Introduction
Adhesion proteins of the cadherin family are
frequently deregulated during cancer growth and progression. The
prototype cadherin of epithelial tissues is E-cadherin, a major
constituent of adherens junctions, which participates in cell-cell
adhesion and cell polarity, is involved in differentiation and cell
signaling and also acts as a tumor suppressor through its negative
impact on cell migration and invasion (1). Disruption of E-cadherin function by
mutation, promoter hypermethylation or loss of heterozygosity has
been frequently reported in lobular breast cancer and other types
of tumors. Ovarian carcinomas are an exception, since E-cadherin is
expressed in all stages of tumorigenesis, including metastases and
tumor cells in effusions (2,3).
Loss of E-cadherin expression is generally
accompanied by increased expression of the mesenchymal cadherins
CDH2 (N-cadherin) and/or cadherin-11 (CDH11, OB-cadherin). This
‘cadherin switch’ is a major characteristic of the
epithelial-mesenchymal transition during the progression of a
number of tumor types. Carcinomas expressing N-cadherin exhibit
reduced adhesion between tumor cells, but are more able to interact
with N-cadherin-positive stromal or endothelial cells (4). In addition, N-cadherin activates
signaling pathways leading to enhanced cell migration, invasion and
survival (5).
CDH11 is normally expressed in mesoderm-derived
tissues, particularly osteoblasts, but is also upregulated in
epithelial cancer and stromal cells in carcinomas (6). Increased CDH11 expression was reported
in brain tumors and prostate cancer, where it leads to preferential
metastasis to the bone (7,8), and in highly invasive breast cancer cell
lines, where CDH11 was shown to promote motility and invasive
potential (9,10). In other tumor entities, such as
osteosarcomas, melanomas and head and neck cancers, a
tumor-suppressive effect of CDH11 was reported, with decreased
expression in metastases compared with that in primary tumors
(6).
The currently available information on the role of
CDH11 in ovarian cancer is sparse. In a microarray analysis of
serous ovarian carcinomas, CDH11 mRNA expression was increased in
metastases compared with that in primary tumors (11). In our own experimental study on the
role of the transcription factor c-Fos in ovarian cancer cells,
c-Fos overexpression resulted in decreased adhesive properties of
the tumor cells, accompanied by downregulation of CDH11 and other
adhesion proteins (12). In order to
further investigate the role of this adhesion protein in ovarian
cancer, we analyzed CDH11 mRNA and protein expression in tissue
samples of ovarian tumors of different histological subtypes.
Materials and methods
Patients
Tissue samples of 213 patients with epithelial
ovarian tumors were included in our western blot analysis,
including benign cystadenomas (n=5), borderline tumors (n=19),
invasive primary carcinomas (n=178) and recurrent carcinomas
(n=11). The cohort characteristics are summarized in Table I. CDH11 mRNA expression was analyzed
in 51 samples, including 6 cystadenomas, 20 borderline tumors and
25 invasive primary carcinomas. CDH11 immunohistochemistry was
performed on 3 cystadenomas, 12 borderline ovarian tumors and 8
invasive carcinomas. Surgery was performed at the University
Medical Centre Hamburg-Eppendorf between 1994 and 2012, or at the
Albertinen Hospital in Hamburg, between 2013 and 2014. All the
patients provided written informed consent for examining their
tissue samples and reviewing their medical records, according to
our Investigational Review Board and Ethics Committee guidelines. A
detailed database including clinicopathological factors,
histological classifications and therapeutic procedures was
generated. The clinical outcomes of all the patients were monitored
from the date of surgery until December, 2013.
 | Table I.Cohort characteristics of primary
carcinomas (n=178). |
Table I.
