Introduction
Contiguous connexins (Cxs) may form homomeric or
heteromeric gap junction hemichannels (connexons) on the cell
membrane (1). Two connexons of
adjacent cells form a gap junction channel, through which gap
junction intercellular communication (GJIC) is possible via the
passage of ions and second messengers (1). GJIC plays a crucial role in maintaining
cell homeostasis, cell growth control and development (1).
First described in cultured hepatoma cells (2), the association between GJIC and
carcinogenesis has since been described in various types of
tumours, such as cervical (3,4), mammary (5), bronchial (6) and colorectal carcinoma (7). Depending on tumour progression, GJIC has
different functions (8). Cxs affect
cell growth by affecting the expression of cell cycle regulatory
genes, such as cyclin A, D1 and D2, and cyclin-dependent kinases
(8). Cx43 transfection in previously
deficient tumour cell lines led to growth inhibition and an
accumulation of classical tumour suppressors, such as p27 and Rb
protein (8). Lack of GJIC leads to an
intracellular accumulation of growth factors (2) and suppressed contact inhibition, causing
cell proliferation (9). King et
al (4) described a correlation
between endogenous Cx43 mRNA and protein expression and increased
growth control, with decreased growth capacity in HeLa cervical
cancer cells. Cx43 knockout in mice leads to astrocytes exhibiting
altered expression of genes associated with apoptosis, cell growth,
transcription factors (10,11), and increased susceptibility of mice to
pulmonary neoplasia (6). Cx26 and
Cx43 expression in MDA-MB-231 breast cancer cells (5) provided similar results. Cx26 is
responsible for contact growth inhibition in HeLa and HepG2 cells
(3,12). Cx45 may form heteromeric gap junctions
along with Cx43, and may affect intercellular contacts during
carcinogenesis (13–15). Cx subtypes increase the attachment of
tumour cells to the stroma (8) and
the endothelial barrier (16),
thereby promoting invasion and metastatic spread. Cx26 was
identified in melanoma cells and surrounding small vessel
endothelia (17), as well as in
squamous cell lung carcinoma and its associated lymph node
metastases (LMNs) (18). Cx26- and
Cx43-negative primary breast cancers developed Cx26- and
Cx43-positive LMNs (19). Glioma
cells establish functional gap junctions comprising Cx43 with
astrocytes in the adult brain, thus facilitating direct parenchymal
invasion (20).
For oral squamous cell carcinoma (OSCC), conflicting
Cx expression data were reported by Ozawa et al (21) and Villaret et al (22), who described Cx26 and Cx30 expression
in OSCC tissues. In a previous histomorphometric study, we analysed
the protein expression of Cx subtypes 26, 43 and 45 in tissue
samples of OSSC, corresponding LMNs and dysplasia-free oral mucosa
in 35 patients (23). In addition to
significantly different expression patterns between the studied
tissue types, high membrane Cx43 expression in OSCC tissues was
found to be associated with poor prognosis (23). Xia et al (24) previously reported reduced Cx43 protein
concentration, despite normal mRNA levels, in an induced rat tongue
carcinogenesis model. The present study aimed to confirm the
expression of the described Cx subtypes at the mRNA level by
conducting a reverse transcription quantitative polymerase chain
reaction (RT-qPCR) analysis in 15 tissue sample pairs of OSCC and
corresponding oral mucosa.
Materials and methods
Patients
Tissue samples from 15 patients suffering from
primary OSCC were analysed. All the patients were diagnosed and
treated according to the guidelines of the national German Oral
Cancer Association (25). The
resection margins and presence of LMNs were histologically
investigated in all the patients. Metastases to the lung, liver and
bone marrow were evaluated by chest radiography, abdominal
ultrasound examination and bone scans in all the patients. The
patients' characteristics are summarised in Table I. The patients provided written
informed consent prior to participating in the study. This study
was conducted in line with the ethical standards of the Declaration
of Helsinki and was approved by the local Ethics Committee at the
George-August-University of Goettingen (vote number 11/6/05).
 | Table I.Descriptive statistics and results
from multivariate regression models with expression levels as the
dependent variable. |
Table I.
