384 | 386 | 0.75
(0.55–1.01) | – | 0.58
(0.35–0.94) | – | 0.71
(0.53–0.94) | – | 0.66
(0.41–1.06) | – |
| SOC | |
| HB | 5 | 1,152 | 1,202 | 0.87
(0.72–1.05) | 0.583 | 0.95
(0.58–1.55) | 0.906 | 0.88
(0.74–1.06) | 0.534 | 1.01
(0.62–1.64) | 0.951 |
| PB | 2 | 584 | 771 | 0.84
(0.67–1.06) | 0.226 | 0.67
(0.46–0.97) | 0.362 | 0.80
(0.64–1.00) | 0.188 | 0.72
(0.51–1.04) | 0.566 |
Test for heterogeneity
When we included all the eligible articles, there
was significant heterogeneity for heterozygote (P=0.046) and
dominant (P=0.089) model comparisons. Subsequently, we used a
meta-regression analysis to investigate the source of heterogeneity
by ethnicity, cancer type and source of controls. Finally, we found
that the cancer type (τ2=0, P=0.008) contributed to significantly
altered heterogeneity, which may account for 100% source of
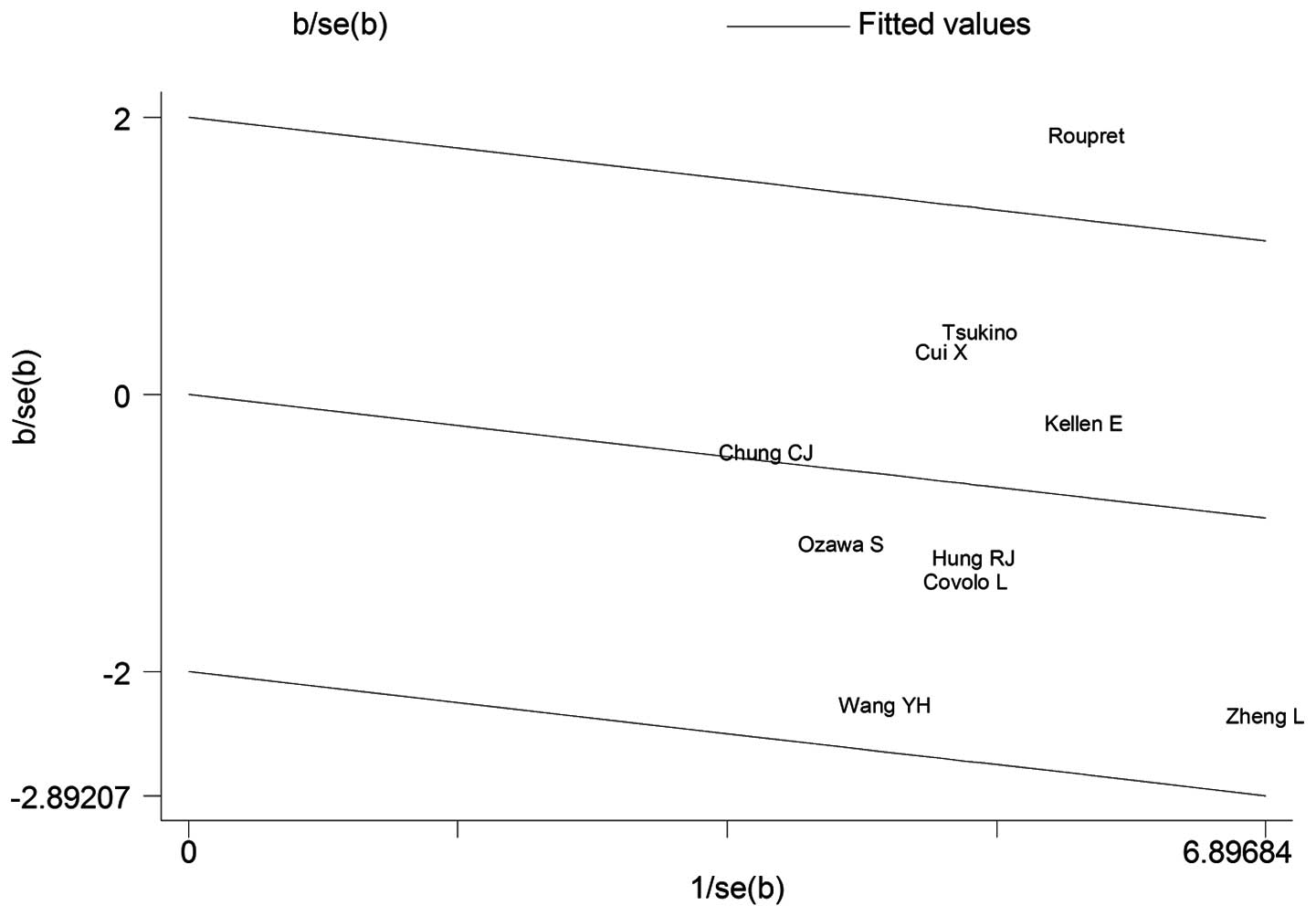
heterogeneity. In addition, a Galbraith plot was also created and
the result demonstrated that the study of Roupret et al
(16) was the source of
heterogeneity (Fig. 4). When 7
studies on BC were included, no heterogeneity was observed in any
of the genetic models.