Cohort characteristics of primary
carcinomas (n=178).
| Characteristics | No. (%) |
|---|
| Age (years) |
|
| Mean | 59.4 |
|
Median | 61.0 |
|
Range | 21–90 |
| FIGO stage |
|
| I | 8 (4.5) |
| II | 7 (3.9) |
| III | 126 (70.8) |
| IV | 31 (17.4) |
|
Unknown | 6 (3.4) |
| Grade |
|
| 1 | 9 (5.1) |
| 2 | 46 (25.8) |
| 3 | 119 (66.9) |
| Not
determined | 4 (2.2) |
| Lymph node
status |
|
| N0 | 45 (25.3) |
| N1 | 101 (56.7) |
| NX | 32 (18.0) |
| Postoperative
residual tumour |
|
|
Microscopic | 100 (56.2) |
| ≤1
cm | 29 (16.3) |
| >1
cm | 15 (8.4) |
| Not
determined | 34 (19.1) |
| Histological
subtype |
|
|
Serous | 148 (83.2) |
|
Mucinous | 4 (2.2) |
|
Endometroid | 11 (6.2) |
|
Others | 13 (7.3) |
| Not
determined | 2 (1.1) |
| Progression-free
survival, months |
|
| Mean | 28.5 |
|
Median | 15.9 |
|
Range | 0–176 |
| Overall survival,
months |
|
| Mean | 40.6 |
|
Median | 30.4 |
|
Range | 1–176 |
Tissue samples and protein
extraction
The tissue samples were collected intraoperatively
and were immediately cryoconserved at −80°C. In order to assure a
tumor cell content of ≥70%, every sample was assessed on cryo-cut
sections stained with haematoxylin and eosin. If necessary, stromal
parts were removed. Approximately 100 mg of tumor tissue were used
for protein extraction, which was performed as previously described
(13).
Western blot analysis
Western blot analysis was generally performed as
previously described (13). Equal
amounts of protein (20 µg) from each sample were loaded per well
and equal loading was verified by immunoblotting with anti-GAPDH
antibody (FL335; Santa Cruz, Heidelberg, Germany; 1:5,000).
Proteins from a tumor with known moderate CDH11 expression served
as control on each gel. Following electrophoresis and blotting to
polyvinylidene difluoride membranes, the membranes were stored at
4°C in blocking solution. The rabbit anti-OB-cadherin antibody
(P707; Cell Signaling Technology Inc., Danvers, MA, USA) was added
to this blocking solution to a final concentration of 0.064 µg/ml
and incubated overnight. Peroxidase-conjugated anti-rabbit IgG
(sc-2054; dilution, 1:8,000; Santa Cruz) served as the secondary
antibody and was visualized by chemiluminescence reagent (Super
Signal West Pico kit; Pierce, Rockford, IL, USA) on medical X-ray
films (Fujifilm Europe, Düsseldorf, Germany). The band intensities
were quantified by densitometry (Imaging Densitometer GS-700;
BioRad, Munich, Germany) and calculated as % intensity of the
control tumor sample (CT).
RNA extraction, cDNA synthesis and
reverse transcription quantitative polymerase chain reaction
(RT-qPCR)
RNA extraction from frozen ovarian tumor tissue and
subsequent quality analysis were performed as previously described
(14). RNA (5 µg) was
reverse-transcribed using the Maxima First Strand cDNA Synthesis
kit (Thermo Fisher Scientific, Pinneberg, Germany) and the obtained
cDNA was diluted (1:5) for further RT-qPCR analysis. The following
primers were used for amplification of the CDH11 sequences and the
housekeeping gene GAPDH: CDH11: forward, 5′-CCCAGTACACGTTGATGCCT-3′
and reverse, 5′-GACGTTCCCACATTGGACCT-3′; GAPDH: forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′.
RT-qPCR was performed using the capillary-based Light Cycler
(Roche, Basel, Switzerland) and the SYBR Premix Ex Taq (Takara Bio,
Saint-Germain-en-Laye, France) with 1 µl cDNA. The samples were
analyzed in duplicates and the results were averaged. CDH11
expression was normalized to the reference gene GAPDH and the
relative expression of CDH11 in each sample was compared with the
expression in one borderline sample, which was used as control
(fold change = 1) based on the ΔΔCt method.
