Introduction
Exogenously administered 5-aminolevulinic acid (ALA)
is metabolized through the assembly of eight ALA molecules to a
heme precursor protoporphyrin IX (PpIX), and subsequently to heme
through the insertion of an iron ion. In cancer cells, however,
PpIX accumulates due to the Warburg effect, abnormalities in iron
metabolism and errors in iron insertion (1). As PpIX is a fluorescent compound, PpIX
in cancer is visualized as a useful intraoperative diagnostic
agent. This procedure is known as photodynamic diagnosis (PDD)
using ALA (2). In Europe and Japan,
ALA has been approved as an intraoperative diagnostic agent for
brain tumors. Photodynamic diagnosis can be applied not only to
brain tumors, but also to various types of cancer, including those
of the bladder (3), prostate
(4,5),
stomach (6) and colon (7), and thus, clinical studies are currently
underway to expand its application.
Specific PpIX accumulation in cancer cells following
ALA administration remains intracellular for ~10 h before being
exported extracellularly by adenosine triphosphate-binding cassette
transporter G2 (ABCG2) (1,8). Our previous study indicated that the
measurement of changes in urinary porphyrin metabolites, such as
uroporphyrins and coproporphyrins, following ALA administration
could be useful in screening for bladder cancer (9). Such changes are possibly attributed to
the fact that cancer cells have abnormalities in heme metabolism.
However, PpIX is known to be a major porphyrin metabolite
accumulating in tumor tissue following ALA administration. Based on
these findings, we hypothesized that the measurement of plasma
concentration of PpIX, a molecule that is released from the tumor
tissue into the plasma may provide more direct information than
that of urinary porphyrin metabolites. Therefore, changes in plasma
concentrations of porphyrin metabolites were measured, particularly
PpIX, following the administration of ALA and evaluated the
effectiveness of this assay as a screening test for cancer
patients.
Materials and methods
Materials
ALA hydrochloride was used (1.0 g; Cosmo Bio Co.,
Tokyo, Japan). Oral ALA solution was prepared by dissolving ALA in
a 5% glucose solution and was ingested 3 h before endoscopic
examination.
Subjects and experimental
protocol
Similar to our previous study (9), the ingestion of ALA and PDD procedures
were approved by the Ethics Committee of Kochi Medical School
(Kochi, Japan) on December 26, 2006 (nos. 18–27). The present study
involved 73 patients with bladder cancer, consisting of the
patients described in the previous study and 7 additional patients,
and 25 healthy adults consisting of the healthy subjects included
in the previous study and 5 additional subjects in whom no primary
or recurrent cancer had been detected by transurethral biopsy of
the bladder for 2–3.5 years. The patients received a prior
explanation of the study and provided written informed consent. The
background characteristics of the patients are shown in Table I. A brief summary of the protocol is
as follows: ALA (1.0 g) was administered orally 3 h before
endoscopic examination. Peripheral blood (5 ml) was collected in
EDTA-2Na tubes at 4 and 8 h after ALA administration and
centrifuged to obtain plasma for high-performance liquid
chromatography (HPLC) analysis of ALA and porphyrin metabolites,
including uroporphyrin I (UPI), UPIII, coproporphyrin I (CPI),
CPIII and PpIX.
 | Table I.Characteristics of the bladder cancer
patients. |
Table I.
Characteristics of the bladder cancer
patients.
|
| TNM staging |
|---|
|
|
|
|---|
| Characteristics | pTa | pTis | pT1 | pT2–4 |
|---|
| Age, median years
(range) | 70.5 (49.1–85.0) | 72.5 (48.0–86.0) | 76.0 (64.0–90.0) | 79.0 (53.0–87.0) |
| Male/female, n | 34/5 | 10/2 | 8/3 | 9/2 |
HPLC analysis
Determination of ALA
Plasma ALA was determined using the method of Endo
et al (10), with appropriate
modifications. Briefly, 0.2 ml of plasma was mixed with 25% w/v TCA
solution by agitation and was centrifuged. Subsequently, 0.01 ml of
the resulting supernatant was transferred to a test tube, incubated
with 0.24 ml of Milli-Q water, 0.25 ml of 200 mM acetic acid-sodium
acetate buffer (pH 3.8), 1.25 ml of solution A (68.4 mM sodium
chloride containing 15% v/v acetylacetone and 10% v/v ethanol) and
0.25 ml of solution B (3.3% w/w formaldehyde) in boiling water for
15 min, and immediately cooled in ice water. This reaction mixture
was applied to a HPLC system (Inertsil ODS-3, 5 µm, 4.6×150 mm; GL
Sciences, Tokyo, Japan) for analysis.