Sensitivity analyses
We next conducted a leave-one-out sensitivity
analysis to determine whether a particular study or studies would
result in heterogeneity. It was demonstrated that the study of
Roupret et al (16) may
qualitatively alter the pooled ORs for the Arg213His polymorphism.
Following exclusion of this study, the degree of heterogeneity
significantly decreased. The same method was used to assess the
stability of our results when we included 7 articles on BC.
Consequently, the sensitivity analysis confirmed that the results
of this meta-analysis were statistically robust.
Publication bias
Begg's funnel plot and Egger's test were performed
to assess the publication bias. The shape of funnel plots did not
reveal any evidence of obvious asymmetry in any of the comparison
models (Fig. 5) and the results of
the Begg's test did not show any evidence of publication bias.
Similar results were obtained when we investigated the association
between Arg213His polymorphism and risk of BC.
Discussion
SULT1A1 is a phase II detoxification enzyme, which
has the ability to sulphate phenolic and steroid compounds
(24). SULT1A1 activity varies
several fold among individuals and exhibits a strong familial
segregation, suggesting that genetic factors play a significant
role in determining the enzymatic activity of SULT1A1 (25). A common single-nucleotide
polymorphism in the coding region of SULT1A1 (Arg213His) has been
identified and was reported to be associated with decreased
platelet enzymatic activity and thermostability. This mutation may
affect an individual's capacity to efficiently sulfate endogenous
compounds, drugs and xenobiotics and, consequently, increase an
individual's susceptibility to cancer (26). To date, a number of case-control
studies have been conducted to investigate the association of this
polymorphism with UC in humans. Unfortunately, the link remains
unclear and ambiguous. To address this issue, we conducted the
present meta-analysis based on 10 studies, including a total of
2,495 cases and 2,905 controls. A meta-analysis is a powerful tool,
as it may provide more reliable results compared to a single study
and reduce random error (27).
Finally, our meta-analysis indicated that the Arg213His
polymorphism in the SULT1A1 gene may contribute to a significant
decrease in the overall risk of BC. However, compelling evidence on
a consistent association in UC is currently lacking.
UC is a highly aggressive malignancy that is
associated with significant morbidity and mortality. UC may be
mainly classified as UTUC and BC, according to the anatomical
location. We observed that individuals with the A/A genotype were
possibly associated with a decreased risk of UC when all the
eligible studies were pooled into the meta-analysis. As anatomical
differences may affect the results from meta-analyses, we also
limited our analyses to BC and UTUC. A total of 4 studies in our
meta-analysis enrolled cases including BC as well as UTUC patients.
We were unable to consult the authors for the detailed original
data of their studies; however, through careful reading, we found
that the majority of the cases in 2 Japanese studies (16,17)
were BC patients. Thus, we decided to combine these 2 studies with
5 studies (19–23) that only included BC patients, to
probe the association between the Arg213His polymorphism and BC.
