Introduction
The bone is the most common site of metastasis in
prostate cancer patients, with bone metastases occurring in ∼65–75%
terminal cases of prostate cancer. Androgen deprivation therapy
(ADT) is commonly used for advanced-stage disease, such as that
involving multiple bone metastases, with significant effects on the
primary tumor as well as the bone metastases. Symptoms, such as
pain, improve in almost all patients, with a median survival period
of 36 months and a 5-year survival rate of 25% (1–3).
However, decreases in the effectiveness of ADT are increasingly
being reported, with cancer ultimately progressing to
castration-resistant prostatic cancer (CRPC) and pain control is a
compelling issue in CRPC patients with bone metastasis. Unlike drug
treatment with analgesics, radiation therapy is extremely effective
in achieving pain relief in bone metastasis patients. However, the
use of hemibody irradiation to treat widespread bone metastases may
induce severe bone marrow toxicity (4, 5).
Strontium-89 (Sr-89) is a pure β-emitter with a half-life of 50.5
days. Blake et al (6)
demonstrated that Sr-89 follows the biochemical pathway of calcium
within the bone, is preferentially taken up at sites of increased
bone turnover, irrespective of the primary tumor where it remains
over a more prolonged period of time compared to the normal bone
tissue from which it is rapidly washed; therefore, maximal activity
is observed at sites of bone metastases following the
administration of this drug. The range of β particles in bone
tissues is short (3 mm); hence, Sr-89 is associated with a
relatively low rate of hematological toxicities (6).
Outside Japan, Sr-89 has been used since the 1990's
and its efficacy has been demonstrated. However, this drug was only
introduced in Japan in July, 2007 and thus far there have been few
reports on the administration of strontium in Japanese prostate
cancer patients. Therefore, in order to elucidate the effectiveness
and adverse events of Sr-89 for the treatment of bone metastasis
from prostate cancer in Japanese patients, we retrospectively
reviewed cases treated at our institution.
Materials and methods
Patients
A total of 18 prostate cancer patients with painful
bone metastases, as diagnosed on bone scintigraphy, who were
treated with Sr-89 at the National Kyushu Cancer Center, Fukuoka,
Japan, between February, 2008 and April, 2014, were retrospectively
reviewed. Written informed consent was obtained from all the
patients prior to the initiation of treatment and the study
protocol was approved by the Institutional Ethics Committee. The
inclusion criteria were persistent pain despite the use of
analgesics and a life expectancy of >3 months. The main
exclusion criteria were disseminated intravascular coagulation, a
significantly degraded renal function or severe bone marrow
suppression (white blood cell count <2,000/mm3,
platelet count <50,000/mm3 and hemoglobin level <8
g/dl). Pain response assessments and blood tests were conducted
prior to and biweekly after Sr-89 injection for 3 months. Sr-89 was
administered via intravenous injection at a dose of 2 MBq/kg to a
maximum of 141 MBq per patient. If the patient received ≥2 courses
of Sr-89, only the first injection of Sr-89 was included in the
analysis.
Pain assessment
Information regarding pain and analgesic effect was
obtained via a physician interview using the visual analog scale
(VAS). Patients who exhibited a reduction in the VAS score from
baseline were defined as pain responders, while the remaining
subjects were classified as pain non-responders.
Prostate-specific antigen (PSA) and
serum alkaline phosphatase (ALP) response and toxicity
Patients who exhibited a reduction in the PSA level
from baseline, even if only marginal, were defined as PSA
responders, whereas the remaining subjects were defined as PSA
non-responders. Similarly, patients who exhibited a reduction in
the serum ALP level from baseline, were defined as ALP responders,
while the remaining subjects were classified as ALP non-responders.
The toxicities were graded using the Common Terminology Criteria
for Adverse Events, version 4.0.