Determination of porphyrin
metabolites
Plasma UPI, UPIII, CPIand CPIII were determined
using the method of Kondo et al (11) with partial modification. Plasma (0.1
ml) was agitated for 1 min with 0.8 ml of a mixture of ethyl
acetate-acetic acid-25% w/v TCA (8:1:1 by vol.) and centrifuged at
20,000 × g for 3 min at 4°C. After collecting the supernatant in a
glass tube, the resulting precipitate was agitated for 1 min with
0.2 ml of ethyl acetate-acetic acid solution (4:1 by vol.) and
centrifuged to obtain the supernatant (this step was repeated
twice). The three batches of supernatant were combined, incubated
at 40°C in a heating block, and evaporated to dryness under
nitrogen gas. The porphyrin-containing solid was dissolved in 0.2
ml of acetonitrile-50% v/v acetic acid solution (4:1 by vol.) and
analyzed with a HPLC system (Symmetry C18, 5 µm, 3.9×150 mm; Ex.
404 nm, Em. 620 nm; Waters, Milford, MA, USA). The elution
conditions were as follows: Solvent A, 1 M ammonium acetate
containing 10.5% acetonitrile (pH 5.2); solvent B, 50 mM ammonium
acetate containing 80% acetonitrile (pH 5.2); and gradient, 100%
solvent A at 1 ml/min for 10 min, followed by a linear increase of
solvent B from 20 to 27.2% over 14.5 min and 100% solvent B for 10
min.
For the determination of PpIX, 0.1 ml of plasma was
vigorously agitated for 30 sec with 0.01 ml of 50% v/v acetic acid
and 0.3 ml of N,N-dimethylformamide-2-propanol
(DMF-IPA) solution (100:1 by vol.) and centrifuged at 20,000 × g
for 5 min at 4°C to collect supernatant. The precipitate was
extracted with 0.15 ml of DMF-IPA solution by repeating the same
procedures as above. The two batches of supernatant were mixed and
analyzed with an HPLC system using a Capcell Pak C18 UG120 column
(5 µm, 4.6 × 150 mm, Shiseido, Tokyo, Japan), mobile phase of 10 mM
