Introduction
Methylenetetrahydrofolate reductase (MTHFR) is a key
enzyme in the remethylation reaction, that catalyses the reduction
of methylenetetrahydrofolate to methyltetrahydrofolate, which is
the methyl donor for the conversion of homocysteine to methionine
(1). An increased plasma total
homocysteine (tHcy) is a risk marker for cardiovascular disease,
neural tube defects and other birth defects (2). There is evidence that increased serum
Hcy levels are associated with declining cognitive function and
dementia (3). Deficiency of B
vitamins, in particular folate, and/or mutations in genes coding
for enzymes or proteins involved in metabolism, are major causes of
elevated concentrations of tHcy (4–8).
There are three universally common polymorphisms in the gene for
MTHFR and two of them, 677C>T (rs1801133) and 1298A>C
(rs1801131), are generally known to affect Hcy concentration to a
varying degree (1,5,9–13).
However the third, 1793G>A (rs2274976), is less common and has
not been well studied, and thus whether it affects tHcy
concentration remains controversial (14–16).
Subjects with the TT genotype have normal tHcy if
their folate status is optimal (17). The 1298A>C polymorphism is
considered not to cause elevated tHcy concentrations, except when
present with the 677T-allele in ‘compound heterozygotes’ (18,19).
Our previous study investigated the impact of MTHFR
haplotypes on plasma tHcy concentrations in Swedish children and
adolescents, and evidence was found for a tHcy-raising effect of
the 1298C-allele and a tHcy-lowering effect of the 1793A-allele
(20). It was hypothesised that in
adults too, the haplotype-based approach in combination with data
on serum (S)-folate and S-cobalamin levels would facilitate the
elucidation of the impact of the MTHFR 1298A>C and
1793G>A polymorphisms on tHcy levels. The present study reports
the findings in a representative epidemiological sample of healthy
Spanish adult subjects from the Canary Islands.
Materials and methods
Subjects
The blood samples for DNA analysis were obtained
from 723 subjects (395 females and 328 males) belonging to the
Canary Islands Nutrition Study (ENCA). Serum samples were obtained
from 523 subjects (297 females and 226 males). ENCA is a
cross-sectional study from the Canary Islands (Spain) which was
conducted to survey the nutritional status and selected metabolic
and genetic variables of the population of the Canary Islands. The
sampling procedures and participation rates have been described
previously (21,22). The present study was approved by
the Research Ethics Committee of the Hospital Universitario Insular
of Gran Canaria (Las Palmas, Canary Islands, Spain). Written
informed consent was obtained from the participants.
Homocysteine and B vitamin analyses
As described previously (21), the blood samples were obtained in
the morning after subjects had fasted for 12 h. The S-folate levels
were measured at the Haematology Unit of the Hospital Universitario
Insular of Gran Canaria (Canary Islands, Spain) through an
automated ionic capturing method with Abbott AXSYM equipment
(Abbot, Berkshire, England, UK). Cobalamin was analysed via the
micro-particle enzyme immune assay method with Abbott AXSYM
equipment also at the Haematology Unit. Homocysteine was analysed
at the University of Barcelona’s Clinical Hospital (Barcelona,
Spain), with polarized fluorescence immunoassay in an AXSYM (Abbot)
analyser. The vitamin and tHcy values were available for 523
subjects.
DNA extraction and genotyping
Total blood DNA was extracted and purified from 200
μl whole blood anticoagulated with EDTA, using the QIAamp DNA Blood
Mini kit according to the manufacturer’s instructions (Qiagen Inc.,
Valencia, CA, USA). The purity was assessed by the ratio of
A280/A260, which was typically 1.7–1.8. All
of the polymerase chain reaction amplifications were performed with
HotStar Taq DNA polymerase kit (Qiagen Inc., Valencia, CA, USA) and
an Eppendorf Mastercycler. The reaction volume was 50 μl for all
polymorphisms. MTHFR 677C>T was amplified according to
the Pyrosequencing® Assay Protocol ‘Genotyping of the
C677T variant in the human methylenetetrahydrofolate reductase
(MTHFR) gene’, version 1 (Biotage AB, Uppsala, Sweden;
www.biotage.com). Approximately 30 ng of genomic DNA
was used as a template. For the MTHFR 1298A>C and
1793G>A polymorphisms, our own genotyping procedures were used,
using the Pyrosequencing platform as described previously (23).
Statistics
For examining of the Hardy-Weinberg equilibrium, a
χ2 test was applied. Plasma tHcy, S-folate and
S-cobalamin concentrations required transformation in order to
achieve normal distribution. Following ln transformation, the
residuals demonstrated a satisfactory pattern and ln values were
used in all statistical analyses. In all tables and figures,
untransformed data are provided.