Statistical analysis
The statistical analyses were conducted using the
JMP® Pro software package, version 9.0.2 (SAS Institute,
Inc., Cary, NC, USA). The Mann-Whitney U test and
χ2 test were used to assess differences between
pain responders and non-responders and between patients with and
those without myelosuppression. The significance of the clinical
parameters associated with improvements in bone pain and
myelosuppression following Sr-89 treatment was assessed using a
logistic regression analysis. A P-value of <0.05 was considered
to indicate a statistically significant difference.
Results
Patient characteristics
The baseline characteristics of the 18 patients who
underwent Sr-89 therapy are summarized in Table I. With respect to analgesic
treatment, opioids were used in 11 patients (61.1%). Regarding
prior treatment for prostate cancer, all the patients had a history
of combined androgen blockade (CAB) therapy and 8 patients (44.4%)
had a history of bone-modifying agent (BMA) administration. The
anticancer agent docetaxel was used in 4 cases (22.2%) and
estramustine phosphate was administered to 3 patients (16.7%). A
total of 6 patients (33.3%) received palliative external-beam
radiotherapy (EBRT) to symptomatic sites of bone metastasis prior
to Sr-89 treatment.
 | Table IBaseline patient characteristics. |
Table I
Baseline patient characteristics.
| Characteristics | Patient no. (%)
(n=18) |
|---|
| Age, years | |
| Median
(range) | 76 (60–86) |
| PSA, ng/ml | |
| Median
(range) | 50.628
(1.764–2,017.400) |
| ALP, IU/l | |
| Median
(range) | 973 (248–2,944) |
| Site of
metastasis | |
| Bone | 11 (61.1) |
| Bone+lymph
node | 7 (38.9) |
| Analgesics | |
| NSAIDs | 11 (61.1) |
| Opioid +
NSAIDs | 7 (38.9) |
| Prior treatment | |
| Combined
androgen blockade | 18 (100.0) |
| Radiation
therapy | 9 (50.0) |
| Bone–modifying
agents | 8 (44.4) |
| Steroids | 7 (38.9) |
| Docetaxel | 4 (22.2) |
| Radical
prostatectomy | 3 (16.7) |
| Estramustine
phosphate | 3 (16.7) |
|
Ethinylestradiol | 3 (16.7) |
| Palliative
radiation therapy | 6 (33.3) |
Patient characteristics according to
the pain response
The results regarding pain response are shown in
Table II. A total of 13 patients
(72.2%) achieved a pain response, whereas 5 patients (27.8%) were
classified as pain non-responders. The correlations between the
patient characteristics and pain response are presented in Table III. According to the logistic
regression analysis, the pre-administration characteristics,
including age, PSA, ALP, history of BMA, opioid use and palliative
radiation therapy, duration from CAB nadir and the time since pain
onset, were not found to be significant predictors of the pain
response. Similarly, the post-administration characteristics,
including pain flares and the PSA and ALP response, were not
significant predictors of the pain response.
 | Table IIPatient characteristics according to
the pain response. |
Table II
Patient characteristics according to
the pain response.
| Characteristics | Response (n=13) | No response
(n=5) | P–value |
|---|
| Age, years | | |
0.522 |
| Median
(range) | 76 (60–86) | 73 (63–79) | |
| PSA, ng/ml | | |
0.888 |
| Median
(range) | 25.448
(1.822-1,130.300) | 795.710
(1.764-2,017.400) | |
| ALP, IU/l | | |
0.255 |
| Median
(range) | 912 (248–2,204) | 1,034
(373–2,944) | |
| History of opioid
use | | |
0.952 |
| Yes | 5 | 2 | |
| No | 8 | 3 | |
| History of palliative
radiation therapy | | |
0.710 |
| Yes | 4 | 2 | |
| No | 9 | 3 | |
| History of BMA | | |
0.410 |
| Yes | 5 | 3 | |
| No | 8 | 2 |
| Time since pain
onset, days | | |
0.402 |
| Median
(range) | 489 (21–2,359) | 168 (26–1,349) | |
| Time since CAB nadir,
days | | |
0.805 |
| Median
(range) | 684 (92–2,340) | 658 (-163-2,147) | |
| Pain flare | | |
0.648 |
| Yes | 4 | 1 |
| No | 9 | 4 | |
| PSA response | | |
0.183 |
| Increase | 10 | 4 | |
| Decrease | 3 | 1 | |
| ALP response | | |
0.730 |
| Increase | 4 | 3 | |
| Decrease | 9 | 2 |
 | Table IIIUnivariate analysis of the
correlations between patient characteristics and pain response. |
Table III
Univariate analysis of the
correlations between patient characteristics and pain response.
