Introduction
Enteral nutrition is preferred in patients who are
malnourished or undergoing cancer treatment and during the
perioperative period (1–3). Safe and reliable methods for the
placement of feeding tubes, such as percutaneous endoscopic
gastrostomy (PEG) (4), laparoscopic
gastrostomy (5) and nasogastric tube
(NGT) feeding (6), have
revolutionized nutritional therapy in such patients. However, NGT
and PEG are not feasible in cases where tube or endoscopic therapy
is not possible due to obstruction of the pharynx or esophagus; in
addition, NGT compromises the patients' quality of life.
Jejunostomy is often indicated in cases where gastrostomy is not
feasible, including gastric and esophageal cancer, where gastric
tube reconstruction is planned.
In this study, we describe a minimally invasive
method of laparoscopic jejunostomy (Lap-J) for obstruction due to
upper gastrointestinal malignancies. In addition, we evaluated the
nutritional benefits of Lap-J during neoadjuvant chemotherapy (NAC)
in cases of obstructing esophageal cancer.
Patients and methods
Patients
We conducted a non-randomized retrospective study of
26 patients who underwent Lap-J for obstruction due to esophageal
or gastric cancer at the National Defense Medical College Hospital
between January, 2008 and February, 2015. The indications for Lap-J
included previous gastric or duodenal surgery, gastroparesis, high
risk of aspiration and esophageal or gastric cancer where
gastrostomy was technically impossible or contraindicated. The
contraindications for Lap-J were unsuitability for general
anesthesia and gastrointestinal disease. In order to evaluate the
nutritional benefits of Lap-J during NAC, we compared nutritional