Descriptive statistics and results
from multivariate regression models with expression levels as the
dependent variable.
|
|
| Cx26 | Cx43 | Cx45 |
|---|
|
|
|
|
|
|
|---|
| Parameters | Descriptive
statistics | β ± SE | P-value | β ± SE | P-value | β ± SE | P-value |
|---|
| Tumour tissue |
| −8.4±2.9 | 0.01 | 0.7±0.3 | 0.04 | 10.5±1.5 | <0.01 |
| Age (years) | 59.0±12.9 | −0.1±0.2 | 0.45 | −0.02±0.02 | 0.22 | −0.1±0.1 | 0.50 |
| Gender |
| −3.7±6.3 | 0.58 | −0.6±0.6 | 0.33 | 1.8±7.2 | 0.81 |
|
Female | 5 (33%) |
|
|
|
|
|
|
| Male | 10 (67%) |
|
|
|
|
|
|
| T status |
| −10.8±26.2 | 0.69 | 0.7±2.7 | 0.80 | −4.7±27.6 | 0.87 |
| Is | 1 (7%) |
|
|
|
|
|
|
| 2 | 5 (33%) |
|
|
|
|
|
|
| 4 | 8 (53%) |
|
|
|
|
|
|
| 4a | 1 (7%) |
|
|
|
|
|
|
| N status |
| 6.4±3.5 | 0.12 | 0.5±0.4 | 0.17 | 0.5±3.7 | 0.90 |
| 0 | 9 (60%) |
|
|
|
|
|
|
| 1 | 3 (20%) |
|
|
|
|
|
|
| 2b | 2 (13%) |
|
|
|
|
|
|
| 2c | 1 (7%) |
|
|
|
|
|
|
| Nicotine abuse |
| −0.7±9.1 | 0.94 | 0.01±0.9 | 0.99 | −14.3±8.0 | 0.14 |
|
Yes | 14 (93%) |
|
|
|
|
|
|
| No | 1 (7%) |
|
|
|
|
|
|
| Alcohol abuse |
| 6.9±3.5 | 0.22 | −1.0±0.5 | 0.05 | −4.2±4.3 | 0.37 |
|
Yes | 10 (67%) |
|
|
|
|
|
|
| No | 5 (33%) |
|
|
|
|
|
|
| Stage |
| 8.4±24.4 | 0.74 | −0.6±2.5 | 0.82 | 1.3±25.4 | 0.96 |
| 0 | 1 (7%) |
|
|
|
|
|
|
| 2 | 5 (33%) |
|
|
|
|
|
|
| 4 | 9 (60%) |
|
|
|
|
|
|
| Grade |
| 1.0±4.8 | 0.84 | −0.7±0.5 | 0.18 | 4.4±4.2 | 0.34 |
| 1 | 2 (13%) |
|
|
|
|
|
|
| 2 | 11 (74%) |
|
|
|
|
|
|
| 3 | 2 (13%) |
|
|
|
|
|
|
Biopsies
One biopsy from OSCC tissue and one from tumour-free
oral mucosa were obtained from each patient during tumour ablation.
All the biopsies were 2–3 mm in diameter. Sampling was performed
according to a predefined, standardized working instructions, based
on size and location of sampling, by the same two experienced
examiners (F.F. and R.M.G.). The biopsies were incubated in
RNAlater® (Ambion/Applied Biosystems, Darmstadt, Germany) overnight
and shock-frozen in liquid nitrogen for storage at −80°C until RNA
isolation.