Analysis of covariance (ANCOVA) was used to examine
differences in tHcy between the age groups, gender, ln S-folate, ln
S-cobalamin and the MTHFR genotypes and haplotypes. Gender
had a significant effect on tHcy concentrations and therefore the
subjects were stratified by gender. Age group, ln S-folate and ln
S-cobalamin all had a significant effect on the tHcy levels and
therefore all ANCOVA calculations adjusted for age in four groups
(18–25, 25–45, 45–55 and 65–75 years), ln S-folate and ln
S-cobalamin. It was previously found in this cohort that alcohol
intake, smoking and BMI did not predict homocysteine concentrations
(21), therefore these variables
were not included in the present study.
When analysing one genotype’s effect on tHcy,
adjustments were made for the other two MTHFR genotypes,
either by including them in the ANCOVA’s or by stratification, as
indicated. Since S-folate and S-cobalamin levels did not differ
between males and females, cut-offs for quintiles were generated
using all of the subjects. Logistic regression was performed for
analysis of the effect of MTHFR 677TT genotype on
S-cobalamin levels. S-cobalamin was stratified into two groups
below and above 150 pmol/l, which has been previously suggested as
a cut-off level for deficiency (24). All of the mean values are estimated
marginal means. To examine for a linear trend in ln tHcy
concentrations between quintiles of S-folate or S-cobalamin in the
subgroups MTHFR 677 CC+CT or MTHFR 677TT, one way
ANOVA was performed. Statistical significance was interpreted as
values of P<0.05 and confidence intervals at 95%. Statistical
analyses were performed using SPSS 15.0 for Windows (SPSS, Inc.,
Chicago, IL, USA).
Results
MTHFR genotypes and haplotypes
Basic clinical characteristics of the studied
population have been published previously (21). Briefly, noted characteristics of
the population were as follows: there was a significant difference
in median tHcy between males and females (13.1 and 10.9 μmol/l,
respectively) and also in median folate intake between males and
females (161.6 and 141.9 μg/day, respectively); there were no
significant differences for cobalamin intake, S-folate, erythrocyte
folate or S-cobalamin. The genotype prevalences and allele
frequencies for all three studied MTHFR polymorphisms in the
723 ENCA subjects are revealed in Table I. All loci were in Hardy-Weinberg
equilibrium when analysing the entire population.
 | Table IGenotype prevalences and allele
frequencies of the three studied MTHFR polymorphisms in 723
subjects in the Canary Islands Nutrition study from the Canary
Islands (Spain). |
Table I
Genotype prevalences and allele
frequencies of the three studied MTHFR polymorphisms in 723
subjects in the Canary Islands Nutrition study from the Canary
Islands (Spain).
| Polymorphism | Genotype | Frequency | χ2 |
|---|
| 677C>T | C/C | 291 (40.2) | 1.203 |
| C/T | 346 (47.9) | |
| T/T | 86 (11.9) | |
| p (C) | 928 (0.642) | |
| q (T) | 518 (0.358) | |
| 1298A>C | A/A | 384 (53.1) | 0.026 |
| A/C | 287 (39.7) | |
| C/C | 52 (7.2) | |
| p (A) | 1055 (0.730) | |
| q (C) | 391 (0.270) | |
| 1793G>A | G/G | 687 (95.0) | 0.471 |
| G/A | 36 (5.0) | |
| A/A | − (−) | |
| p (G) | 1410 (0.975) | |
| q (A) | 36 (0.025) | |
The frequency of the MTHFR 677T minor allele
was q=0.357 and 0.360, respectively, in subjects below and above 40
years of age (χ2=0.01). In females aged below and above
40 years of age, the frequency of the MTHFR 677T-allele was
q=0.377 and 0.356, respectively (χ2=0.76), and in males
below and above 40 years of age the frequency was q=0.333 and
0.364, respectively (χ2=1.13).
tHcy and MTHFR genotypes or
haplotypes
A one-way ANCOVA was performed with MTHFR
677C>T as the fixed factor and gender, age in the four groups,
ln S-folate, ln S-cobalamin, and genotypes of MTHFR
1298A>C and 1793G>A as covariates. Adjusted R2 for
the model was 0.333. The MTHFR 677C>T polymorphism
demonstrated a statistically significant interaction with gender
(P=0.02) so in the following studies the results were therefore
stratified according to gender. Adjusted for covariates, the
MTHFR 677C>T polymorphism had a significant effect on
tHcy in males (P=0.005) and adjusted R2 for the model
was 0.342. Adjusted for covariates, the MTHFR 677C>T had
no significant effect in females.