| Characteristics | Odds ratio | P–value | 95% CI |
|---|
| Age |
0.958 |
0.539 |
0.834–1.100 |
| PSA |
1.002 |
0.191 |
1.000–1.004 |
| ALP |
1.001 |
0.386 |
0.999–1.002 |
| History of opioid
use |
1.067 |
0.952 |
0.129–8.795 |
| History of
palliative radiation therapy |
1.500 |
0.711 |
0.176–12.778 |
| History of BMA |
2.400 |
0.416 |
0.291–19.7899 |
| Time since pain
onset |
0.999 |
0.538 |
0.998–1.001 |
| Duration from CAB
nadir |
1.000 |
0.735 |
0.998–1.001 |
| Pain flare |
0.563 |
0.650 |
0.047–6.771 |
| PSA response |
1.200 |
0.888 |
0.094–15.264 |
| ALP response |
3.375 |
0.266 |
0.396–28.751 |
Toxicities
The toxicities occurring within 3 months following
Sr-89 therapy are listed in Table
IV. While no patients exhibited leukocyte toxicities in this
study, 2 patients experienced myelosuppression, involving anemia
and thrombocytopenia, requiring the transfusion of red cell or
platelet concentrate following administration of Sr-89. Of the 18
patients, 5 (27.8%) reported pain flares, all of whom were
successfully treated with rescue drugs alone.
 | Table IVToxicity within 3 months after
strontium–89 administration. |
Table IV
Toxicity within 3 months after
strontium–89 administration.
| Grade, no.
(n=18) |
|---|
|
|
|---|
|
Toxicitiesa | 3 | 4 | 3 or 4 (%) |
|---|
| Leukopenia | 0 | 0 | 0 (0.0) |
| Anemia | 0 | 2 | 2 (11.1) |
|
Thrombocytopenia | 1 | 0 | 1 (5.6) |
| Pain flare | | | 5 (27.8) |
Patient characteristics according to
bone marrow suppression
The results regarding bone marrow suppression are
shown in Table V. A total of 16
patients (88.9%) did not exhibit bone marrow suppression, whereas
only 2 patients experienced bone marrow suppression. The
correlations between the patient characteristics and bone marrow
suppression are presented in Table
VI. According to the logistic regression analysis, of the
pre-administration characteristics, only ALP was a significant
predictor of bone marrow suppression in the univariate and the
multivariate analyses (P=0.006).
 | Table VPatient characteristics according to
the degree of bone marrow suppression. |
Table V
Patient characteristics according to
the degree of bone marrow suppression.