Consequently, our findings demonstrated that the Arg213His
polymorphism was significantly associated with a decreased risk of
BC. To eliminate the potential effect of UTUC cases and determine
the association more precisely, we excluded the 2 Japanese studies
in the subgroup analysis by cancer type. As expected, the results
were in accordance with the abovementioned observations. Of note, a
contradictory and interesting association was observed in the UTUC
subgroup. Our results suggested that individuals with the A/A
genotype had a higher risk of UTUC compared to subjects carrying
the wild genotype, raising the question of why our results on UTUC
did not confirm the protective effect of the Arg213His polymorphism
with respect to BC risk. This discrepancy may be attributed to the
differences between BC and UTUC. Although UTUC and BC both
originate from the urothelium and may share certain risk factors or
molecular disruption pathways, each has its own distinct
characteristics. In comparison to UC of the bladder, primary UTUCs
are less common, representing only 5% of all UCs and <10% of
renal tumors (28). The annual
incidence of UTUC is low compared to that of BC. Catto et al
(29,30) provided evidence that the extent of
the mutator and methylator phenotypes in UC differed with tumor
location and suggested that carcinogens may affect the urinary
tract in different ways. Furthermore, several studies demonstrated
that patients with UTUC have a higher risk, compared to those with
BC, for hereditary non-polyposis colorectal cancer and
microsatellite instability is more common in UTUC compared to BC
(31,32). Green et al (33) described UTUC and BC as disparate
twins in their recent review and revealed that there were
practical, anatomical, biological and molecular differences between
these two types of cancer. In addition, the study of Liang et
al (34) strongly suggested
that the urothelia of the bladder and upper urinary tract represent
two separate cell lineages and are most likely maintained by
distinct stem cell populations. Therefore, we hypothesized that
genetic polymorphisms of enzymes metabolizing carcinogens may yield
products of different activity and lead to opposite cancer risks.
In other words, histologically identical tumors may arise through
different molecular mechanisms. In two Japanese studies, although
the cases included BC as well as UTUC patients, the sum of the UTUC
patients was inadequate to have statistical power affecting the
overall results. It is noteworthy that certain other factors may
also result in this discrepancy. For UTUC, there was only one study
included in the analysis with limited sample sizes and the subjects
were all French. In addition, we found that the DNA of the cases in
this UTUC study was extracted from tissues, whereas the DNA in the
majority of the other studies was extracted from the blood. It is
widely accepted that DNA from white blood cells rather than tissue
should be used for determining genetic polymorphisms, as loss of
heterozygosity has been noted in exfoliated cells (35). Taken together, a relatively small
sample size, differences in country and race and diverse methods
were likely to affect our results. Therefore, a larger sample size
and a higher number of articles are required to verify the
association between the Arg213His polymorphism and UTUC.
Different ethnicities may have disparate genetic
backgrounds, which affect the association between polymorphism and
cancer susceptibility. We performed a stratified analysis by
ethnicity; however, we were unable to identify any positive
association among Asians and Caucasians, whether 10 or 7 articles
were included in the analyses. The number of subjects was
relatively limited, without sufficient statistical power to
investigate the true association. Furthermore, in view of the
diversity of possible comparisons and the unavoidable flexibility
in selecting and defining the correlations, the associations may
not be necessarily reliable. For example, selection bias, matching
criteria, misclassifications on disease status and genotyping may
play a role. In addition, we mentioned that only 5 studies
investigated Asian populations and only 4 studies included
Caucasian populations. Therefore, larger-scale studies and combined
analysis are required to further investigate ethnic differences in
the effect of the Arg213His polymorphism on the risk of UC. In the
subgroup analysis by source of controls, a significantly decreased
UC risk was observed among studies using population-based controls.
Some bias may exist in hospital-based studies, as such controls may
represent a sample of an indistinct reference population instead of
the general population, particularly when the genotypes
investigated were associated with the condition of the
hospital-based controls. Thus, a proper and representative
cancer-free control sample is crucial for reducing bias in such
genotype association studies.