Immunohistochemistry
For CDH11 immunohistochemistry, paraffin-embedded
tissue sections of 4 µm were deparafinized and incubated overnight
at 4°C with the goat polyclonal CDH11 antibody (cat. no. AF1790;
R&D Systems, Abingdon, UK), diluted to 1:150 in antibody
diluent (Dako, Glostrup, Denmark) without prior antigen retrieval
steps. For detection, the washed slides were incubated with
biotin-labelled anti-mouse immunoglobulin (IgG), preformed
ABC-Complex (Vectastain; Vector Laboratories, Burlingame, CA, USA)
and DAB-substrate kit (Vectastain; Vector Laboratories). All the
slides were counterstained with haematoxylin. For negative
controls, normal rabbit IgG (Dako) was used instead of primary
antibody. The endothelial antigen CD31 was visualized using the
monoclonal mouse antibody anti-CD31 (cat. no. M0823; Dako, Hamburg,
Germany), diluted 1:60. Images were captured using an AxioVision 40
microscope (Carl Zeiss Imaging Solutions, Munich, Germany) and
photoshop software (Adobe Systems Inc., San Jose, CA, USA).
Results
CDH11 mRNA expression in ovarian
tumors of different malignant potential
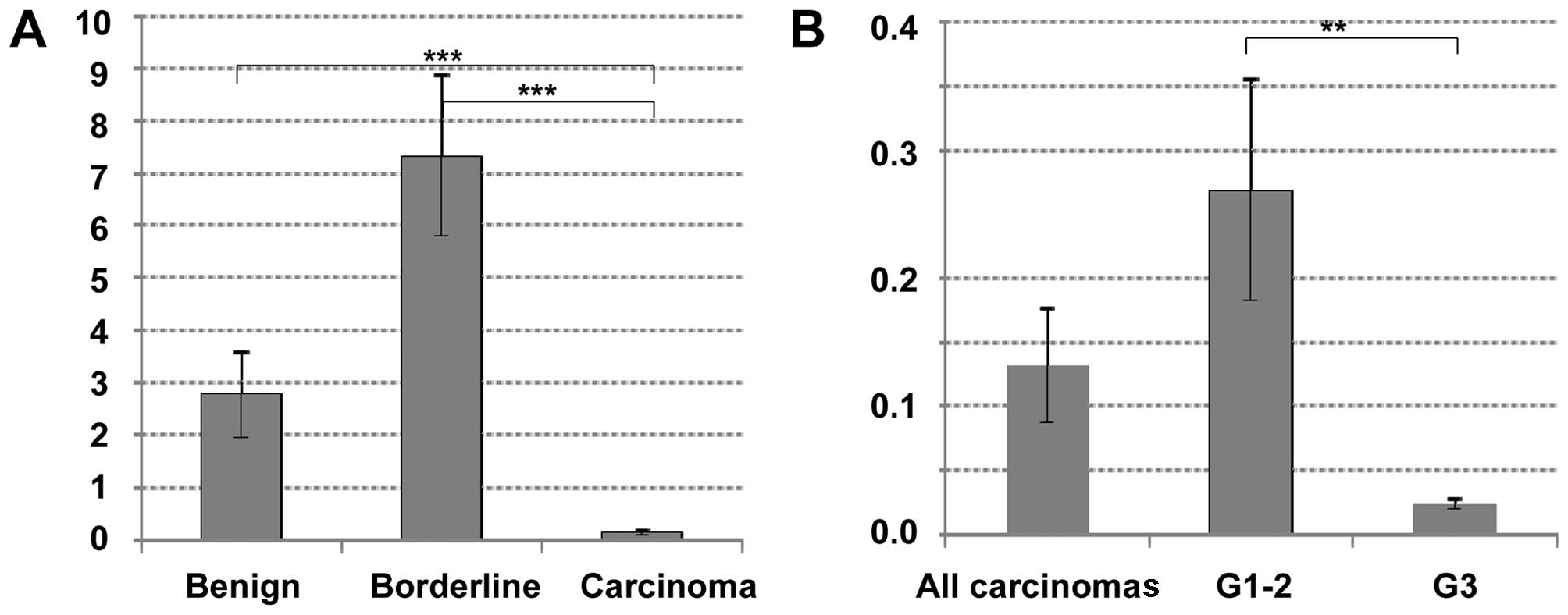
In a first approach, CDH11 mRNA expression was
investigated in 6 cystadenomas/cystadenonofibromas, 20 borderline
tumors and 25 invasive ovarian carcinomas. Interestingly, there was
a distinct difference between these tumor types, with significantly
lower CDH11 expression in carcinomas compared to borderline tumors
(P<0.00001) or cystadenomas (P<0.00001). There was no
statistically significant difference between benign or borderline
tumors (P=0.14, Fig. 1A). Among
invasive carcinomas, CDH11 expression was lower in poorly
differentiated tumors (grade 3) compared with that in highly or
moderately differentiated carcinomas (grade 1–2, P=0.004, Fig. 1B). No significant correlation to age,
stage, nodal involvement or histological tumor type was observed
(data not shown).