parameters such as body weight, serum total protein, albumin,
lymphocyte count and prognostic nutritional index (7) between patients who underwent Lap-J prior
to NAC (Lap-J group, n=9) and those who received NAC without Lap-J
(control group, n=56).
Chemotherapy with cisplatin plus 5-fluorouracil
(5-FU) was repeated twice every 3 weeks. A dose of 80
mg/m2 cisplatin was administered by intravenous drip
infusion for 2 h on day 1; 5-FU was administered at a dose of 800
mg/m2 by continuous infusion on days 1–5.
Surgical procedure
Following induction of general anesthesia, the
patient was placed in a supine position. The surgeon and
laparoscopist stood on the right side of the patient and the first
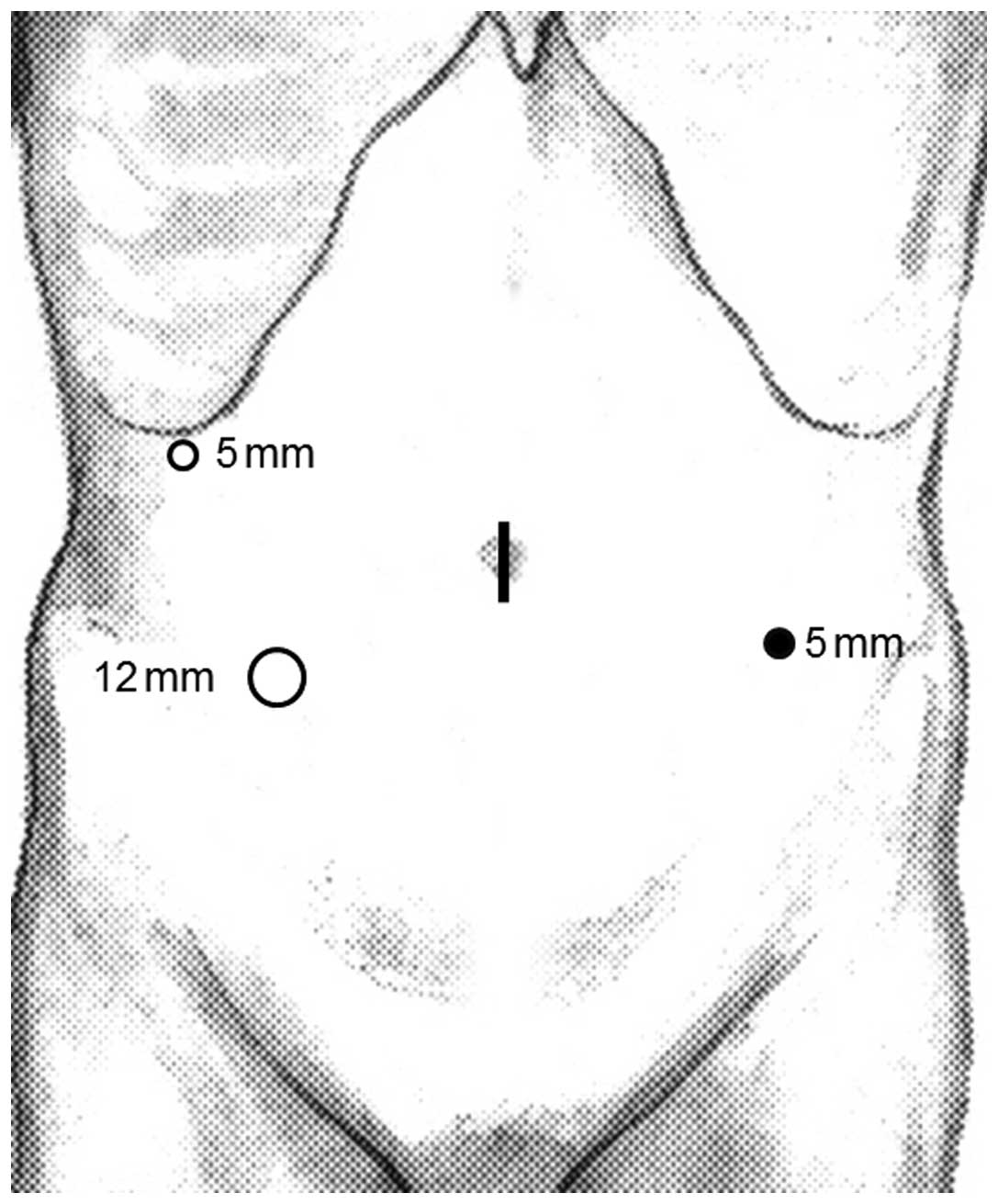
assistant on the left side. A camera port was inserted through a
median umbilical incision. Next, a 12 mmHg pneumoperitoneum was
induced and 3 additional ports (2 ports with a diameter of 5 mm and
1 port with a diameter of 12 mm) were inserted under laparoscopic
imaging into the right upper, left lower and right lower quadrants,
as shown in Fig. 1.
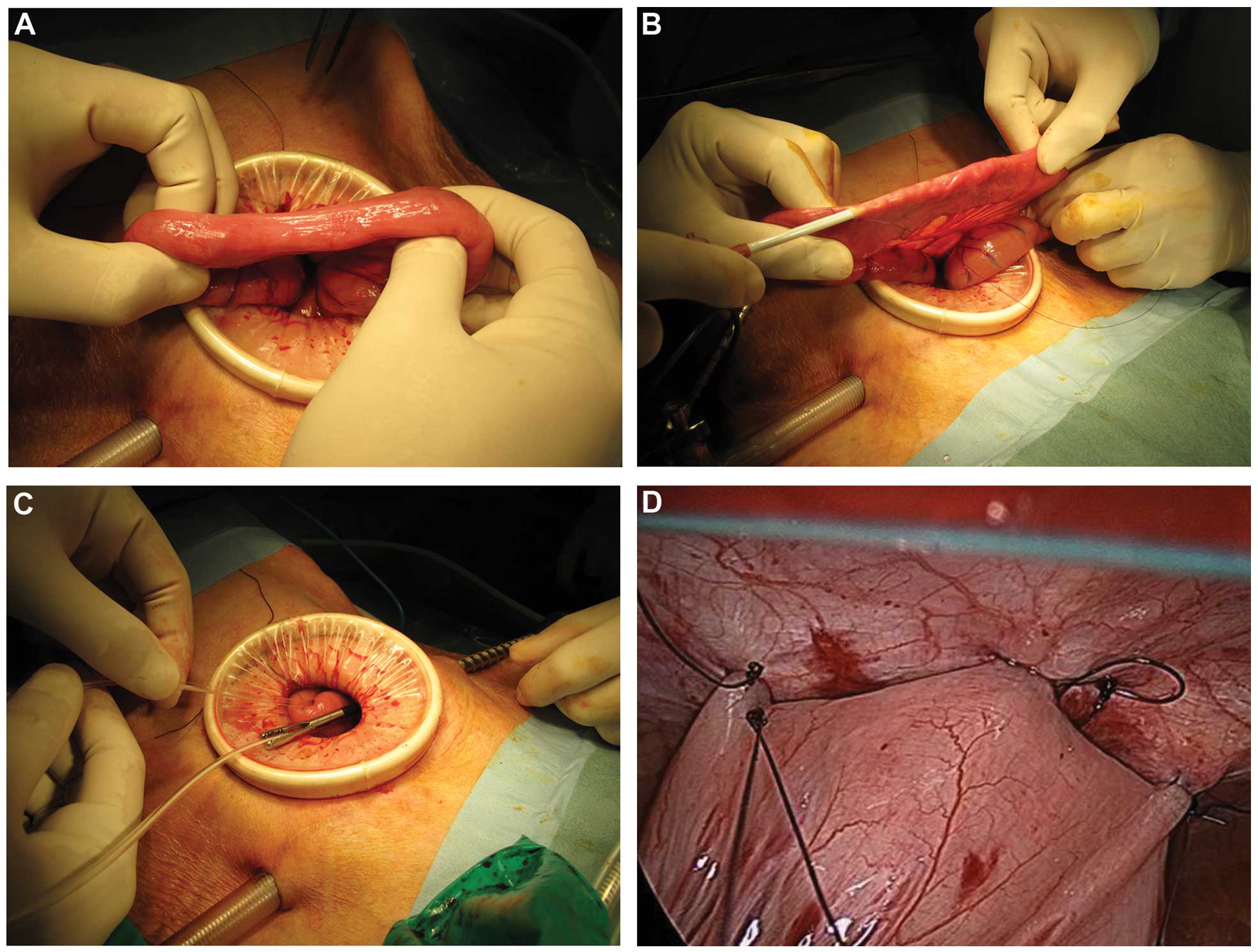
Following exploration of the peritoneal cavity, the
jejunum 20–30 cm distant from the Treitz ligament was pulled out
through the umbilical trocar incision, which was extended to ~2 cm
by enlarging the median fascia and skin incision before covering
with an Alexis wound retractor (Applied Medical, Tokyo, Japan)
(Fig. 2A). After a serosal suture
(4–0 PDS; Ethicon, Tokyo, Japan) was placed, a trocar with a
peel-away sheath (Covidien, Tokyo, Japan) was used to penetrate the
subserosa for ~8 cm prior to penetrating the jejunal lumen
(Fig. 2B). The peel-away sheath was