To isolate the single effect of the MTHFR
1298A>C polymorphism on tHcy, a one-way ANCOVA was performed
separately in the genotype groups 677CC and 677CT, with 1298A>C
as a fixed factor and age in the four groups, ln S-folate, and ln
S-cobalamin as covariates (Table
II). The 1298A>C polymorphism had a significant effect on
tHcy in males with the 677CT genotype, with a mean tHcy of 1.7
μmol/l higher than the 1298AA wildtype subjects. In males with the
677CC genotype, 1298A>C had no significant effect on tHcy
concentrations. In females, there was no significant effect of
MTHFR 1298A>C on tHcy concentrations.
 | Table IItHcy concentrations according to
MTHFR 1298A>C genotype in subjects with the MTHFR
677 CC or CT genotype. |
Table II
tHcy concentrations according to
MTHFR 1298A>C genotype in subjects with the MTHFR
677 CC or CT genotype.
| Subjects | Genotype | No. | Mean (95% CI) | P-value |
|---|
| Females
(n=250) |
| 677 CC | 1298 AA | 35 | 12.2
(11.3–13.2) | 0.187 |
| 1298 AC | 51 | 11.2
(10.4–11.9) | |
| 1298 CC | 15 | 11.1
(9.6–12.5) | |
| 677 CT | 1298 AA | 93 | 11.7
(11.1–12.4) | 0.702 |
| 1298 AC | 56 | 11.1
(10.8–12.4) | |
| Males (n=183) |
| 677 CC | 1298 AA | 35 | 13.2
(12.2–14.2) | 0.423 |
| 1298 AC | 41 | 12.9
(12.0–13.8) | |
| 1298 CC | 9 | 14.8
(12.8–16.7) | |
| 677 CT | 1298 AA | 59 | 13.6
(12.5–14.6) | 0.025 |
| 1298 AC | 39 | 15.3
(14.1–16.6) | |
To investigate the effect of the MTHFR
1793G>A polymorphism on tHcy concentrations, one-way ANCOVA’s
were performed with the diplotypes CCG/CCG and CCG/CCA as fixed
factors and age in the four groups, ln S-folate and ln S-cobalamin
as covariates. There was no significant effect on tHcy of the
MTHFR 1793G>A genotype in neither females nor males, but
the sample number of subjects was small. When the study population
was stratified in younger vs. older subjects (below and above 52
years of age according to the mean age of menopause) and
MTHFR 677C>T genotype (CC+CT vs. TT) ANCOVA (with
MTHFR 1298A>C, ln S-folate and ln S-cobalamin as
cofactors) demonstrated a significant Hcy-lowering effect of the
MTHFR 1793 GA genotype in males <52 years, with a mean of