tetrabutylammonium hydroxide solution (pH 7.5) (flow rate, 1.0
ml/min; elution temperature, 40°C), and fluorescence detector (Ex.
400 nm, Em. 630 nm).
Statistical analysis
Mann-Whitney's U and Steel-Dwass' tests were used
for the analysis. P<0.05 was considered to indicate a
significant concentration. Diagnostic efficacy was evaluated using
the area under the curve (AUC) on receiver-operating
characteristics (ROC) analysis.
Results
Plasma concentrations of ALA, PpIX and
porphyrins
The background characteristics of patients with
bladder cancer are summarized in Table
I. Measurements of the plasma ALA, PpIX and total porphyrin
concentrations at 4 and 8 h after the oral administration of ALA in
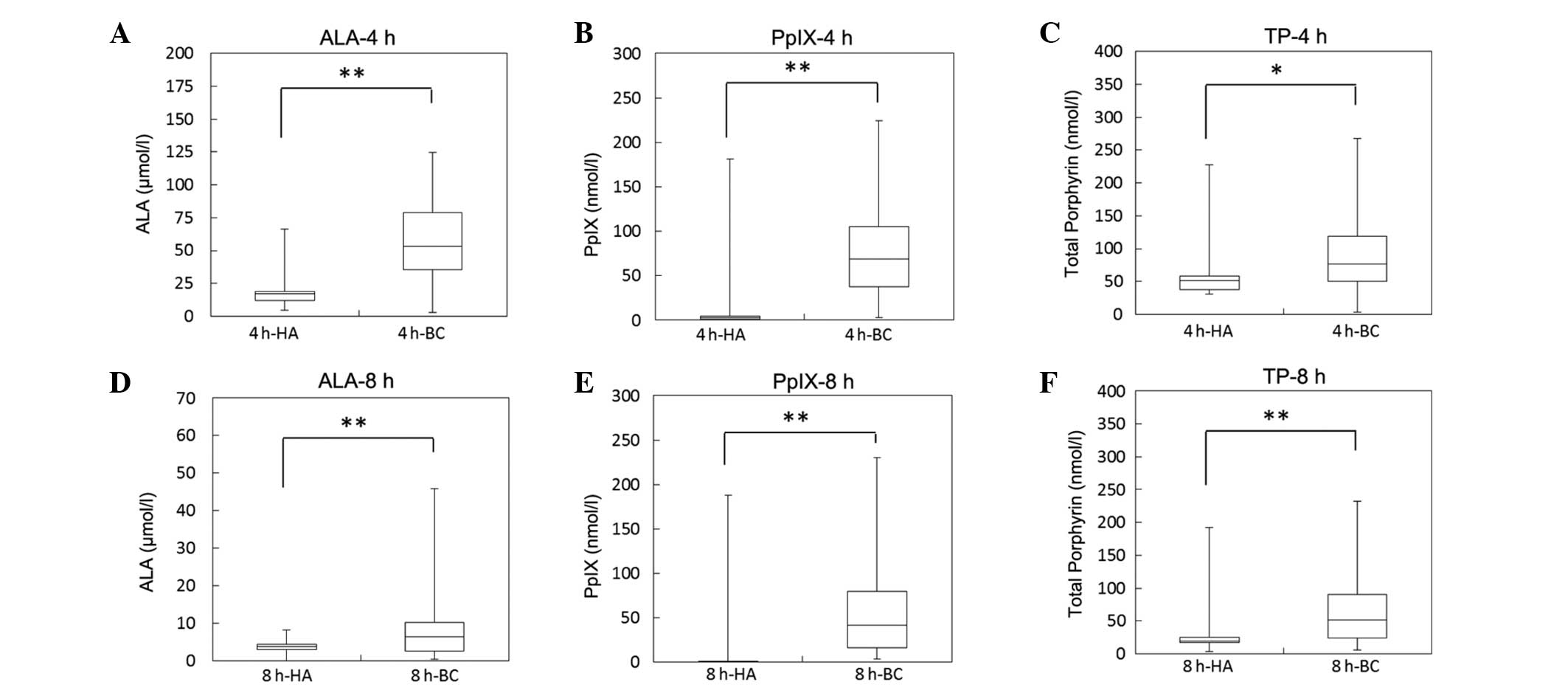
these cancer patients and healthy adults are shown in Fig. 1. The median plasma ALA concentration
at 4 and 8 h after ALA administration was 17.0 (4.3–66.4) and 3.8
(0–8.2) µmol/l, respectively, in healthy adults (HA group),
compared to 53.0 (2.6–124.9) and 6.4 (0.4–45.9) µmol/l,
respectively in bladder cancer patients (BC group), demonstrating
that plasma ALA concentrations were significantly higher in the BC
group compared to the HA group at 4 and 8 h after ALA
administration (Fig. 1A). The median
plasma PpIX concentrations at 4 and 8 h after ALA administration
was 2.8 (0.8–180.9) and 0.2 (0–187.6) nmol/l, respectively, in the
HA group, compared to 69.1 (2.8–224.5) and 41.8 (4.0–229.9) nmol/l,
respectively, in the BC group. Similar to the ALA concentrations,
plasma PpIX concentrations were significantly higher in the BC
compared with in the HA group at 4 and 8 h after ALA administration
(Fig. 1B). Additionally, similar to
the PpIX concentrations, total porphyrin concentrations in plasma
were also significantly higher in the BC compared with the HA group
(Fig. 1C).