RNA isolation
RNA was isolated using the RNeasy Mini kit (Qiagen,
Hilden, Germany) according to the manufacturers' recommendations
and stored at −80°C. Subsequently, the samples were treated with
DNase I to remove genomic DNA contaminations. The RNA quality was
determined using the Agilent 2100 Bioanalyzer (Agilent
Technologies, Boeblingen, Germany) microfluidic electrophoresis.
The analysed tissue sample pairs (OSCC and oral mucosa) had
comparable RNA integrity numbers.
RT-qPCR
For verification of Cx subtype expression, RNA
samples were converted into cDNA using the Bio-Rad iScript cDNA
Synthesis kit (Bio-Rad Laboratories, Munich, Germany) and
quantified on a Bio-Rad MyiQ Real-Time PCR Detection system with
the Bio-Rad iQ SYBR Green Supermix. The primers are listed
below:
GAPDH: 5′-GAGTCAACGGATTTGGTCGT-3′,
5′-GACAAGCTTCCCGTTCTCAG-3′; Cx26: 5′-ACTCCACCAGCATTGGAAAG-3′,
5′-TGGGAGATGGGGAAGTAGTG-3′; Cx43: 5′-AGCAGTCTGCCTTTCGTTGT-3′,
5′-TCTGCTTCAAGTGCATGTCC-3′; and Cx45: 5′-GCACTGCCAGTAGCAAATCA-3′,
5′-CCAACAGCATCCCTGAAGAT-3′.
Relative gene expression was quantified using the
∆∆Cq method. Since GAPDH is not differentially expressed in OSCC
tissue (26), it was used as a
housekeeping gene, based on which the Cx gene expression was
normalized.
Statistical analysis
Relative gene expression normalized to GAPDH and
log2-transformed was calculated from Cq values and PCR
efficiency (27). The expression
change between OSCC and oral mucosa and the effect of
clinicopathological routine parameters was separately assessed for
Cx26, Cx43 and Cx45 by a multivariate linear regression model. To
account for the matched-pair situation (i.e., OSCC and normal
mucosa from the same patient), patients were included as
random-effects term to each model. For tissue and other dichotomous
model parameters, the regression coefficients may be considered as
log2 fold changes and are reported with their related
standard errors. The significance level was set to α=5% for each
test. For a stronger statistical perspective, the P-values for the
tissue effects were adjusted by the method of Bonferroni and Holm
(28). All the analyses were
performed using R software, version 3.1 (www.r-project.org). The multivariate regression models
were fitted with the lmer function of the lme4 package for R.
Results
Differential Cx expression in OSCC and
normal oral mucosa
The multivariate model analysis yielded a
significant differential expression between the two types of
tissues (OSCC and oral mucosa) for each of the three Cx subtypes.
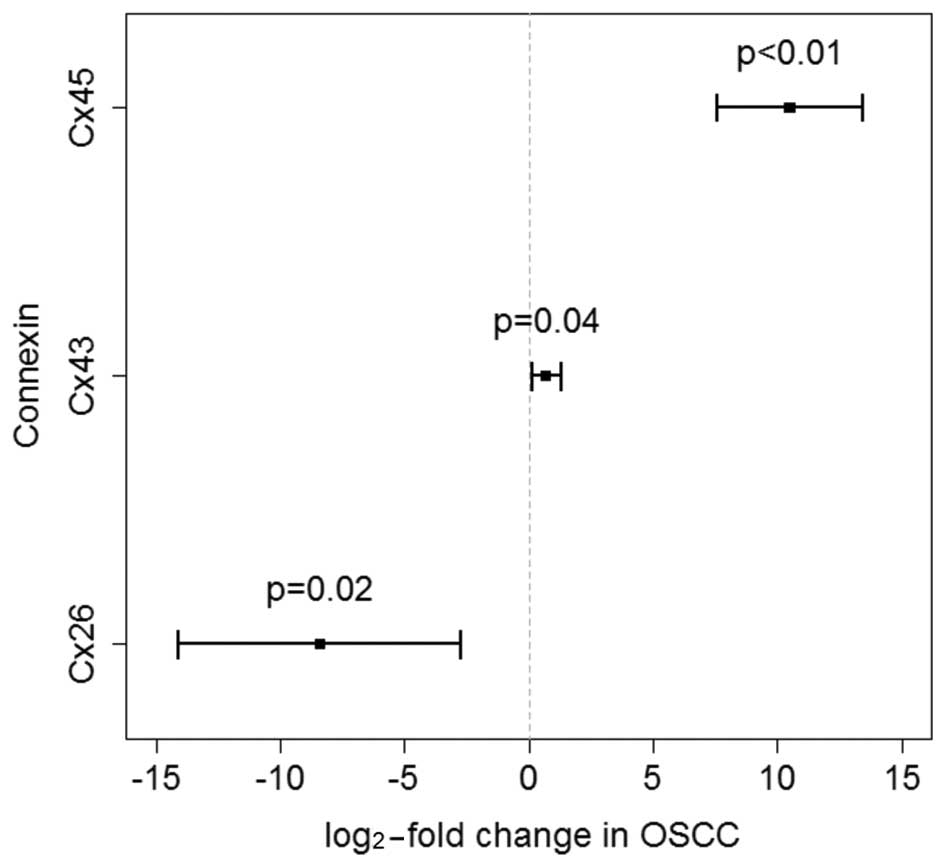
In detail, Cx45 exhibited a strong overexpression of 10.5-log-fold
(95% CI: 7.6–13.4) in OSCC samples and was differentially expressed
compared with tumour-free oral mucosa controls (P<0.01). Cx26