removed and the jejunal tube was inserted to 30 cm. Intraluminal
placement was confirmed by flushing with saline. The tube was held
by a laparoscopic grasper inserted through the left lower trocar
(Fig. 2C). The loop of bowel was
gently returned to the abdomen and the feeding tube was drawn
through the abdominal wall via the left lower incision. The jejunum
was laparoscopically sutured to the anterior abdominal wall using 5
or 6 sutures (3-0 Monocryl; Ethicon) (Fig. 2D).
Statistical analysis
Data are expressed as means ± standard deviation.
Comparisons between the two groups were performed using the
Wilcoxon signed-rank test. Data were analyzed using the MedCalc
v9.0 statistical software package (MedCalc, Mariakerke, Belgium). A
P-value of <0.05 was considered to indicate statistically
significant differences.
Results
Short-term outcomes of Lap-J
We safely performed the Lap-J procedure in 26
patients with obstructing upper gastrointestinal malignancies
(Table I). The primary indications
were esophageal (n=22) and gastric cancer (n=4). The mean age was
69.4±6.0 years (range, 48–79 years). All the patients had stage III
or IV disease, except for 1 case of stage I gastric cancer. Nine
esophageal cancer patients had NAC followed by surgery after Lap-J.
The mean operative time was 81.6±29.6 min (range, 35–155 min). The
mean estimated intraoperative blood loss was 1.2±0.4 ml (range,
0–15 ml). All the patients were able to stand, walk and resume
enteral nutrition on postoperative day 1, without complications.
The mean postoperative hospital stay was 21.6±18.3 days (range,
1–84 days). The hospital stay was prolonged in 20 cases, the causes
of which are listed in Table II.