Thus far, the single most important risk factor in
the development of UC is exposure to cigarette smoke. It is
estimated that ~80% of all UC may be attributed to cigarette
smoking (34). It was previously
reported that smoking increases the risk of developing BC and UTUC
by as high as 4- and 6-fold, respectively (37). Arylamines, which are found in
tobacco smoke, proceed via a two-step pathway involving cytochrome
P450 1A2-catalyzed N-hydroxylation followed by an O-esterification
step catalyzed by N-acetyltransferases (NATs) and/or SULTs. As a
consequence of these reactions, in UCs, the aryl nitrenium ions
generated from N-hydroxylamines are considered to be the ultimate
reactive intermediates responsible for carcinogenic activity. In
this study, we performed a stratified analysis by smoking status
based on 6 studies that presented detailed data of smoking status
and drew the conclusion that the Arg213His polymorphism was
associated with a decreased risk of UC in non-smokers, but not in
smokers. A possible explanation is that the association between
this polymorphism and the decreased risk of UC may be masked by the
overwhelming accumulated exposure to tobacco carcinogens, so that
the association is more evident in non-smokers. In addition,
passive smoking should be taken into consideration. Non-smokers may
be exposed to second-hand smoking. When tobacco smoke is inhaled,
some of the carcinogens are absorbed through the lungs into the
blood. However, these results require confirmation by further
large-scale studies.
The significance of heterogeneity, which may affect
the results of this meta-analysis, must be addressed. Obvious
heterogeneity between studies was observed when we included all
eligible studies. Consistently, through meta-regression analysis
and creation of a Galbraith plot, we observed that the study of
Roupret et al (18), which
was the only study that included UTUC patients as case group, was
the main source of heterogeneity. The degree of heterogeneity
significantly decreased after this study was excluded. The most
likely interpretation may be the differences between BC and UTUC,
as mentioned above. In addition, the differences in the
pathological stages of the patients and the genotyping methods,
such as Taqman and PCR-RFLP, may also lead to heterogeneity.
Furthermore, despite the overall robust statistical
evidence generated through this analysis, certain limitations were
identified. First, our results were based on unadjusted estimates,
while a more precise analysis should be conducted if all individual
raw data were available, which would allow for adjustment by other
covariates, including age, gender, alcohol consumption, cigarette
consumption and other lifestyle habits. Second, only studies that
were indexed by the selected databases were included for the
meta-analysis and some relevant published studies or unpublished
studies with null results were missed, which may have biased our
results. Third, UC is a multifactorial disease that results from
complex interactions between several genetic and environmental
factors, suggesting that there is not a single genetic or
environmental factor significantly affecting the susceptibility to
UC. Thus, the combined effects of different gene polymorphisms
should be further analyzed. In our meta-analysis, as SULT1A1 and
NAT2 are both involved in the metabolism of arylamines and NAT2 has
been reported to play a role in carcinogenesis, we aimed to discuss
the combined effects of SULT1A1 and NAT2 genetic polymorphisms.
However, we were unable to do so, as only 3 studies provided
detailed data of these two genes. Fourth, to the best of our
knowledge, this is the first meta-analysis regarding the
comprehensive assessment of the association between the Arg213His
polymorphism and the risk of UC. Thus, the number of published
studies was not sufficient for a comprehensive analysis,
particularly for UTUC. Therefore, more studies with larger sample
sizes and detailed information are required. In addition, the
patients exhibited different histological stages and grades or
other coexisting conditions, which may have affected our results.
Furthermore, other pathological types of urinary BC should be
considered, which may be an interference factor.
In conclusion, the results from the present
meta-analysis indicated an association between the Arg213His
polymorphism and a decreased risk of BC. However, there was
insufficient evidence to support a consistent association between
this polymorphism and UC, partly due to the differences between BC
and UTUC. To advance the understanding of this association, the
following recommendations have been made: First, decrease
false-positive and -negative results through stratifying large
samples by age, gender, dietary habits, lifestyle and ethnicity.
Second, histopathological and clinical data may be used to
subclassify the type and stage of UC to obtain a more homogeneous
population for the analysis. Thirdly, given the fact that UC is a
type of polygenic disorder, the combined effects of different gene
polymorphisms require further analysis. Finally, more case-control
studies or updated meta-analyses should be conducted to elucidate
the possible roles of the Arg213His polymorphism in the etiology of
UC.