exhibited a downregulation of −8.4-log-fold (95% CI: −14.1 to −2.7)
in cancer tissues (P=0.01). Compared with oral mucosa controls, the
gene expression for Cx43 was marginally upregulated (P=0.04) in
OSCC tissues (0.7-log-fold; 95% CI: 0.1–1.3; Fig. 1). Following a
Bonferroni/Holm-adjustment, the P-values for the tissue effects
remained statistically significant. The gene expression of the
described Cx subtypes did not differ between the early and late
stages of malignancy, and there were no significant effects by the
clinicopathological routine parameters T status, N status and
American Joint Committee on Cancer stage. Moreover, Cx subtype
expression was not found to be correlated with alcohol and tobacco
abuse, gender, or histopathological grade. Only for Cx43, the
P-value for the effect of alcohol abuse (P=0.0518) exhibited a
trend for a possible downregulation of this Cx in patients abusing
alcohol (Table I).
Discussion
In a previous histomorphometrical analysis (23), we investigated the protein expression
patterns of the Cx subtypes 26, 43 and 45 in tissue samples of
OSCC, dysplasia-free oral mucosa and LNM in 35 primary OSCC
patients and observed differential expression profiles between the
tissue types. Moreover, high membrane Cx43 expression in OSSC
tissues was associated with poor prognosis, and exhibited a similar
prognostic tendency in microscopically unchanged oral mucosa of
same patients (23). The present
study was performed on tissue samples of tumours at different
stages. However, the primary goal was not to investigate the effect
of tumour stage, but rather to determine whether the Cx subtype
expression differed at the mRNA level between OSSC and oral mucosa.
Since differences between the transcriptional and protein levels of
Cx43 have been described in an experimental rat tongue
carcinogenesis model (24), we
further aimed to confirm the expression of Cx subtypes at the mRNA
level in OSCC tissues.
Cx45 mRNA has been shown to be more strongly
overexpressed in OSCC tissues compared with intraindividual mucosa
controls (P<0.01). In contrast to LNM, which exhibited a
significant increase in the Cx45 protein level (23), Cx45 protein expression was not found
to be significantly different between OSCC tissues and
dysplasia-free oral mucosa in our previous investigation (23). There is only limited evidence of the
relevance of Cx45 for carcinogenesis in recent publications. Cx45
is variably expressed in human lung fibroblasts and lung carcinoma
cells (29), and was detected in
normal lung tissue and advanced-stage mouse lung carcinomas
(30). Cx45 has been extensively
investigated regarding its co-expression with Cx43 resulting in
altered gap junctions. Cx45 was found to be upregulated in heart
failure, compared with Cx43 (31).