|
Characteristics | Myelosuppression
(n=2) | No myelosuppression
(n=16) | P–value |
|---|
| Age, years | | |
0.206 |
| Median
(range) | 68 (63–73) | 76 (60–86) | |
| PSA, ng/ml | | | 0.092 |
| Median
(range) | 822.360
(795.710–849.010) | 33.702
(1.764–2,017.400) | |
| ALP, IU/l | | | 0.035 |
| Median
(range) | 2,403
(1,861–2,944) | 896
(248–2,204) | |
| History of opioid
use | | | 0.732 |
| Yes | 1 | 6 | |
| No | 1 | 10 | |
| History of
palliative radiation therapy | | |
0.595 |
| Yes | 1 | 5 | |
| No | 1 | 11 | |
| History of BMA | | |
0.867 |
| Yes | 1 | 7 | |
| No | 1 | 9 | |
| Time since pain
onset, days | | |
0.482 |
| Median
(range) | 342 (26–658) | 459 (21–2,359) | |
| Time since CAB
nadir, days | | |
0.673 |
| Median
(range) | 566 (474–658) | 796
(-163–2,340) | |
 | Table VIAnalysis of the correlations between
the patient characteristics and bone marrow suppression. |
Table VI
Analysis of the correlations between
the patient characteristics and bone marrow suppression.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
|
Characteristics | Odds ratio | P–value | 95% CI | Odds ratio | P–value | 95% CI |
|---|
| Age |
0.898 |
0.290 |
0.695–1.096 |
| PSA |
1.001 |
0.234 |
0.999–1.004 |
| ALP |
1.004 |
0.006 |
1.000–1.012 |
1.004 |
0.006 |
1.000–1.012 |
| History of opioid
use |
1.667 |
0.735 |
0.058–47.734 |
| History of
palliative radiation therapy |
2.200 |
0.605 |
0.076–63.848 |
| History of BMA |
1.286 |
0.867 |
0.045–36.557 |
| Time since pain
onset |
0.999 |
0.469 |
0.993–1.001 |
| Time since CAB
nadir |
0.999 |
0.450 |
0.996–1.001 |
Association between changes in the PSA
and ALP values and the pain response
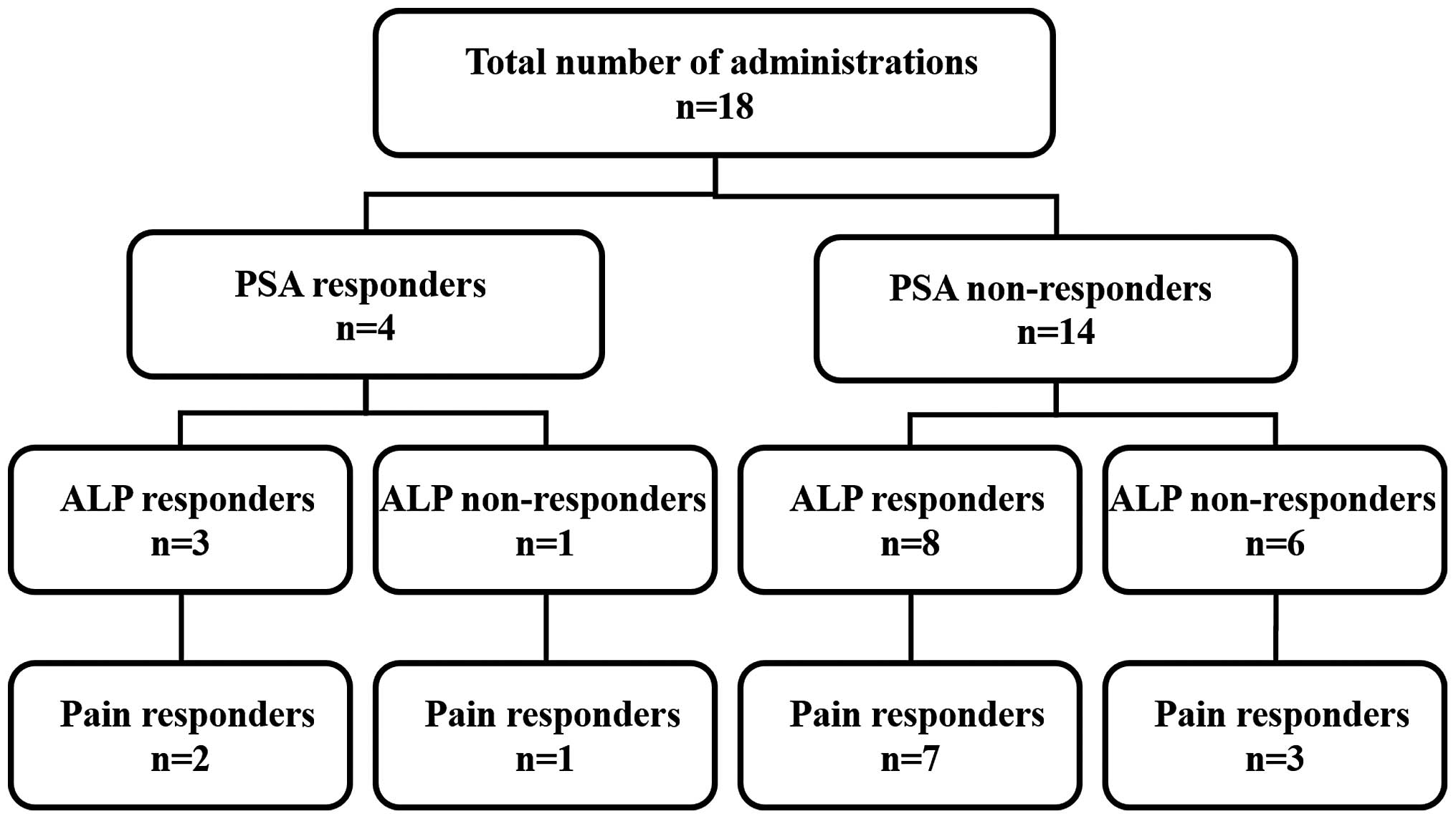
Among the 18 patients, the PSA level decreased in 4
subjects, among whom the ALP level decreased in 3 patients, 2 of
whom reported improvements in bone pain. The PSA level increased in
14 patients, among whom the ALP level decreased in 8 patients, 7 of
whom experienced pain improvement (Fig. 1).
Discussion