Stages of bladder cancer progression
and plasma concentrations of ALA and PpIX
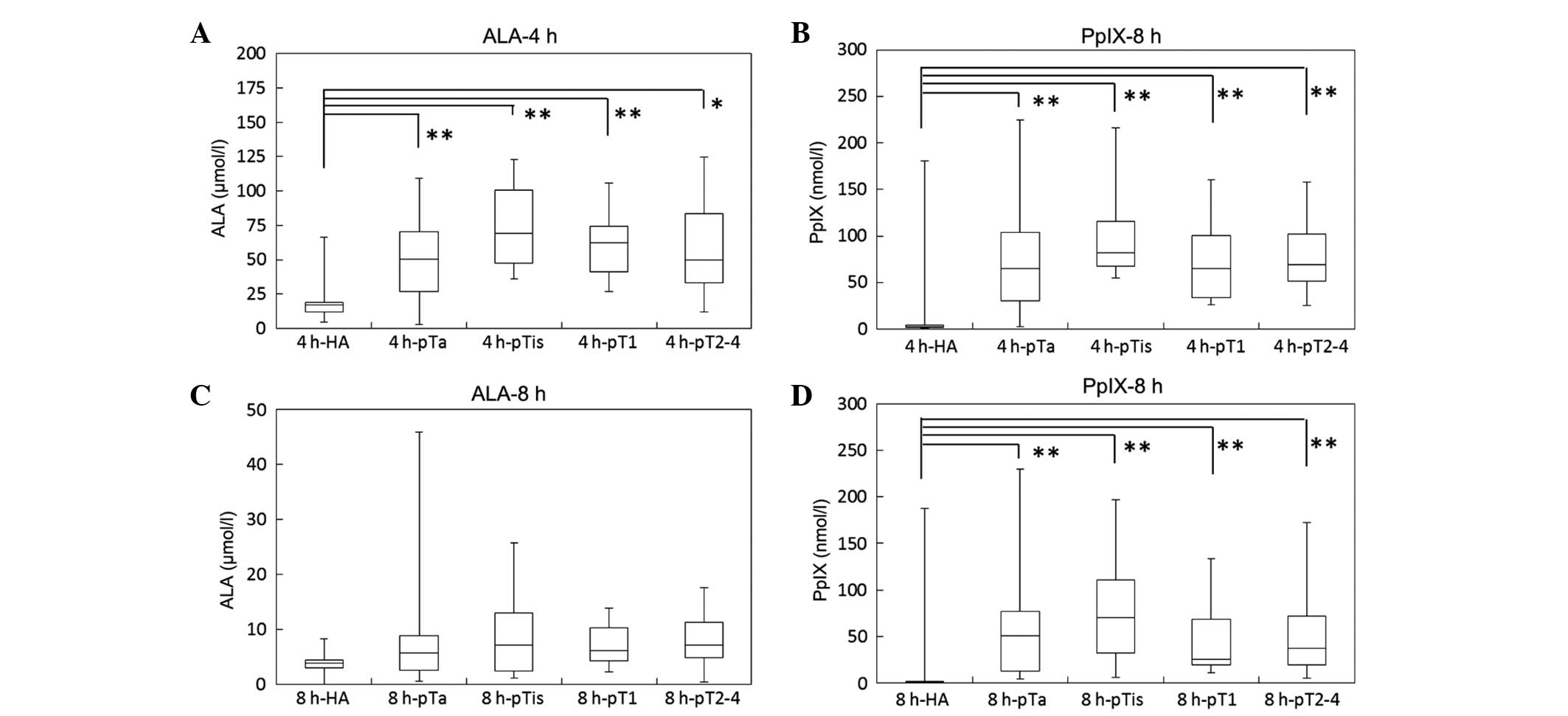
For the BC group, the data shown in Fig. 1 were classified into four groups
according to the stage of bladder cancer (pTa, pTis, pT1 and
pT2–4), and compared with those for the HA group (Fig. 2). The median plasma ALA concentration
at 4 h after ALA administration was 50.6 (2.6–109.2), 69.3
(35.7–122.7), 62.3 (27.1–105.8), and 49.7 (12.2–124.9) µmol/l in
the pTa, pTis, pT1, and pT2–4 groups, respectively. The median
plasma PpIX concentrations at 4 h after ALA administration was 65.2
(2.8–224.5), 82.1 (55.4–216.3), 65.4 (26.5–160.6), and 68.9
(25.3–158.3) nmol/l in the pTa, pTis, pT1, and pT2–4 groups,
respectively. Plasma concentrations of ALA and PpIX at 4 h after
ALA administration were significantly increased in all the stages
of bladder cancer compared with those in the HA group. However,
there were no marked differences in plasma concentrations of ALA
and PpIX concentration across the tumor stages. A similar tendency
of ALA and PpIX plasma concentrations were observed 8 h after ALA
administration.
Plasma concentrations of UPI, UPIII,
CPI and CPIII
Plasma concentrations of porphyrin metabolites, such
as UPI, UPIII, CPI and CPIII, following ALA administration were
analyzed (Fig. 3). The median
concentrations of these porphyrin metabolites in plasma were
markedly lower than those in urine, corresponding to only
1/60–1/1,800 of the urinary concentrations described in our
previous study (9).