2.4 μmol/l lower than the males with the MTHFR 1793 GG
genotype (Table III).
 | Table IIItHcy concentrations according to
MTHFR 1793G>A genotype in males and females below and
above 52 years old. Stratified according to MTHFR 677C>T
genotype. |
Table III
tHcy concentrations according to
MTHFR 1793G>A genotype in males and females below and
above 52 years old. Stratified according to MTHFR 677C>T
genotype.
| Sex | Age (years | MTHFR 677
genotype | MTHFR
1793G>A | Mean (95% CI) | P-value |
|---|
| Males | <52 | CC+CT | GG | 12.7
(12.1–13.2) | 0.042 |
| | | GA | 10.2
(8.0–12.5) | |
| | TT | GG | 21.7
(14.9–28.5) | |
| Males | >52 | CC+CT | GG | 15.5
(14.6–16.5) | 0.742 |
| | | GA | 16.2
(12.3–20.1) | |
| | TT | GG | 15.5
(11.1–19.9) | |
| Females | <52 | CC+CT | GG | 10.9
(10.5–11.4) | 0.920 |
| | | GA | 11.0
(9.2–12.9) | |
| | TT | GG | 11.6
(9.9–13.3) | |
| Females | >52 | CC+CT | GG | 13.0
(12.3–13.7) | 0.447 |
| | | GA | 12.0
(9.3–14.6) | |
| | TT | GG | 12.3
(10.3–14.3) | |
To investigate the effect of the MTHFR
haplotypes on tHcy, ANCOVA was performed with ln tHcy as a
dependent factor, haplotype was entered as fixed factor, and age in
the four groups, ln S-folate and ln S-cobalamin were entered as
covariates. With the two-locus haplotypes 677T-1298A and 677C-1298C
as fixed factors, an R2 of 0.352 was obtained in males
(P=0.013) for the TA haplotype whereas in females, no haplotype was
significantly correlated with tHcy. In a similar ANCOVA utilizing
the three-locus haplotypes, 677T-1298A-1793G and 677C-1298C-1793G
as fixed factors, no additional power over that of the MTHFR
677C>T genotype was obtained. The haplotype containing the
mutated 1793A-allele was excluded from the analysis due to its low
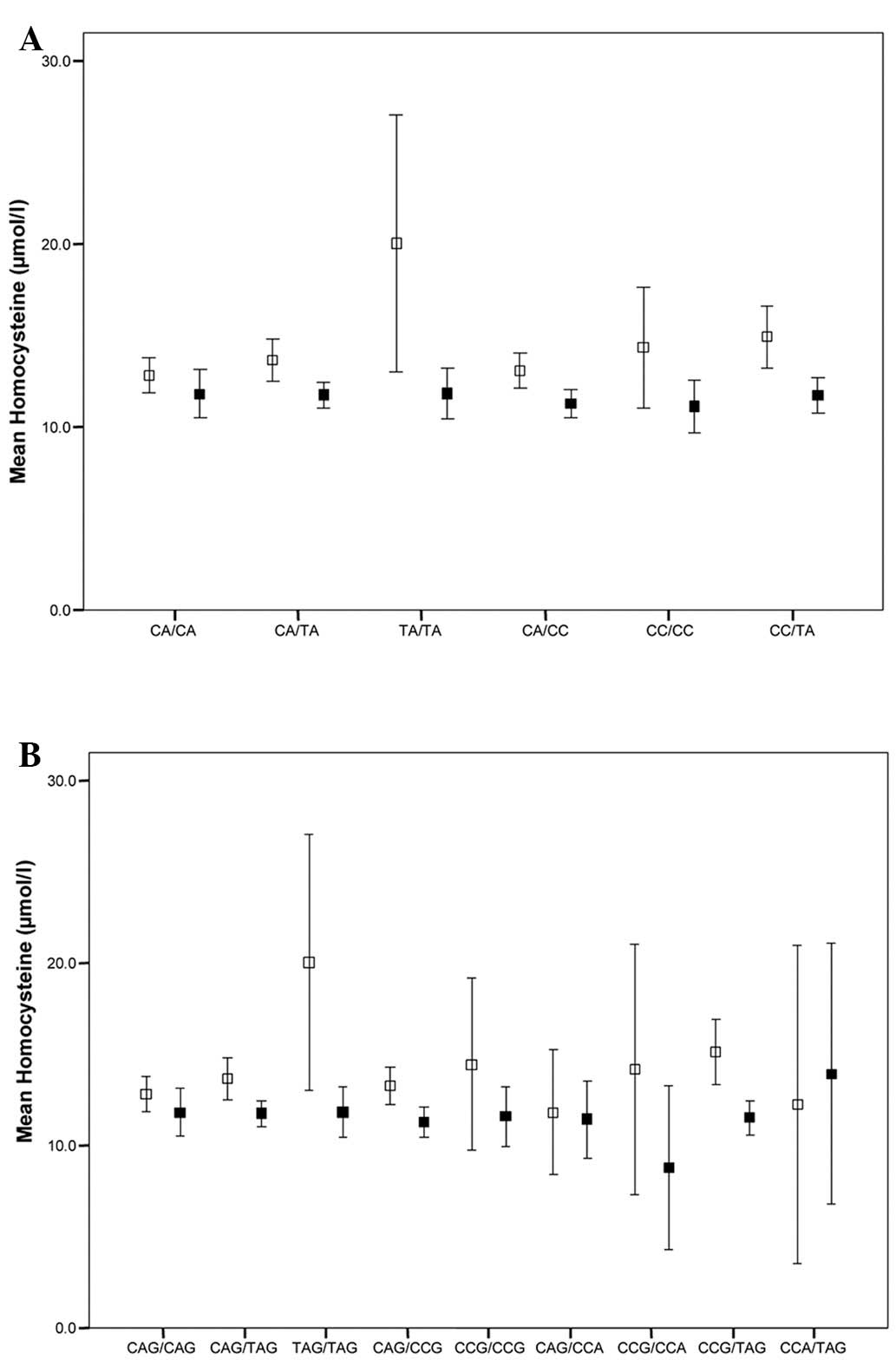
prevalence. Fig. 1 summarizes the
mean tHcy concentrations in all the different diplotype
subgroups.
tHcy and nutrigenetic interactions
S-cobalamin was divided into groups of above and
below 150 pmol/l and logistic regression was performed to elucidate
the effect of MTHFR 677TT on S-cobalamin. It was revealed
that the MTHFR 677TT genotype was not statistically
significantly associated with S-cobalamin.