The use of Sr-89 chloride therapy to treat painful
bone metastases was first reported in 1942 by Pecher (7). Since then, several phase III studies
(8–11) have demonstrated the usefulness and
cost-effectiveness of Sr-89 as either an alternative or adjunct to
EBRT in males with bone pain resulting from metastatic prostate
cancer. In Japan, a phase III clinical trial of Sr-89 was conducted
in 1995; however, approval for this drug was not obtained, due to
problems with the evaluation method (12). Although Sr-89 subsequently obtained
manufacturing approval in July, 2007, thus far there have been only
few reports describing its administration to Japanese prostate
cancer patients, compared to the findings reported in Europe and
the United States of America. Therefore, in order to elucidate the
effectiveness and adverse events of Sr-89 for the treatment of bone
metastasis from prostate cancer in Japanese patients, we
retrospectively reviewed relevant cases treated at our
institution.
As regards the palliative efficacy of Sr-89, 13
patients (72.2%) in this study achieved a pain response (Table II), based on decreases in their
VAS score. The majority of the previous studies have used scoring
systems; however, differences in the criteria have complicated the
analyses of data, although the definitions of a complete response
and lack of response are straightforward. The proportion of
patients classified as complete responders to Sr-89 ranges between
8 and 77% (mean, 32%), while that of patients exhibiting no
response ranges between 14 and 52% (mean, 25%). Within this range,
44% of patients display some degree of response to Sr-89 treatment,
resulting in a mean overall response rate of 76% (13). Therefore, the administration of
Sr-89 for the treatment of prostate cancer in Japanese patients is
expected to exert an analgesic effect and achieve some pain
relief.
We then investigated the characteristics of patients
who experienced pain relief following Sr-89 administration.