CDH11 protein expression in ovarian
tumors
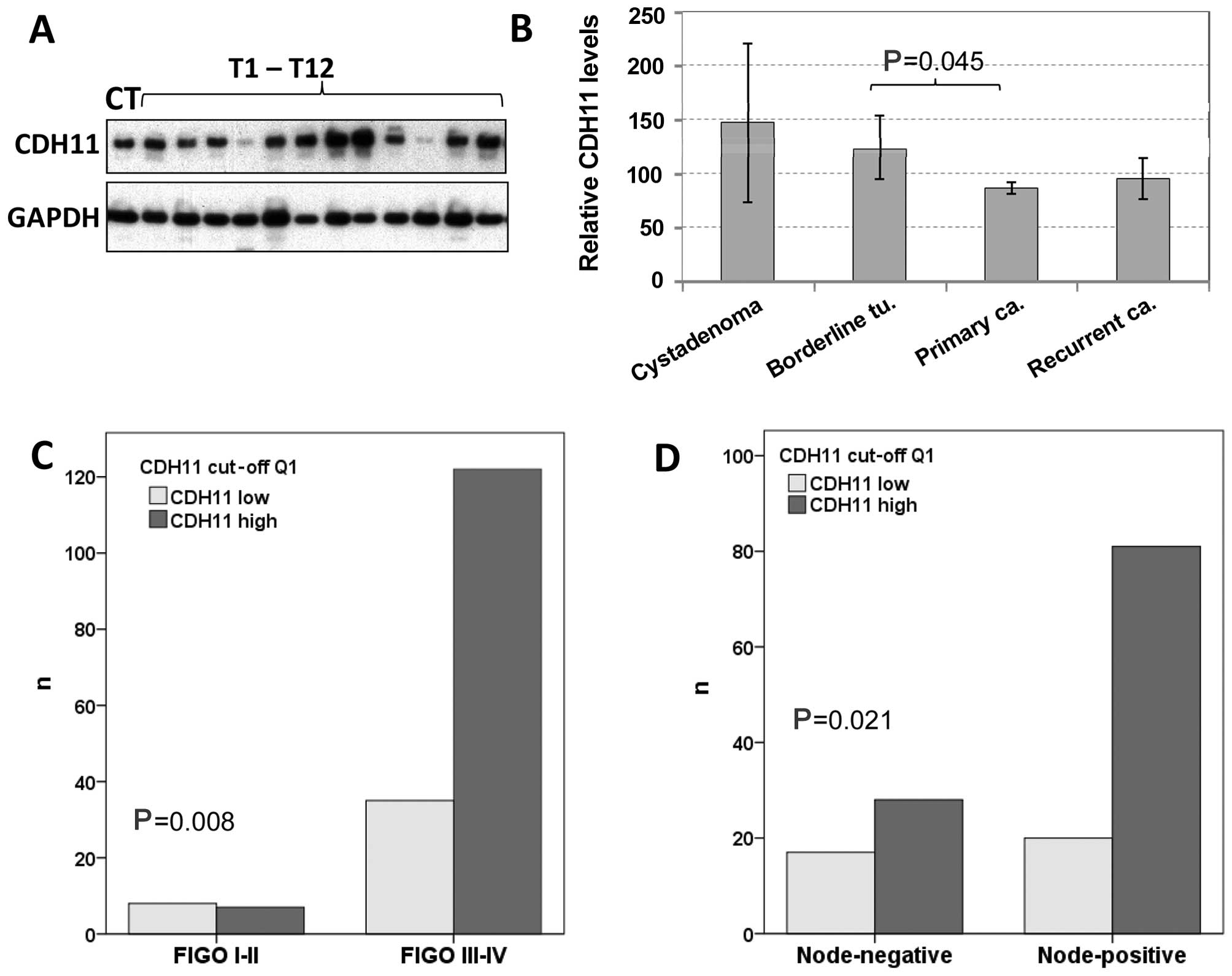
By western blot analysis, CDH11 was detected in
almost all 213 tissue samples in varying amounts (Fig. 2A). Compared to the CT included in all
the experiments, the protein expression levels ranged from 0.03 to
400% (mean, 90.5%; median, 72.0%). Regarding the tumor types, the
mean CDH11 protein expression decreased with increasing malignancy,
being 147% in benign tumors, 124% in borderline tumors and 86% in
carcinomas (Fig. 2B). The difference
between invasive cancer and borderline tumors was statistically
significant (P=0.045). Recurrent tumors exhibited higher CDH11
protein levels (96%) compared with primary carcinomas (86%), but
this difference did not reach statistical significance.
In 39 tumor samples representing all tumor types,
CDH11 expression was analyzed at the protein and mRNA levels. By
the Pearsons correlation test, there was no significant correlation
between mRNA and protein data in this sub-cohort (r=0.280;
P=0.084).
Correlations of CDH11 protein
expression with clinical or histological parameters and prognosis
in ovarian cancer
Based on CDH11 protein expression values, the cohort
of 178 primary carcinomas was first divided into 4 quartiles of