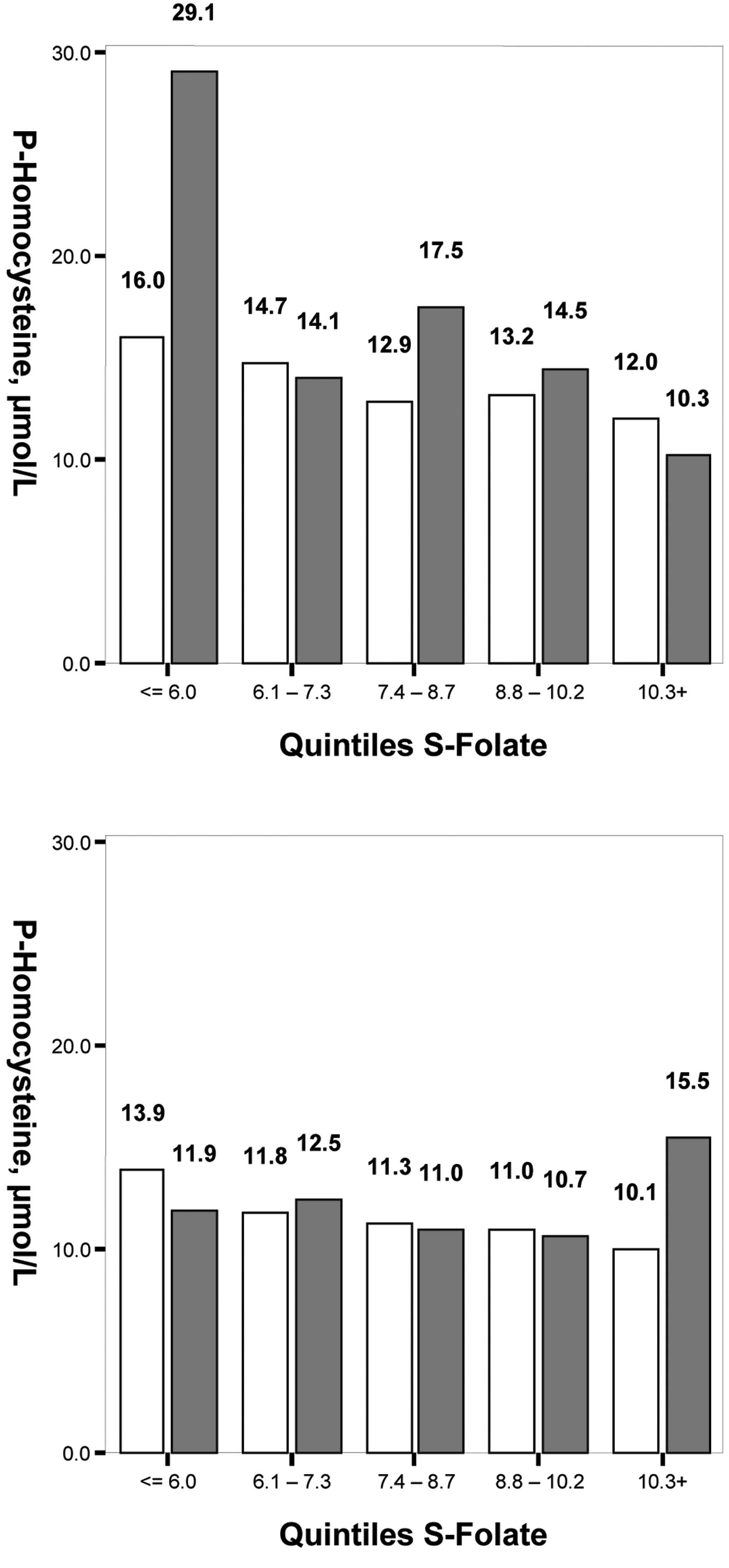
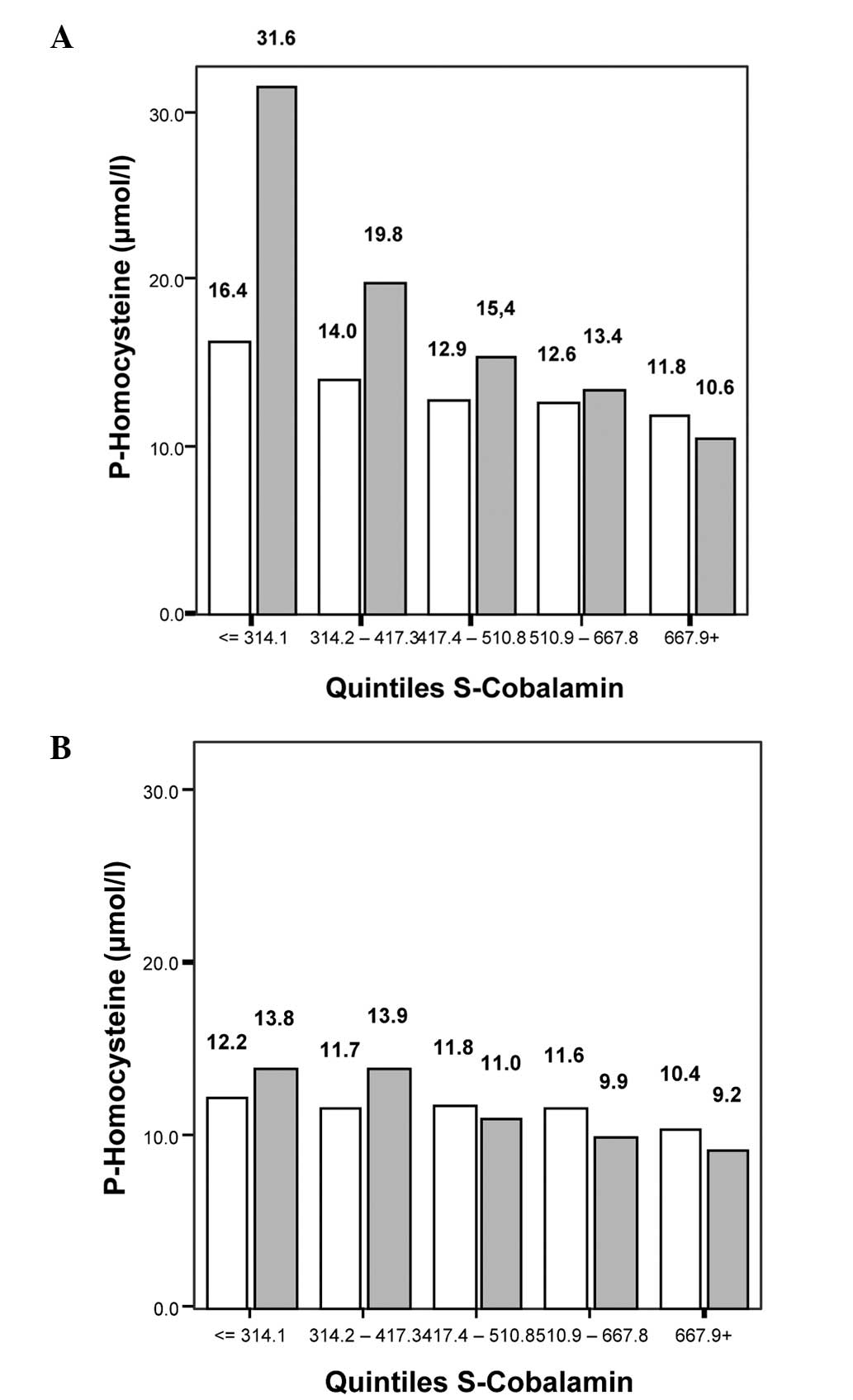
Fig. 2 reveals that
the mean tHcy levels increased with lower quintiles of S-folate,
statistically significantly in the MTHFR 677 CC+CT subgroups
(P<0.001 for both genders). The mean difference between
Q1 and Q5 in males was +4.0 μmol/l in the
CC+CT subgroup and +18.8 μmol/l in the TT subgroup, and in females
it was +3.8 μmol/l in the CC+CT subgroup and -3.6 μmol/l for the TT
group.
Fig. 3 demonstrates
that the mean tHcy levels also increased with lower quintiles of
S-cobalamin. In the MTHFR 677 CC+CT subgroup, P<0.001 for
males and P=0.005 for females; in the MTHFR 677TT subgroup,
P=0.005 for males and P=0.015 for females. The mean tHcy difference
in males between the cobalamin quintiles Q1 and
Q5 was +4.6 μmol/l in the CC+CT subgroup and +21 μmol/l
in the TT subgroup, and in females it was +1.8 μmol/l in the CC+CT
subgroup and +4.6 μmol/l in the TT subgroup.
tHcy in pre- and postmenopausal
females
The female subjects were divided into two groups,
below and above 52 years of age and ANCOVA was performed (Table IV). There was a significant
difference in tHcy concentrations between the two age groups
(P<0.001). None of the polymorphisms MTHFR 677C>T,
1298A>C and 1793G>A were significantly correlated with tHcy
in the two groups (data not shown).
 | Table IVtHcy concentrations in females and
males below and above 52 years old. |
Table IV
tHcy concentrations in females and
males below and above 52 years old.
| Age (years) | no. | Mean (95% CI) | P-value |
|---|
| Females |
| <52 | 194 | 10.8
(10.4–11.2) | <0.001 |
| >52 | 103 | 13.2
(12.6–13.8) | |
| Males |
| <52 | 140 | 14.4
(13.2–15.6) | 0.006 |
| >52 | 86 | 14.9
(13.3–16.4) | |
Discussion
The present study aimed to clarify the nutrigenetic
impact on tHcy concentrations of the MTHFR genotypes and
haplotypes adjusted for the known covariates age, sex and serum
concentration of folate and cobalamin in the Spanish adult
population. Within this population, tHcy levels are known to be
dependent on both these vitamins (21).