Subsequent therapy was initiated 11.5±6.3 days (range, 3–27 days)
after Lap-J, excluding cases receiving palliative care. Three cases
developed diarrhea that improved with reduced feeding speed and 2
cases developed obstruction of the jejunostomy tube and tubes were
exchanged using a guide wire. There was no Lap-J-related
mortality.
 | Table I.Demographic data of patients who
underwent Lap-J. |
Table I.
Demographic data of patients who
underwent Lap-J.
| Variables | No. of patients
(n=26) |
|---|
| Age, years | 69.4±6.0 (48–79) |
| Gender |
|
| Male | 22 |
|
Female | 4 |
| BMI,
kg/m2 | 19.3±2.9
(14.6–24.0) |
| Location |
|
|
Esophageal cancer | 22 |
| Gastric
cancer | 4 |
| Stage |
|
| I | 1 |
| II | 0 |
| III | 15 |
| IV | 10 |
| Type of
treatment |
|
|
Surgery | 2 |
|
Chemotherapy followed by
surgery | 9 |
|
Chemoradiation therapy | 6 |
|
Chemotherapy | 4 |
| Radiation
therapy | 1 |
| Best
supportive care | 4 |
 | Table II.Surgical outcomes of Lap-J. |
Table II.
Surgical outcomes of Lap-J.
| Variables | No. |
|---|
| Operative time,
min | 81.6±29.6
(35–155) |
| Blood loss, g | 0.9±2.9 (0–15) |
| Start of enteral
feeding, days | 1.2±0.4 (1–2) |
| Start of subsequent
therapy, days | 11.5±6.3 (3–27) |
| Hospital stay
following Lap-J, days | 21.6±18.3 (1–84) |
| Complications |
|
| Diarrhea | 3 |
| Obstruction of the
tube | 2 |
| Leakage | 0 |
| Dislodgement | 0 |
| Perforation | 0 |
| Bowel necrosis | 0 |
| Bowel torsion | 0 |
| Herniation | 0 |
Nutritional benefits of Lap-J during
NAC for obstructing esophageal cancer
The nutritional parameters prior to and following
NAC in the two groups are presented in Table III. Although there was a significant
decrease in body weight and serum total protein following NAC in
the control group, no such changes were observed in the Lap-J
group. No change in serum albumin, lymphocyte count, or prognostic
nutritional index following NAC was observed in either group.
 | Table III.Nutritional data prior to and
following NAC for esophageal cancer in patients who did and those
who did not undergo Lap-J. |
Table III.
Nutritional data prior to and
following NAC for esophageal cancer in patients who did and those
who did not undergo Lap-J.
|
| With Lap-J (n=9) | Without Lap-J
(n=56) |
|---|
|
|
|
|
|---|
| Variables | Prior to NAC | Following NAC | P-value | Prior to NAC | Following NAC | P-value |
|---|
| Body weight, kg | 47.3±8.2 | 47.7±6.3 | 0.6072 | 58.3±5.5 | 56.4±4.8 | 0.0020 |
| Total protein,
g/dl | 6.89±0.46 | 6.90±0.46 | 0.9291 | 6.61±0.53 | 6.26±0.53 | 0.0010 |
| Albumin, g/dl | 3.78±0.15 | 3.69±0.13 | 0.4496 | 3.83±0.37 | 3.72±0.47 | 0.0815 |
| Lymphocyte count,
/µl | 1,349.2±425.8 | 1,962.7±783.4 | 0.0873 | 1,539.8±500.5 | 1,550.5±447.3 | 0.8735 |
| PNI | 38.4±4.3 | 37.9±3.9 | 0.6205 | 39.1±3.8 | 38.0±4.7 | 0.0837 |
Discussion
In this study, we demonstrated a simple and safe
technique for Lap-J with jejunopexy. There was no procedure-related
mortality and enteral nutrition was resumed the following day in
all the patients.