equal size with low, moderate, strong and very strong CDH11
expression. Comparing these 4 groups by Chi-square tests or using
the median value as cut-off, there was no significant association
with grade, age, International Federation of Gynecology and
Obstetrics stage or lymph node involvement (data not shown). Yet,
if the first quartile was compared with the upper 3 quartiles, low
CDH11 expression was found to be significantly correlated with
early stage and negative lymph node status (Fig. 2C and D). Regarding the histological
subgroups, there was a non-significant tendency showing lower CDH11
expression in endometrioid carcinomas compared with serous tumors
(data not shown). No other significant associations with clinical
or histological parameters were observed in this group. By
Kaplan-Meier analysis and log-rank test using different CDH11
cut-off values, no significant correlation with recurrence-free or
overall survival was identified (data not shown).
Localization of CDH11 protein
expression detected by immunohistochemistry
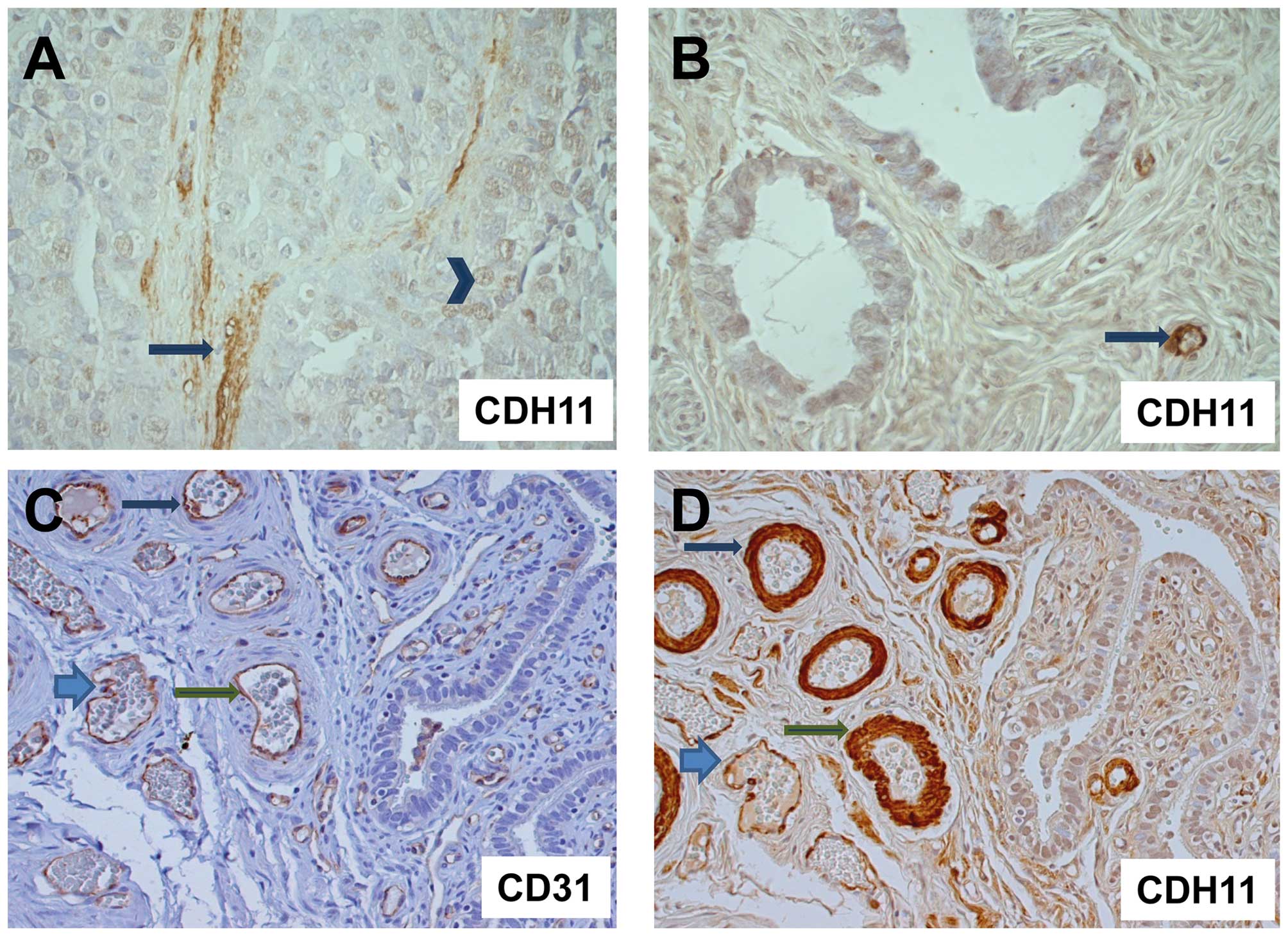
Since CDH11 expression had been reported not only in
tumor cells, but also in stromal or endothelial cells, we analyzed
representative tumors from each group (8 invasive carcinomas, 12
borderline tumors and 3 cystadenomas) for CDH11 expression by
immunohistochemistry. Strikingly, invasive carcinoma cells were
either CDH11-negative or displayed only weak nuclear or cytoplasmic
CDH11 expression. We did not detect any membranous CDH11 staining
in tumor cells. By contrast, strong CDH11 staining was observed in
the neighboring vessel walls and stromal matrix (Fig. 3). The comparison with
immunohistochemical detection of the endothelial marker CD31 in
parallel sections demonstrated CDH11 expression in endothelia and
vascular smooth muscle cells around the blood vessels (Fig. 3C). Isotypic controls were always
negative (data not shown).
Discussion
The function of CDH11 attracted interest during our
prior experimental studies, in which decreased adhesive properties
of ovarian cancer cells upon c-Fos overexpression were
demonstrated, accompanied by downregulation of various adhesion
proteins, including CDH11. Based on the results from other tumor
entities and the first data on ovarian tumors (11), we investigated CDH11 mRNA and protein
expression in a large cohort of ovarian carcinomas, borderline
tumors and benign cystadenomas. By western blot analysis and
RT-qPCR, we detected decreasing CDH11 expression with increasing
malignancy, with the highest expression observed in benign tumors.