Our allele figures for the MTHFR 677C>T
polymorphism are consistent with another study, which investigated
the prevalence of MTHFR 677C>T in Spain (25). A study from Majorca (26) reported different frequencies of the
q allele between younger and elderly subjects, a genetically
implausible finding not supported by the present data from the
Canary Islands, or a study comparing the MTHFR 677T-allele
frequencies between newborn and >80-year-old Swedish subjects,
which were found to be q=0.291 and 0.270, respectively (27). The present figures on q among
younger (below 40 years) vs. elderly (above 40 years) differed only
marginally and in a way that evidently invites to the assumption of
‘regression towards the mean’.
In the present study, an important finding was the
significant gender difference in the effect of the MTHFR
677C>T polymorphism on tHcy concentrations, as is consistent
with several other studies (28–30).
In Spanish females with the MTHFR 677TT genotype, the
reduced levels of 5′-methyl-tetrahydrofolate did not affect tHcy
levels if adjusted for major covariates. One possible explanation
may be estrogen. Lower levels of tHcy are observed in pregnant
females, and in premenopausal and postmenopausal females undergoing
hormone replacement therapy (31)
(Table IV). Estrogen induces the
expression of the gene for phosphatidylethanolamine
N-methyltransferase (PEMT) which catalyzes the biosynthesis of
phosphatidylcholine, a precursor for betaine. Betaine is an
alternative source of methyl groups in the remethylation process of
Hcy, and it has been proposed that premenopausal females may supply
choline from endogenous biosynthesis (32). By contrast, it has been noted that
deletion of the PEMT gene in mice reduces Hcy levels by 50%
(33), which would argue against
estrogen induction of PEMT as an explanation of the present
findings. When the effect of the 677T-allele on tHcy in Swedish
children and adolescents was studied, a significant tHcy-raising
effect of the T-allele in girls was identified, but it was of
smaller magnitude in μmol/l than in the boys of the same ages
(20). Yang et al
demonstrated that the adverse impact of the MTHFR 677TT
genotype on homocysteine concentrations was attenuated by dietary
folate intake (34), but in this
cohort females actually have lower folate intake than males
(21) which precludes this as an
explanation. Therefore, the causes of the described male/female
differences remain elusive and it may well be that alternative food
sources (for instance, choline) contributing to the methyl group
balance, unaccounted for in this as well as in the majority of
other epidemiological studies, may explain, in part, such gender
differences (33). Other studies
have also demonstrated that the effect of the MTHFR
677C>T polymorphism may vary between populations, for example,
people in Mexico City have a high prevalence of the MTHFR
677C>T polymorphism but they also have a low influence of the
polymorphism on Hcy concentrations (35).
Notably, another finding was that in the subjects
with the MTHFR 677TT genotype, there was an interaction of
tHcy with not only low S-folate, but also with low S-cobalamin
(Fig. 3) as has also been observed
in other studies (24,36–38).
The association of low S-cobalamin with increased tHcy was more
robust than that of low S-folate (Figs. 2 and 3). The subjects with the MTHFR
677TT genotype raised their tHcy levels quantitatively more with
lower cobalamin quintiles than did subjects with the 677CC or CT
genotypes, and the effect was of a greater magnitude (in μmol/l) in
males than in females.
The findings suggest that in the Canary Islands both
folate and cobalamin are major tHcy-determinants in both males and
females, and both vitamins should be included in nutrigenetic
studies on MTHFR 677C>T, often regarded as biochemically
responsive only to S-folate levels.
The MTHFR 1793A-allele was in complete
linkage disequilibrium with the 1298C-allele. Consistent with our
previous Swedish study (20), it
was identified that the 1793 A-allele has a lowering effect on tHcy
levels, however in this study it was only statistically significant
in males <52 years of age. The 1298A>C polymorphism had a
minor elevating effect on tHcy in males, who were 677CT/1298AC
compound heterozygotes.
Nutrigenetic interactions and their effect on
biomarkers are commonly overlooked. Nevertheless, even small
effects may be uncovered in well-characterized populations and, as
it appears from our studies, in younger populations and
particularly in males. Several body functions decrease with age,
e.g. glomerular filtration rate and tubular function which raise
tHcy concentrations. In addition, the older population may be
taking vitamin supplementation or drugs affecting Hcy metabolism,
for instance, Spaniards >65 years of age take a mean of three or
more different medications per day (39,40).