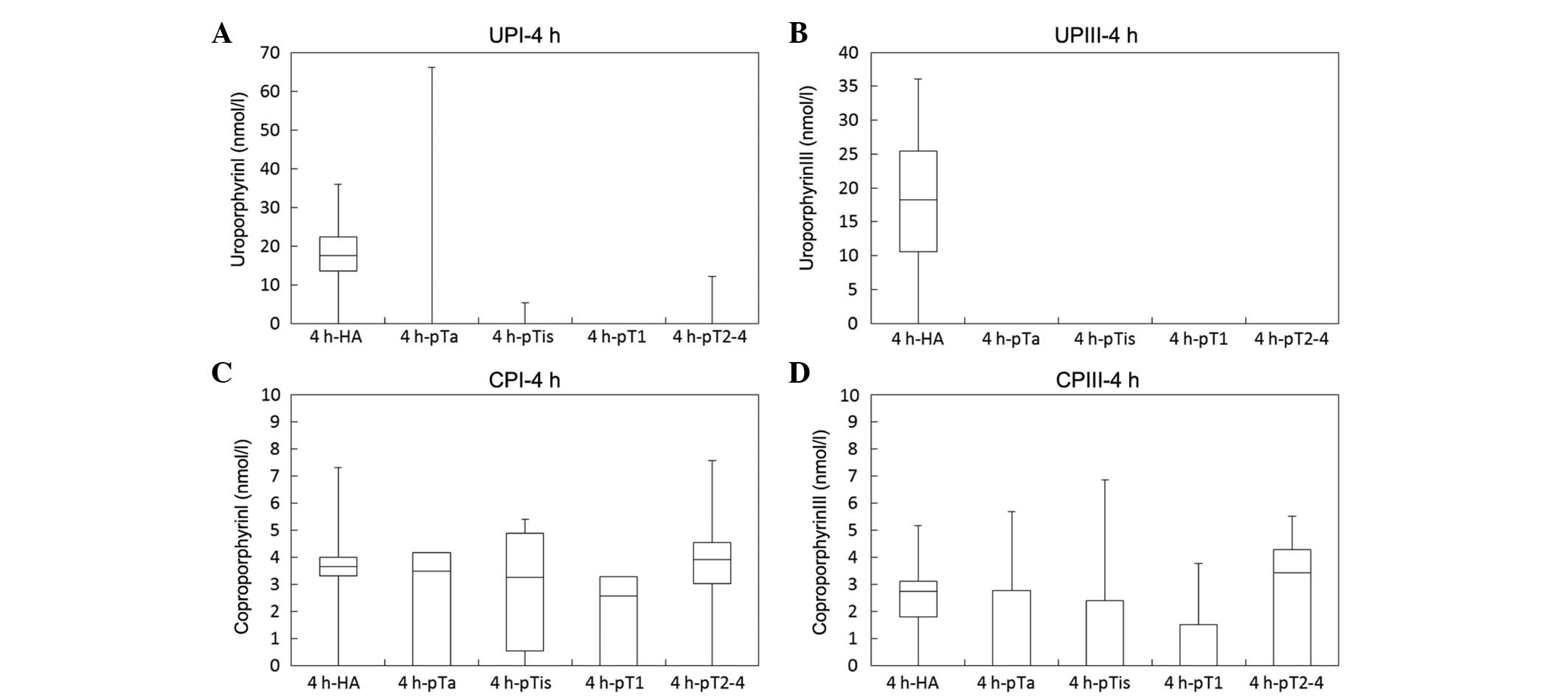 | Figure 3.Plasma concentrations of porphyrin
metabolites (UPI, UPIII, CPI and CPIII) by stage of bladder cancer
An oral dose of 1.0 g of ALA hydrochloride was administered to
healthy adults (HA, n=25) and bladder cancer patients (n=73) who
were classified into 4 groups according to the stage of disease
progression: pTa, pTis, pT1 and pT2–4. The plasma concentrations of
porphyrin metabolites (UPI, UPIII, CPI and CPIII) at (A and B) 4
and (C and D) 8 h after ALA administration were measured by HPLC.