By immunohistochemistry we demonstrated that, within the tumor
tissue, CDH11 was predominantly expressed in blood vessel walls and
stromal extracellular matrix components. This finding suggests that
the difference in CDH11 expression between benign, borderline and
malignant tumors, as demonstrated by RT-qPCR and western blot
analysis, may be attributed to the different tumor-stroma ratios,
with the largest fraction of stromal cells found in benign
tumors.
CDH11 expression in myofibroblasts or vascular
smooth muscle cells has been previously reported (15,16), where
it promotes cell proliferation and migration. Upregulation of CDH11
expression in differentiated myofibroblasts was detected during
dermal wound healing (15), where it
contributed to cell contraction. Interestingly, CDH11-mediated
cell-cell contacts in fibroblasts lead to strong upregulation of
vascular endothelial growth factor-D expression, indicating that it
may be involved in the angiogenic process (17). The CDH11 overexpression in fibroblasts
in the vicinity of tumor cells concurs with the observation that
the tumor microenvironment shares several characteristics with a
chronic wound (18,19).
In contrast to stromal cells, we did not identify
any membranous CDH11 expression in epithelial ovarian tumor cells
of different malignancy, but only weak or absent cytoplasmic or
nuclear immunoreactivity. A similar staining pattern was reported
in glioma cells, where an anti-invasive function of CDH11 was
hypothesized (20).
Regarding the role of CDH11 in human malignant
tumors, conflicting results were reported: A tumor suppressor
function of CDH11 expression was reported in retinoblastomas
(21) and gliomas (20), whereas high CDH11 expression
correlated with a more malignant subtype in colorectal cancer
(22), prostate cancer (8), breast cancer cell lines (10) and osteosarcomas (23). The latter immunohistochemical study is
in contrast to an RT-PCR-based investigation, which reported a
correlation of CDH11 expression with a good prognosis in this tumor
type (24). In the light of our
results, this contradiction may be associated with methodical
differences.
In ovarian tumors, CDH11 mRNA expression was
previously reported to be upregulated in metastatic lesions
compared to primary tumors (11). In
the present study, high CDH11 protein expression, as shown by
western blot analysis, was associated with advanced stage and nodal
involvement, which points to the same direction. Since the
tumor-stroma ratio does not differ in carcinomas of different stage
or nodal status, the RT-qPCR or western blot analysis results are
comparable within this tumor group. However, although these
correlations suggest a possible oncogenic role of this adhesion
molecule in ovarian cancer, the corresponding follow-up data show
that CDH11 expression is not significantly associated with
prognosis in ovarian carcinomas.
In conclusion, to the best of our knowledge, this is
the first study of CDH11 expression in a large cohort of ovarian
tumors of different histological subtypes. Among ovarian tumors,
CDH11 is predominantly expressed in stromal cells (myofibroblasts,
vascular smooth muscle cells and endothelial cells), leading to the
highest expression in benign tumors with a high stromal fraction.
Although in malignant ovarian cancer, high CDH11 expression
correlated with characteristics associated with an unfavorable
outcome, the lack of a prognostic significance in the survival
analysis indicates that it is not a suitable marker or therapeutic
target in this type of tumor.
Acknowledgements
We would like to thank Maila Rossberg and Kathrin
Eylmann for their excellent technical assistance.
References
|
1
|
Berx G and van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davidson B, Berner A, Nesland JM, et al:
E-cadherin and alpha-, beta-, and gamma-catenin protein expression
is up-regulated in ovarian carcinoma cells in serous effusions. J
Pathol. 192:460–469. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sundfeldt K, Piontkewitz Y, Ivarsson K, et
al: E-cadherin expression in human epithelial ovarian cancer and
normal ovary. Int J Cancer. 74:275–280. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hazan RB, Kang L, Whooley BP and Borgen
PI: N-cadherin promotes adhesion between invasive breast cancer
cells and the stroma. Cell Adhes Commun. 4:399–411. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qian X, Anzovino A, Kim S, et al:
N-cadherin/FGFR promotes metastasis through
epithelial-to-mesenchymal transition and stem/progenitor cell-like
properties. Oncogene. 33:3411–3421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Roy F: Beyond E-cadherin: Roles of
other cadherin superfamily members in cancer. Nat Rev Cancer.