Consequently, the patients administered Sr-89 in this study were
divided into two groups, namely the pain response group and the
non-response group (Table II). No
significant differences were observed between the two groups. The
correlations between the patient characteristics and the pain
response were subsequently analyzed using a logistic regression
analysis. However, there were no significant differences in the
pre-administration factors, including age, PSA, ALP, history of
opioid use, palliative radiation and BMA, time since the CAB nadir
and time since pain onset, according to the univariate and
multivariate analyses (Table
III). Based on these results, Sr-89 was shown to be effective
in pain management for prostate cancer patients with bone
metastasis, although it is difficult to identify patients who will
benefit from this drug prior to treatment. In a study by
Kraeber-Bodéré F et al (14), the patients were classified into
two groups according to the number of foci suggestive of metastases
on bone scintigraphy, namely the ≤10 (moderate bone involvement)
and >10 (extensive bone involvement) foci groups. In that study,
the overall response rate (77 and 75%, respectively) was not
significantly different between the two groups, whereas the rate of
complete response was significantly higher in the group with ≤10
foci (54 vs. 24%; P=0.005) (14).
Sr-89 is effective in patients with early-stage bone metastasis,
while the pain relief effect is lower and adverse events are more
severe in patients with end-stage bone metastasis (15–17).
Therefore, Sr-89 therapy is considered to exert a positive effect
against pain, even when the bone metastasis is not extensive.
However, a pain relief effect may also be obtained in patients with
extensive bone metastasis, although it is difficult to precisely
predict the effect prior to Sr-89 administration (18). These reports support the present
findings.
We then examined the adverse events associated with
the administration of Sr-89 (Table
IV). Finlay et al (13)
reported that the toxic effects associated with Sr-89 are mild and
reversible. Approximately 15% of the patients experience increased
pain or flares after 1–5 days, which may last for 4 days. A
previous trial reported that pain flares were associated with a
good response to Sr-89 (19); by
contrast, a multicenter trial reported that such flares are not
associated with the effect of Sr-89 treatment (20). Therefore, the clinical significance
of pain flares remains unclear. In this study, 5 of the 18 patients
(27.8%) reported pain flares (Table
IV); however, pain flares were not found to be a significant
predictive factor of the pain response (P=0.650, Table III). Finlay et al
(13) reported toxic hematological
effects to be the most commonly observed side effects, with a
reduction in the white blood cell count of 11–65% in 12–80% of the
patients. In addition, the platelet count has been reported to
decrease by an average of 29% in 29–80% of the patients, whereas
changes in the red cell count are negligible or non-existent.
Generally, these blood parameters return to normal without
intervention. Moreover, previous trials have found no significant
changes in the hematological variables associated with Sr-89 use
(13). In this study, grade 4
anemia was observed in 2 patients (11.1%) and grade 3
thrombocytopenia in 1 patient (5.6%), as shown in Table IV. These cases overlapped and all
the patients required blood transfusions. The patients who were
administered Sr-89 were divided into two groups, namely the
myelosuppression and no myelosuppression groups (Table V), with significant differences in
the PSA and ALP values between the two groups (P=0.092 and 0.035,
respectively). The correlations between the patient characteristics
and myelosuppression were subsequently analyzed using a logistic
regression analysis, which identified ALP level as the only
significant factor affecting the incidence of bone marrow
suppression in the univariate and multivariate analyses (P=0.006,
Table VI). Based on these
results, physicians should pay more attention when administering
Sr-89 to patients with high ALP values, which suggest extensive
bone metastasis, due to the potential for bone marrow suppression.
Furthermore, Sr-89 should be administered before bone metastases
become widespread, as less favorable responses and higher toxicity
have been reported in patients with end-stage disease. For example,
a study by Rogers et al (21) on 60 patients with widespread
symptomatic disease treated with Sr-89 at a dose of 66.6–173.9 MBq
(median, 133.2 MBq) (1.8–4.7 mCi [median, 3.6 mCi]) reported an
overall response rate of 67% at 7–11 weeks. In that study, 3
patients (6%) exhibited severe thrombocytopenia and bleeding
diathesis at the time of death at 10, 12 and 16 weeks after
injection. Lee et al (22)
further reported unfavorable results in 28 patients with end-stage
disease treated with Sr-89 at a dose of 81.4–162.8 MBq (2.2–4.4
mCi). In that report, only 29% of the patients experienced moderate
to significant pain relief, 32% exhibited some relief and 50%
reported no pain relief. That group of patients exhibited a median
survival of only 23 weeks and 32% of the subjects required
additional palliative EBRT. In addition, the patients displayed a
significant reduction in their blood counts. Among patients with
end-stage bone metastasis, due to bone marrow suppression following
prior treatment with external irradiation and chemotherapy and the
infiltration of the bone marrow by the tumor, the standby capacity
of the bone marrow may already significantly decreased prior to
Sr-89 administration. Therefore, bone marrow suppression may be
synergistically enhanced in patients with extensive bone metastasis
compared to those with fewer bone metastatic lesions, as the degree
of bone marrow suppression is associated with the proportion of
Sr-89 accumulated and retained in the bone, which is relatively
increased in such patients.