*P<0.05 and **P<0.01 by Steel-Dwass's test. UP, uroporphyrin;
CP, coproporphyrin; ALA, 5-aminolevulinic acid; HPLC,
high-performance liquid chromatography. |
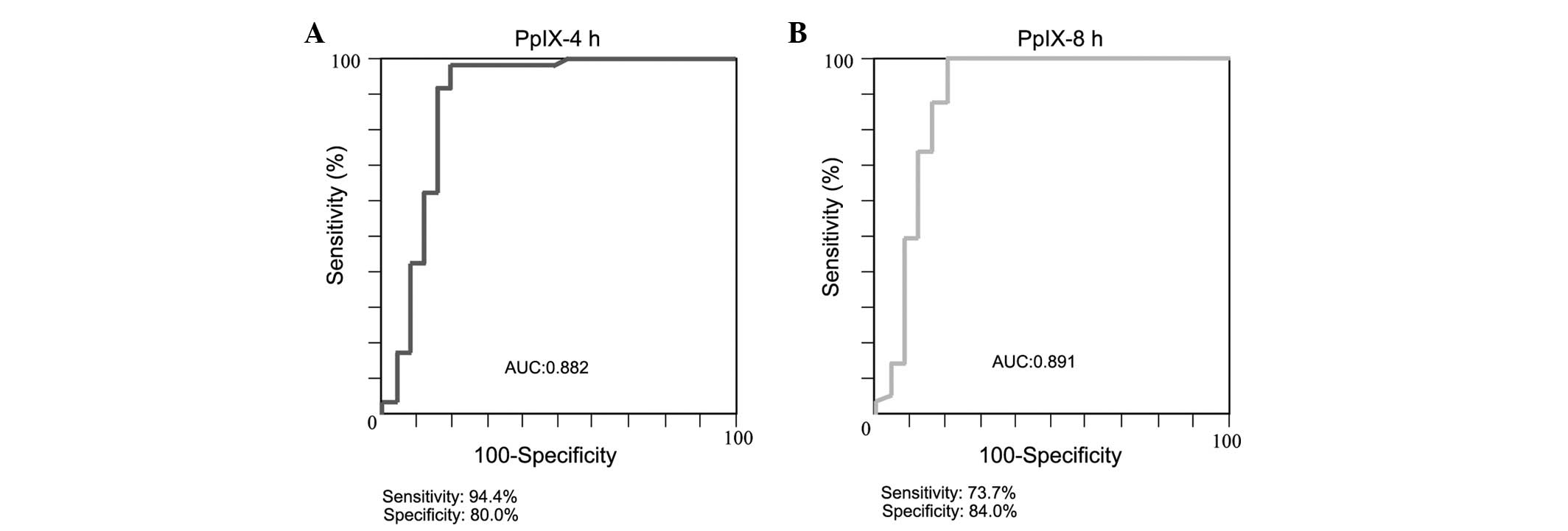
Analysis of ROC curves
An ROC analysis was performed of plasma PpIX
concentrations (Fig. 4). A cut-off
value of 20 nmol/l was determined by the ROC analysis. The AUC,
sensitivity and specificity of the PpIX measurement at 4 h
post-administration were 0.882, 94.4 and 80.0%, respectively,
whereas those of the measurement at 8 h post-administration were
0.891, 73.7 and 84.0%, respectively.
Discussion
Patients with bladder cancer, regardless of the
stage of progression, showed significantly higher plasma PpIX
concentrations following ALA administration compared to healthy
adults, demonstrating the potential usefulness of the plasma PpIX
concentration following ALA administration in screening for bladder
cancer. In addition, 4 h after plasma PpIX concentration showed a
greater diagnostic efficacy than 8 h after plasma PpIX
concentration. To the best of our knowledge, there is no tumor
marker for bladder cancer that can be detected in plasma.
Therefore, this is the first study of a plasma tumor marker for
bladder cancer.
There are marked differences in the urinary
excretion of UPs and CPs following ALA administration between
healthy subjects and bladder cancer patients. The plasma
concentrations of UPs and CPs measured in the present study were
markedly lower, corresponding to only 1/60–1/1,800 of the reported
urinary concentrations, and were <1/10 of the plasma PpIX
concentrations. There was a >10 times difference in the plasma
PpIX concentrations between healthy adults and bladder cancer
patients. Although it is difficult to compare the suitability of
urine and plasma samples for cancer screening, the measurement of
plasma PpIX could be more advantageous than that of urinary
porphyrins in terms of sensitivity.