The diffusion capacity of cationic fluorescent dyes over
heteromeric gap junctions comprising Cx45 and Cx43 was found to be
reduced when Cx45 is overexpressed (15), leading to altered intercellular
voltage gating mechanisms (14). The
relative upregulation of Cx45 in comparison with Cx43 was shown to
cause an increased susceptibility to cardiac arrhythmias in
vivo (13).
The Cx26 mRNA level was downregulated in OSCC
tissues, as opposed to oral mucosa controls (P=0.01). In our
histomorphometrical analysis, no Cx26 protein expression was
detected in oral mucosa (23);
however, it was increased in primary OSCC and exhibited the highest
levels in local LNMs. Different studies indicated that Cx26 may be
involved in tumour cell invasion and metastasis. Kanczuga-Koda
et al (19) described Cx26
overexpression in corresponding LNMs compared with primary mammary
carcinoma. Saito-Katsuragi et al (17) demonstrated a significant Cx26
expression in tumour cells and tumour-related microvessel
endothelia during metastasis of human malignant melanoma, whereas
no Cx26 expression was found in control tissues from either healthy
dermis or nevus cell nevi. In this context, it appears likely that
neoplastic cells use Cx26 to form homomeric gap junctions with
tumour-associated microvessel endothelia, thus improving
perivascular accumulation and preparing extravasation.
In the present investigation, Cx43 gene expression
was marginally upregulated in OSCC tissues, unlike that in oral
mucosa controls (P=0.04). In our previous analysis (23), the cytoplasmic Cx43 protein level was
found to be increased in primary OSCC compared with matching oral
mucosa. Moreover, membrane Cx43 expression was reduced in OSCC
tissues compared with oral mucosa controls, suggesting a loss of
gap junctions comprising Cx43, leading to a loss of GJIC. A
reduction of Cx43 during carcinogenesis was previously demonstrated
(32). However, it has not been fully
elucidated whether this loss is due to increased degradation of gap
junction channels, or faulty transcription and post-transcriptional
modifications within the Cxs. It was recently suggested that
post-transcriptional, translational and degradation regulations
play a similarly important gene transcription role in the
determination of protein concentrations (33). Despite reduced Cx43 protein levels,
normal high mRNA levels were detected (24). Budunova et al (34) investigated the expression of Cxs 26,
43 and 31.1 in mouse hyperplastic skin, papilloma and SCC. In
addition to the high levels of Cx26 and Cx43 mRNA in most of the
SCC, the authors observed decreased protein levels of both Cx
subtypes in tumour plasma membranes, and concluded that the
expression of these two Cxs in SCC was impaired at the
post-translational level (34).
The exact functions of connexins and GJIC during
oral carcinogenesis remain unclear. Connexin regulation at the
transcriptional level appears to be an early event during the
initiation and development of OSCC, and is maintained during tumour
progression. However, the mRNA-protein correlation is variable.
This may be indicative of post-transcriptional, translational and
degradation regulations being relevant for the determination of Cx
protein concentration during oral carcinogenesis.
References
|
1
|
Willecke K, Eiberger J, Degen J, Eckardt
D, Romualdi A, Güldenagel M, Deutsch U and Söhl G: Structural and
functional diversity of connexin genes in the mouse and human
genome. Biol Chem. 383:725–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loewenstein WR and Penn RD: Intercellular
communication and tissue growth. II. Tissue regeneration. J Cell
Biol. 33:235–242. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mesnil M, Krutovskikh V, Piccoli C,
Elfgang C, Traub O, Willecke K and Yamasaki H: Negative growth
control of HeLa cells by connexin genes: Connexin species
specificity. Cancer Res. 55:629–639. 1995.PubMed/NCBI
|
|
4
|
King TJ, Fukushima LH, Donlon TA, Hieber
AD, Shimabukuro KA and Bertram JS: Correlation between growth
control, neoplastic potential and endogenous connexin 43 expression
in HeLa cell lines: Implications for tumor progression.