As laparoscopic feeding jejunostomy was first
described in 1990 (8), several
modified procedures for Lap-J have since been developed (9–12). Duh
et al (13) described
laparoscopy-assisted percutaneous placement of jejunostomy
catheters using T-fasteners to retract and anchor the jejunum. This
procedure is advantageous, as it does not require extension of the
umbilical trocar incision or exposure of the jejunum. However, this
procedure does not penetrate the subserosa for a sufficient length
to avoid the use of the Witzel technique (14). Enterocutaneous fistula formation
around the feeding tube is one of the most serious complications of
jejunostomy. To avoid this complication, the techniques must
involve affixation of the jejunum to the parietal peritoneum at the
puncture site and employment of a subserous tunnel, as in our
technique. Several studies have reported laparoscopy-guided
techniques that exteriorize the proximal jejunum with the insertion
of feeding tubes through an enterotomy and securing by
extracorporeal fixation to the fascia with non-absorbable or
absorbable stitches (15,16).
Obstructing esophageal cancer with planned gastric
tube reconstruction is a common indication for jejunostomy. In
Japan, preoperative chemotherapy with cisplatin plus 5-FU is
considered standard treatment for patients with stage II/III
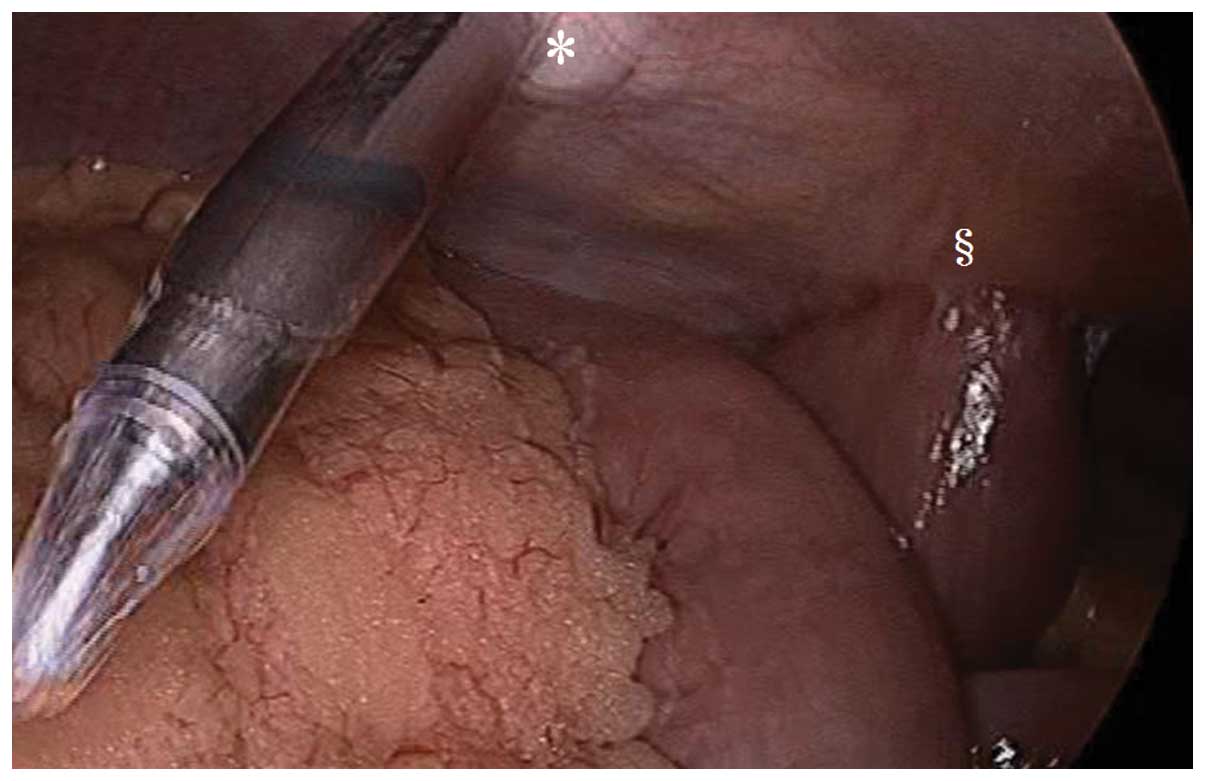
esophageal squamous cell carcinoma (17). We performed Lap-J in 9 patients with
stage II/III esophageal cancer prior to NAC. No abdominal adhesions
were observed in these patients and there were no complications or
difficulties in laparoscopic gastric tube reconstruction and
abdominal lymph node dissection (Fig.