14:121–134. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ,
Chen DT, Yu-Lee LY, Zhang S, Yeh ET, Hu MC, et al: Cadherin-11
promotes the metastasis of prostate cancer cells to bone. Mol
Cancer Res. 6:1259–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang CF, Lira C, Chu K, et al:
Cadherin-11 increases migration and invasion of prostate cancer
cells and enhances their interaction with osteoblasts. Cancer Res.
70:4580–4589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nieman MT, Prudoff RS, Johnson KR and
Wheelock MJ: N-cadherin promotes motility in human breast cancer
cells regardless of their E-cadherin expression. J Cell Biol.
147:631–644. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pishvaian MJ, Feltes CM, Thompson P, et
al: Cadherin-11 is expressed in invasive breast cancer cell lines.
Cancer Res. 59:947–952. 1999.PubMed/NCBI
|
|
11
|
Bignotti E, Tassi RA, Calza S, et al: Gene
expression profile of ovarian serous papillary carcinomas:
identification of metastasis-associated genes. Am J Obstet Gynecol.
196:245.e1–11. 2007. View Article : Google Scholar
|
|
12
|
Oliveira-Ferrer L, Rößler K, Haustein V,
et al: c-FOS suppresses ovarian cancer progression by changing
adhesion. Br J Cancer. 110:753–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mahner S, Baasch C, Schwarz J, et al:
C-Fos expression is a molecular predictor of progression and
survival in epithelial ovarian carcinoma. Br J Cancer.
99:1269–1275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schröder C, Schumacher U, Müller V, et al:
The transcription factor Fra-2 promotes mammary tumour progression
by changing the adhesive properties of breast cancer cells. Eur J
Cancer. 46:1650–1660. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hinz B, Pittet P, Smith-Clerc J,
Chaponnier C and Meister JJ: Myofibroblast development is
characterized by specific cell-cell adherens junctions. Mol Biol
Cell. 15:4310–4320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monahan TS, Andersen ND, Panossian H, et
al: A novel function for cadherin 11/osteoblast-cadherin in
vascular smooth muscle cells: Modulation of cell migration and
proliferation. J Vasc Surg. 45:581–589. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orlandini M and Oliviero S: In fibroblasts
Vegf-D expression is induced by cell-cell contact mediated by
cadherin-11. J Biol Chem. 276:6576–6581. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arnold KM, Opdenaker LM, Flynn D and
Sims-Mourtada J: Wound healing and cancer stem cells: Inflammation
as a driver of treatment resistance in breast cancer. Cancer Growth
Metastasis. 8:1–13. 2015.PubMed/NCBI
|
|
19
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Delic S, Lottmann N, Jetschke K,
Reifenberger G and Riemenschneider MJ: Identification and
functional validation of CDH11, PCSK6 and SH3GL3 as novel glioma
invasion-associated candidate genes. Neuropathol Appl Neurobiol.
38:201–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marchong MN, Yurkowski C, Ma C, Spencer C,
Pajovic S and Gallie BL: Cdh11 acts as a tumor suppressor in a
murine retinoblastoma model by facilitating tumor cell death. PLoS
Genet. 6:e10009232010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Sousa E, Melo F, Wang X, Jansen M, et
al: Poor-prognosis colon cancer is defined by a molecularly
distinct subtype and develops from serrated precursor lesions. Nat
Med. 19:614–618. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng Z, Niu G, Cai L, Wei R and Zhao X:
The prognostic significance of CD44V6, CDH11, and β-catenin
expression in patients with osteosarcoma. BioMed Res Int.
2013:4961932013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakajima G, Patino-Garcia A, Bruheim S, et
al: CDH11 expression is associated with survival in patients with
osteosarcoma. Cancer Genomics Proteomics. 5:37–42. 2008.PubMed/NCBI
|