References
|
1
|
Chung CJ, Huang CY, Pu YS, Shiue HS, Su CT
and Hsueh YM: The effect of cigarette smoke and arsenic exposure on
urothelial carcinoma risk is modified by glutathione S-transferase
M1 gene null genotype. Toxicol Appl Pharmacol. 266:254–259. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milenkovic-Petronic D, Milojevic B, Djokic
M, Sipetic-Grujicic S, Milojevic IG, Bumbasirevic U and Dzamic Z:
The impact of tumor size on outcomes in patients with upper urinary
tract urothelial carcinoma. Int Urol Nephrol. 46:563–569. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tanaka N, Kikuchi E, Kanao K, et al: The
predictive value of positive urine cytology for outcomes following
radical nephroureterectomy in patients with primary upper tract
urothelial carcinoma: a multi-institutional study. Urol Oncol.
32(48): e19–26. 2014. View Article : Google Scholar
|
|
4
|
Peng Q, Mo C, Tang W, et al: DNA repair
gene XRCC3 polymorphisms and bladder cancer risk: a meta-analysis.
Tumour Biol. 35:1933–1944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ross JS, Wang K, Al-Rohil RN, et al:
Advanced urothelial carcinoma: next-generation sequencing reveals
diverse genomic alterations and targets of therapy. Mod Pathol.
27:271–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Glatt H and Meinl W: Pharmacogenetics of
soluble sulfotransferases (SULTs). Naunyn Schmiedebergs Arch
Pharmacol. 369:55–68. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ginsberg G, Guyton K, Johns D, et al:
Genetic polymorphism in metabolism and host defense enzymes:
implications for human health risk assessment. Crit Rev Toxicol.
40:575–619. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YH, Juang GD, Hwang TI, et al:
Genetic polymorphism of sulfotransferase 1A1, cigarette smoking,
hazardous chemical exposure and urothelial cancer risk in a
Taiwanese population. Int J Urol. 15:1029–1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dooley TP, Obermoeller RD, Leiter EH, et
al: Mapping of the phenol sulfotransferase gene (STP) to human
chromosome 16p12.1-p11.2 and to mouse chromosome 7. Genomics.
18:440–443. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pereira WO, Paiva AS, Queiroz JW, et al:
Genetic polymorphism in the sulfotransferase SULT1A1 gene in
cancer. Cancer Genet Cytogenet. 160:55–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Daniels J and Kadlubar S: Sulfotransferase
genetic variation: from cancer risk to treatment response. Drug
Metab Rev. 45:415–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou HC, Lang NP and Kadlubar FF:
Metabolic activation of the N-hydroxy derivative of the carcinogen
4-aminobiphenyl by human tissue sulfotransferases. Carcinogenesis.
16:413–417. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davey Smith G and Egger M: Meta-analyses
of randomised controlled trials. Lancet. 350(1182)1997.
|
|
14
|
Mantel N and Haenszel W: Statistical
aspects of the analysis of data from retrospective studies of
disease. J Natl Cancer Inst. 22:719–748. 1959.PubMed/NCBI
|
|
15
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozawa S, Katoh T, Inatomi H, et al:
Association of genotypes of carcinogen-activating enzymes, phenol
sulfotransferase SULT1A1 (ST1A3) and arylamine N-acetyltransferase
NAT2, with urothelial cancer in a Japanese population. Int J
Cancer. 102:418–421. 2002. View Article : Google Scholar
|
|
17
|
Tsukino H, Kuroda Y, Nakao H, et al:
Cytochrome P450 (CYP) 1A2, sulfotransferase (SULT) 1A1, and
N-acetyltransferase (NAT) 2 polymorphisms and susceptibility to
urothelial cancer. J Cancer Res Clin Oncol. 130:99–106. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rouprêt M, Cancel-Tassin G, Comperat E, et
al: Phenol sulfotransferase SULT1A1*2 allele and enhanced risk of
upper urinary tract urothelial cell carcinoma. Cancer Epidemiol
Biomarkers Prev. 16:2500–2503. 2007.
|
|
19
|
Covolo L, Placidi D, Gelatti U, et al:
Bladder cancer, GSTs, NAT1, NAT2, SULT1A1, XRCC1, XRCC3, XPD
genetic polymorphisms and coffee consumption: a case-control study.