Finally, we investigated the association between
changes in the PSA and ALP levels according to the extent of pain
improvement associated with bone metastasis. As shown in Fig. 1, a PSA response was observed in 4
cases (22.2%) and an ALP response was observed in 11 cases (61.1%);
however, there were no fixed trends in the changes in the PSA or
ALP values according to the pain response. Similar results were
obtained in the logistic regression analysis, as shown in Table III (PSA responders, P=0.888; ALP
responders, P=0.266). Therefore, changes in the characteristics of
the patients are not necessarily associated with the pain
improvement effect. Previous trials on hormone-refractory prostate
cancer patients have reported changes in tumor markers following
treatment with Sr-89. For example, a decrease of >50% was
observed in the serum PSA level in 37% of the patients in a
previous study (20). However,
another study demonstrated a mean increase of 36% in the PSA
concentration from the pretreatment period to 2 months after
treatment, whereas the ALP concentration decreased by 20% (23). Those reports also support the
findings of our study. When Sr-89 was first released in Japan, it
was recommended in the manual that it should be administered only
to patients with bone pain that could not be controlled with other
common pain relief methods. However, the manual was revised in
February, 2013 and now specifies that Sr-89 may be used at any
stage in the analgesic ladder, based on the criteria of the World
Health Organization (WHO). In accordance with this revision, Sr-89
may be administered in the early stage of bone metastasis and
applied clinically more effectively and safely.
Sr-89 is very effective in ameliorating bone pain in
Japanese prostate cancer patients. Although it is difficult to
predict which patients will achieve pain relief from Sr-89 prior to
administration, this drug should be administered during the early
stages of bone metastasis, due to the potential for bone marrow
suppression in patients with high ALP levels.
References
|
1
|
Coleman RE: Metastatic bone disease:
clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80 8 Suppl:1588–1594. 1997. View Article : Google Scholar
|
|
3
|
Zekri J, Ahmed N, Coleman RE and Hancock
BW: The skeletal metastatic complications of renal cell carcinoma.
Int J Oncol. 19:379–382. 2001.PubMed/NCBI
|
|
4
|
Sciuto R, Festa A, Rea S, Pasqualoni R,
Bergomi S, Petrilli G and Maini CL: Effects of low-dose cisplatin
on 89Sr therapy for painful bone metastases from
prostate cancer: a randomized clinical trial. J Nucl Med. 43:79–86.
2002.PubMed/NCBI
|
|
5
|
Bolger JJ, Dearnaley DP, Kirk D, et al:
Strontium-89 (Metastron) versus external beam radiotherapy in
patients with painful bone metastases secondary to prostatic
cancer: preliminary report of a multicenter trial. UK Metastron
Investigators Group. Semin Oncol. 20:32–33. 1993.
|
|
6
|
Blake GM, Zivanovic MA, Blaquiere RM, Fine
DR, McEwan AJ and Ackery DM: Strontium-89 therapy: measurement of
absorbed dose to skeletal metastases. J Nucl Med. 29:549–557.
1988.PubMed/NCBI
|
|
7
|
Pecher C: Biological investigations with
radioactive calcium and strontium: preliminary report on the use of
radioactive strontium in the treatment of metastatic bone cancer.