3) (18). No increase in
laparoscopic operative time was observed (Lap-J vs. control group,
285 vs. 292 min, respectively). In addition, Lap-J prior to NAC was
not associated with a decrease in body weight or serum total
protein during NAC, compared with patients receiving NAC who did
not undergo Lap-J. Thus, Lap-J prior to NAC may be useful in
patients with obstructing esophageal cancer for whom gastric tube
reconstruction is planned.
In conclusion, we described a minimally invasive
jejunostomy technique that may be particularly useful for patients
in whom endoscopic therapy is not feasible due to obstruction from
upper gastrointestinal malignancies.
References
|
1
|
Braga M, Ljungqvist O, Soeters P, Fearon
K, Weimann A and Bozzetti F: ESPEN: ESPEN guidelines on parenteral
nutrition: Surgery. Clin Nutr. 28:378–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aiko S, Kumano I, Yamanaka N, Tsujimoto H,
Takahata R and Maehara T: Effects of an immuno-enhanced diet
containing antioxidants in esophageal cancer surgery following
neoadjuvant therapy. Dis Esophagus. 25:137–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Braga M, Gianotti L, Nespoli L, Radaelli G
and DiCarlo V: Nutritional approach in malnourished surgical
patients: A prospective randomized study. Arch Surg. 137:174–180.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell TR, Brotman M and Norris F:
Percutaneous gastrostomy. A new simplified and cost-effective
technique = Am J Surg. 148:132–137. 1984.
|
|
5
|
Tsujimoto H, Yaguchi Y, Kumano I,
Matsumoto Y, Yoshida K and Hase K: Laparoscopy-assisted
percutaneous gastrostomy tube placement along with laparoscopic
gastropexy. Dig Surg. 28:163–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nally DM, Kelly EG, Clarke M and Ridgway
P: Nasogastric nutrition is efficacious in severe acute
pancreatitis: A systematic review and meta-analysis. Br J Nutr.
112:1769–1778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamura Y, Ashida R, Ito T, Sugiura T,
Mori K and Uesaka K: Preoperative neutrophil to lymphocyte ratio
and prognostic nutritional index predict overall survival after
hepatectomy for hepatocellular carcinoma. World J Surg.
39:1501–1509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Regan PJ and Scarrow GD: Laparoscopic
jejunostomy. Endoscopy. 22:39–40. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albrink MH, Foster J, Rosemurgy AS and
Carey LC: Laparoscopic feeding jejunostomy: Also a simple
technique. Surg Endosc. 6:259–260. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allen JW, Ali A, Wo J, Bumpous JM and
Cacchione RN: Totally laparoscopic feeding jejunostomy. Surg
Endosc. 16:1802–1805. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grondona P, Andreani SM, Barr N and Singh
KK: Laparoscopic feeding jejunostomy technique as part of staging
laparoscopy. Surg Laparosc Endosc Percutan Tech. 15:263–266. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
HanGeurts IJ, Lim A, Stijnen T and Bonjer
HJ: Laparoscopic feeding jejunostomy: A systematic review. Surg
Endosc. 19:951–957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duh QY, SenokozlieffEnglehart AL,
Siperstein AE, et al: Prospective evaluation of the safety and
efficacy of laparoscopic jejunostomy. West J Med. 162:117–122.
1995.PubMed/NCBI
|
|
14
|
Pearce CB and Duncan HD: Enteral feeding.
Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or
jejunostomy: Its indications and limitations. Postgrad Med J.
78:198–204. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gedaly R, Briceño P, Ravelo R and
Weisinger K: Laparoscopic jejunostomy with an 18-mm trocar. Surg
Laparosc Endosc. 7:420–422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Düzgün SA, Bozer M, Coskun A, et al: A
simplified laparoscopic technique for enteral access in cancer
patients. Hepatogastroenterology. 49:1002–1005. 2002.PubMed/NCBI
|
|
17
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:68–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsujimoto H, Ono S, Sugasawa H, et al:
Gastric tube reconstruction by laparoscopy-assisted surgery
attenuates postoperative systemic inflammatory response after
esophagectomy for esophageal cancer. World J Surg. 34:2830–2836.
2010. View Article : Google Scholar : PubMed/NCBI
|