Eur J Epidemiol. 23:355–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kellen E, Zeegers M, Paulussen A, et al:
Does occupational exposure to PAHs, diesel and aromatic amines
interact with smoking and metabolic genetic polymorphisms to
increase the risk on bladder cancer?; The Belgian case control
study on bladder cancer risk. Cancer Lett. 245:51–60. 2007.
View Article : Google Scholar
|
|
21
|
Hung RJ, Boffetta P, Brennan P, et al:
GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with
environmental exposures and bladder cancer risk in a high-risk
population. Int J Cancer. 110:598–604. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cui X, Lu X, Hiura M, et al: Association
of genotypes of carcinogen-metabolizing enzymes and smoking status
with bladder cancer in a Japanese population. Environ Health Prev
Med. 18:136–142. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng L, Wang Y, Schabath MB, Grossman HB
and Wu X: Sulfotransferase 1A1 (SULT1A1) polymorphism and bladder
cancer risk: a case-control study. Cancer Lett. 202:61–69. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu X, Kubota T, Dhakal I, et al: Copy
number variation in sulfotransferase isoform 1A1 (SULT1A1) is
significantly associated with enzymatic activity in Japanese
subjects. Pharmgenomics Pers Med. 6:19–24. 2013.PubMed/NCBI
|
|
25
|
Tengström M, Mannermaa A, Kosma VM,
Hirvonen A and Kataja V: SULT1A1 rs9282861 polymorphism - a
potential modifier of efficacy of the systemic adjuvant therapy in
breast cancer? BMC Cancer. 12:2572012.PubMed/NCBI
|
|
26
|
Coughtrie MW, Gilissen RA, Shek B, et al:
Phenol sulphotransferase SULT1A1 polymorphism: molecular diagnosis
and allele frequencies in Caucasian and African populations.
Biochem J. 337:45–49. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Munafò MR and Flint J: Meta-analysis of
genetic association studies. Trends Genet. 20:439–444.
2004.PubMed/NCBI
|
|
28
|
Patel N, Arya M, Muneer A, et al:
Molecular aspects of upper tract urothelial carcinoma. Urol Oncol.
32(28): e11–20. 2014. View Article : Google Scholar
|
|
29
|
Catto JW, Azzouzi AR, Amira N, et al:
Distinct patterns of microsatellite instability are seen in tumours
of the urinary tract. Oncogene. 22:8699–8706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Catto JW, Azzouzi AR, Rehman I, et al:
Promoter hypermethylation is associated with tumor location, stage,
and subsequent progression in transitional cell carcinoma. J Clin
Oncol. 23:2903–2910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roupret M, Catto J, Coulet F, et al:
Microsatellite instability as indicator of MSH2 gene mutation in
patients with upper urinary tract transitional cell carcinoma. J
Med Genet. 41:e912004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bermejo JL, Eng C and Hemminki K: Cancer
characteristics in Swedish families fulfilling criteria for
hereditary nonpolyposis colorectal cancer. Gastroenterology.
129:1889–1899. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green DA, Rink M, Xylinas E, et al:
Urothelial carcinoma of the bladder and the upper tract: disparate
twins. J Urol. 189:1214–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liang FX, Bosland MC, Huang H, et al:
Cellular basis of urothelial squamous metaplasia: roles of lineage
heterogeneity and cell replacement. J Cell Biol. 171:835–844. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klug SJ, Ressing M, Koenig J, et al: TP53
codon 72 polymorphism and cervical cancer: a pooled analysis of
individual data from 49 studies. Lancet Oncol. 10:772–784. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tyler A: Urothelial cancers: ureter, renal
pelvis, and bladder. Semin Oncol Nurs. 28:154–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Crivelli JJ, Xylinas E, Kluth LA, et al:
Effect of smoking on outcomes of urothelial carcinoma: a systematic
review of the literature. Eur Urol. 65:742–754. 2014. View Article : Google Scholar : PubMed/NCBI
|