Univ Calif Publ Pharmacol. 2:1117–1149. 1942.
|
|
8
|
Lewington VJ, McEwan AJ, Ackery DM, et al:
A prospective, randomised double-blind crossover study to examine
the efficacy of strontium-89 in pain palliation in patients with
advanced prostate cancer metastatic to bone. Eur J Cancer.
27:954–958. 1991. View Article : Google Scholar
|
|
9
|
Porter AT, McEwan AJ, Powe JE, et al:
Results of a randomized phase-III trial to evaluate the efficacy of
strontium-89 adjuvant to local field external beam irradiation in
the management of endocrine resistant metastatic prostate cancer.
Int J Radiat Oncol Biol Phys. 25:805–813. 1993. View Article : Google Scholar
|
|
10
|
McEwan AJ, Amyotte GA, McGowan DG,
MacGillivray JA and Porter AT: A retrospective analysis of the cost
effectiveness of treatment with Metastron
(89Sr-chloride) in patients with prostate cancer
metastatic to bone. Nucl Med Commun. 15:499–504. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malmberg I, Persson U, Ask A, Tennvall J
and Abrahamsson PA: Painful bone metastases in hormone-refractory
prostate cancer: economic costs of strontium-89 and/or external
radiotherapy. Urology. 50:747–753. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kimura Y, Hamamoto K, Furudate M, et al:
Effectiveness of the radioactive strontium (89Sr) chloride agent,
SMS.2P for pain palliation in patients with metastatic bone tumor
in phase III multicenter clinical trial. Jpn J Nucl Med.
33:1347–1358. 1996.(In Japanese).
|
|
13
|
Finlay IG, Mason MD and Shelley M:
Radioisotopes for the palliation of metastatic bone cancer: a
systematic review. Lancet Oncol. 6:392–400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kraeber-Bodéré F, Campion L, Rousseau C,
Bourdin S, Chatal JF and Resche I: Treatment of bone metastases of
prostate cancer with strontium-89 chloride: efficacy in relation to
the degree of bone involvement. Eur J Nucl Med. 27:1487–1493.
2000.PubMed/NCBI
|
|
15
|
McEwan AJ: Use of radionuclides for the
palliation of bone metastases. Semin Radiat Oncol. 10:103–114.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Serafini AN: Therapy of metastatic bone
pain. J Nucl Med. 42:895–906. 2001.PubMed/NCBI
|
|
17
|
Lewington VJ: Bone-seeking radionuclides
for therapy. J Nucl Med. 46 Suppl 1:38S–47S. 2005.PubMed/NCBI
|
|
18
|
Windsor PM: Predictors of response to
strontium-89 (Metastron) in skeletal metastases from prostate
cancer: report of a single centre's 10-year experience. Clin Oncol
(R Coll Radiol). 13:219–227. 2001.PubMed/NCBI
|
|
19
|
Laing AH, Ackery DM, Bayly RJ, et al:
Strontium-89 chloride for pain palliation in prostatic skeletal
malignancy. Br J Radiol. 64:816–822. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Turner SL, Gruenewald S, Spry N and Gebski
VMetastron Users Group: Less pain does equal better quality of life
following strontium-89 therapy for metastatic prostate cancer. Br J
Cancer. 84:297–302. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rogers CL, Speiser BL, Ram PC, et al:
Efficacy and toxicity of intravenous strontium-89 for symptomatic
osseous metastasis. Brachyther Int. 4:133–142. 1998.
|
|
22
|
Lee CK, Aeppli DM, Unger J, Boudreau RJ
and Levitt SH: Strontium-89 chloride (Metastron) for palliative
treatment of bony metastases. The University of Minnesota
experience. Am J Clin Oncol. 19:102–107. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fosså SD, Paus E, Lochoff M, Backe SM and
Aas M: 89Strontium in bone metastases from hormone
resistant prostate cancer: palliation effect and biochemical
changes. Br J Cancer. 66:177–180. 1992.PubMed/NCBI
|