Carcinogenesis. 21:311–315. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McLachlan E, Shao Q, Wang HL, Langlois S
and Laird DW: Connexins act as tumor suppressors in
three-dimensional mammary cell organoids by regulating
differentiation and angiogenesis. Cancer Res. 66:9886–9894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avanzo JL, Mesnil M, Hernandez-Blazquez
FJ, Mackowiak II, Mori CM, da Silva TC, Oloris SC, Gárate AP,
Massironi SM, Yamasaki H and Dagli ML: Increased susceptibility to
urethane-induced lung tumors in mice with decreased expression of
connexin 43. Carcinogenesis. 25:1973–1982. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dubina MV, Iatckii NA, Popov DE, Vasil'ev
SV and Krutovskikh VA: Connexin 43, but not connexin 32, is mutated
at advanced stages of human sporadic colon cancer. Oncogene.
21:4992–4996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cronier L, Crespin S, Strale PO, Defamie N
and Mesnil M: Gap junctions and cancer: New functions for an old
story. Antioxid Redox Signal. 11:323–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trosko JE, Chang CC, Upham BL and Tai MH:
Ignored hallmarks of carcinogenesis: Stem cells and cell-cell
communication. Ann N Y Acad Sci. 1028:192–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iacobas DA, Urban-Maldonado M, Iacobas S,
Scemes E and Spray DC: Array analysis of gene expression in
connexin-43 null astrocytes. Physiol Genomics. 15:177–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iacobas DA, Scemes E and Spray DC: Gene
expression alterations in connexin null mice extend beyond the gap
junction. Neurochem Int. 45:243–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yano T, Hernandez-Blazquez FJ, Omori Y and
Yamasaki H: Reduction of malignant phenotype of HEPG2 cell is
associated with the expression of connexin 26 but not connexin 32.
Carcinogenesis. 22:1593–1600. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Betsuyaku T, Nnebe NS, Sundset R,
Patibandla S, Krueger CM and Yamada KA: Overexpression of cardiac
connexin 45 increases susceptibility to ventricular
tachyarrhythmias in vivo. Am J Physiol Heart Circ Physiol.
290:H163–H171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bukauskas FF, Angele AB, Verselis VK and
Bennett MV: Coupling asymmetry of heterotypic connexin 45/connexin
43-EGFP gap junctions: Properties of fast and slow gating
mechanisms. Proc Natl Acad Sci USA. 99:7113–7118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koval M, Geist ST, Westphale EM, Kemendy
AE, Civitelli R, Beyer EC and Steinberg TH: Transfected connexin 45
alters gap junction permeability in cells expressing endogenous
connexin 43. J Cell Biol. 130:987–995. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogawa K, Pitchakarn P, Suzuki S,
Chewonarin T, Tang M, Takahashi S, Naiki-Ito A, Sato S, Takahashi
S, Asamoto M and Shirai T: Silencing of connexin 43 suppresses
invasion, migration and lung metastasis of rat hepatocellular
carcinoma cells. Cancer Sci. 103:860–867. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saito-Katsuragi M, Asada H, Niizeki H,
Katoh F, Masuzawa M, Tsutsumi M, Kuniyasu H, Ito A, Nojima H and
Miyagawa S: Role for connexin 26 in metastasis of human malignant
melanoma: Communication between melanoma and endothelial cells via
connexin 26. Cancer. 110:1162–1172. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito A, Koma Y, Uchino K, Okada T,
Ohbayashi C, Tsubota N and Okada M: Increased expression of