In the present study, cancer patients showed
significantly higher plasma ALA concentrations compared to the
healthy adults. This is possibly due to potential differences in
the metabolic rate of ALA between normal and cancer cells that have
abnormalities in their overall heme metabolism. However,
considering that the examined cancer patients were older than the
healthy subjects and thus, may have a decreased metabolism and a
reduced metabolic rate of ALA, the present findings warrant
further, careful investigation.
In cancer cells, PpIX is considered to be generated
in the mitochondria. Increased plasma PpIX in cancer patients
possibly originates from cancer cells, in which PpIX is generated
and exported through the transporter. Therefore, plasma PpIX may
provide direct evidence for the presence of tumors.
The measurement of plasma concentrations of PpIX
allows a more direct index of the presence of tumors, as well as
advantages in sensitivity and in that no data correction is
necessary, whereas measurement of the urinary excretion of UPs and
CPs is less invasive than a blood test. For these reasons, it would
be favorable to apply the urine test to a mass screening program,
such as health examinations, and use the blood test for individuals
with abnormal urine on screening and those who are at a higher risk
of cancer to test, such as for the recurrence of cancer.
Although the present study focused on bladder
cancer, the principle that PpIX accumulates specifically in cancer
cells following ALA administration is common to almost all types of
cancer. In order to optimize the assay conditions, further studies
are required to confirm these findings in various types of cancer
and determine the optimal dose of ALA.
Acknowledgements
The present study was supported by the Adaptable and
Seamless Technology Transfer Program through target-driven research
and development from the Japan Science and Technology Agency and
the Grant-in-Aid for Scientific Research (C) (grant nos. 26430141
and 21592050) from the Ministry of Education, Culture, Sports,
Science and Technology.
Glossary
Abbreviations
Abbreviations:
|
ALA
|
5-aminolevulinic acid
|
|
ABCG2
|
adenosine triphosphate-binding
cassette transporter G2
|
|
PpIX
|
protoporphyrin IX
|
References
|
1
|
Ishizuka M, Abe F, Sano Y, et al: Novel
development of 5-aminolevurinic acid (ALA) in cancer diagnoses and
therapy. Int Immunopharmacol. 11:358–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stummer W, Pichlmeier U, Meinel T, et al:
Fluorescence-guided surgery with 5-aminolevulinic acid for
resection of malignant glioma: a randomised controlledmulticentre
phase III trial. Lancet Oncol. 7:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inoue K, Fukuhara H, Shimamoto T, et al:
Comparison between intravesical and oral administration of
5-aminolevulinic acid in the clinical benefit of photodynamic
diagnosis for nonmuscle invasive bladder cancer. Cancer.
118:1062–1074. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoue K, Ashida S, Fukuhara H, et al:
Application of 5-aminolevulinic acid-mediated photodynamic
diagnosis to robot-assisted laparoscopic radical prostatectomy.
Urology. 82:1175–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukuhara H, Inoue K, Satake H, et al:
Photodynamic diagnosis of positive margin during radical
prostatectomy: preliminary experience with 5-aminolevulinic acid.
Int J Urol. 18:585–591. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Namikawa T, Inoue K, Uemura S, et al:
Photodynamic diagnosis using 5-aminolevulinic acid during
gastrectomy for gastric cancer. J Surg Oncol. 109:213–217. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kondo Y, Murayama Y, Konishi H, et al:
Fluorescent detection of peritoneal metastasis in human colorectal
cancer using 5-aminolevulinic acid. Int J Oncol. 45:41–46.
2014.PubMed/NCBI
|
|
8
|
Hagiya Y, Fukuhara H, Matsumoto K, et al:
Expression levels of PEPT1 and ABCG2 play key roles in
5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin
IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn
Ther. 10:288–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inoue K, Ota U, Ishizuka M, et al:
Porphyrins as urinary biomarkers for bladder cancer after
5-aminolevulinic acid (ALA) administration: the potential of
photodynamic screening for tumors. Photodiagnosis Photodyn Ther.
10:484–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Endo Y, Okayama A, Endo G, et al:
Improvement of urinary δ-aminolevulinic acid determination by HPLC
and fluorescence detection using condensing reaction with
acetylacetone and formaldehyde. Jpn J Ind Health. 36:49–56.
1994.
|
|
11
|
Kondo M, Sekine K and Takara S: Occurrence
of anemia and alterations in porphyrin metabolism in chronic
hemodialysis patients. Porphyrins. 3:297–302. 1994.
|