connexin 26 in the invasive component of lung squamous cell
carcinoma: Significant correlation with poor prognosis. Cancer
Lett. 234:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanczuga-Koda L, Sulkowski S, Lenczewski
A, Koda M, Wincewicz A, Baltaziak M and Sulkowska M: Increased
expression of connexins 26 and 43 in lymph node metastases of
breast cancer. J Clin Pathol. 59:429–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin JH, Takano T, Cotrina ML, Arcuino G,
Kang J, Liu S, Gao Q, Jiang L, Li F, Lichtenberg-Frate H, et al:
Connexin 43 enhances the adhesivity and mediates the invasion of
malignant glioma cells. J Neurosci. 22:4302–4311. 2002.PubMed/NCBI
|
|
21
|
Ozawa H, Matsunaga T, Kamiya K, Tokumaru
Y, Fujii M, Tomita T and Ogawa K: Decreased expression of
connexin-30 and aberrant expression of connexin-26 in human head
and neck cancer. Anticancer Res. 27:2189–2195. 2007.PubMed/NCBI
|
|
22
|
Villaret DB, Wang T, Dillon D, Xu J, Sivam
D, Cheever MA and Reed SG: Identification of genes overexpressed in
head and neck squamous cell carcinoma using a combination of
complementary DNA subtraction and microarray analysis.
Laryngoscope. 110:374–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brockmeyer P, Jung K, Perske C,
Schliephake H and Hemmerlein B: Membrane connexin 43 acts as an
independent prognostic marker in oral squamous cell carcinoma. Int
J Oncol. 45:273–281. 2014.PubMed/NCBI
|
|
24
|
Xia J, Liu X, Tao X, Hong Y, Chen X, Dai
Y, Huang Y and Cheng B: Expression of gap junctional protein
connexin 43 during 4-nitroquinoline-1-oxide-induced rat tongue
carcinogenesis. J Mol Histol. 40:183–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolff KD, Follmann M and Nast A: The
diagnosis and treatment of oral cavity cancer. Dtsch Arztebl Int.
109:829–835. 2012.PubMed/NCBI
|
|
26
|
Fialka F, Gruber RM, Hitt R, Opitz L,
Brunner E, Schliephake H and Kramer FJ: CPA6, FMO2, LGI1, SIAT1 and
TNC are differentially expressed in early- and late-stage oral
squamous cell carcinoma - a pilot study. Oral Oncol. 44:941–948.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holm S: A simple sequentially rejective
multiple test procedure. Scandinavian J Stat. 6:65–70. 1979.
|
|
29
|
Zhang ZQ, Hu Y, Wang BJ, Lin ZX, Naus CC
and Nicholson BJ: Effective asymmetry in gap junctional
intercellular communication between populations of human normal
lung fibroblasts and lung carcinoma cells. Carcinogenesis.
25:473–482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Udaka N, Miyagi Y and Ito T: Connexin
expression in mouse lung tumor. Cancer Lett. 246:224–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada KA, Rogers JG, Sundset R, Steinberg
TH and Saffitz J: Up-regulation of connexin 45 in heart failure. J
Cardiovasc Electrophysiol. 14:1205–1212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xing Y, Xiao Y, Zeng F, Zhao J, Xiao C,
Xiong P and Feng W: Altered expression of connexin-43 and impaired
capacity of gap junctional intercellular communication in prostate
cancer cells. J Huazhong Univ Sci Technolog Med Sci. 27:291–294.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vogel C and Marcotte EM: Insights into the
regulation of protein abundance from proteomic and transcriptomic
analyses. Nat Rev Genet. 13:227–232. 2012.PubMed/NCBI
|
|
34
|
Budunova IV, Carbajal S and Slaga TJ: The
expression of gap junctional proteins during different stages of
mouse skin carcinogenesis. Carcinogenesis. 16:2717–2724. 1995.
View Article : Google Scholar : PubMed/NCBI
|