Haplotypes from the MTHFR polymorphisms
677C>T, 1298A>C and 1793G>A (CAG, TAG, CCG, CCA) were
constructed and their impact on tHcy was analysed. A small
additional explanatory power for tHcy concentrations was obtained
using the 677–1298 two-locus haplotype system above that which was
provided by the MTHFR 677C>T genotype alone. In our
previous study in children, the best explanatory power was obtained
by the three locus haplotype (20). It is therefore suggested that, when
investigating the association of the MTHFR polymorphisms to
tHcy in a population sample, the MTHFR haplotypes should be
examined in the calculations and not just the genotypes.
Based on the above results and the findings of our
previous study (20), the
following set of nutrigenetic statements for the Hcy metabolism are
proposed: (i) age, sex and factors linked to the ethnicity of the
studied subjects, appear to be able to override the nutrigenetic
impact of tHcy-raising MTHFR genotypes or haplotypes in
particular settings, exemplified here by Spanish adult females;
(ii) gene-nutrient interactions on plasma tHcy levels thus may or
may not exist in a certain population; (iii) the transferability of
nutrigenetic findings between different communities may therefore
be limited, and may possibly need to be re-evaluated for each
particular community according to age, sex and ethnicity.
In conclusion, the major genetic impact on tHcy
concentrations in Spanish subjects was attributable to the
MTHFR 677C>T polymorphism but in the full cohort the
effect was limited to males only. A haplotype based analysis was
marginally superior to genotype based analyses in accounting for
the MTHFR impact on tHcy. A nutrigenetic interaction with
low S-cobalamin was also demonstrated: in subjects with the lowest
S-cobalamin levels, the tHcy increase was higher among 677 TT
homozygotes than in subjects with the 677 CC or CT genotypes and
the magnitude of this effect was more pronounced in males.
Acknowledgements
This study was supported by grants from
Nyckelfonden, Örebro, Sweden and Forskningskommitteén, Örebro
County Council, Sweden. The authors are grateful to Margareta
Karlsson for technical assistance and Anita Hurtig-Wennlöf for
generous comments as the manuscript evolved. Authors thank the
Canarian Health Service for facilitating all the data from the ENCA
(Canarian Nutrition Survey).
References
|
1
|
Yamada K, Chen Z, Rozen R and Matthews RG:
Effects of common polymorphisms on the properties of recombinant
human methylenetetrahydrofolate reductase. Proc Natl Acad Sci USA.
98:14853–14858. 2001. View Article : Google Scholar
|
|
2
|
Bolander-Gouaille C and Bottiglieri T:
Homocysteine Related Vitamins and Neuropsychiatric Disorders.
Springer; Paris: 2003
|
|
3
|
Garcia A and Zanibbi K: Homocysteine and
cognitive function in elderly people. CMAJ. 171:897–904. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blom HJ: Determinants of plasma
homocysteine. Am J Clin Nutr. 67:188–189. 1998.
|
|
5
|
Frosst P, Blom HJ, Milos R, Goyette P,
Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA,
van den Heuvel LP, et al: A candidate genetic risk factor for
vascular disease: a common mutation in methylenetetrahydrofolate
reductase. Nat Genet. 10:111–113. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lievers KJ, Boers GH, Verhoef P, den
Heijer M, Kluijtmans LA, van der Put NM, Trijbels FJ and Blom HJ: A
second common variant in the methylenetetrahydrofolate reductase
(MTHFR) gene and its relationship to MTHFR enzyme activity,
homocysteine, and cardiovascular disease risk. J Mol Med (Berl).
79:522–528. 2001. View Article : Google Scholar
|
|
7
|
Mudd SH, Uhlendorf BW, Freeman JM,
Finkelstein JD and Shih VE: Homocystinuria associated with
decreased methylenetetrahydrofolate reductase activity. Biochem
Biophys Res Commun. 46:905–912. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Selhub J: Homocysteine metabolism. Annu
Rev Nutr. 19:217–246. 1999. View Article : Google Scholar
|
|
9
|
van der Put NM, Gabreëls F, Stevens EM,
Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP and Blom HJ:
A second common mutation in the methylenetetrahydrofolate reductase
gene: an additional risk factor for neural-tube defects? Am J Hum
Genet. 62:1044–1051. 1998.PubMed/NCBI
|
|
10
|
Weisberg I, Tran P, Christensen B, Sibani
S and Rozen R: A second genetic polymorphism in
methylenetetrahydrofolate reductase (MTHFR) associated with
decreased enzyme activity. Mol Genet Metab. 64:169–172. 1998.
View Article : Google Scholar
|
|
11
|
Viel A, Dall’Agnese L, Simone F,
Canzonieri V, Capozzi E, Visentin MC, Valle R and Boiocchi M: Loss
of heterozygosity at the 5,10-methylenetetrahydrofolate reductase
locus in human ovarian carcinomas. Br J Cancer. 75:1105–1110. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Bree A, Verschuren WM, Bjørke-Monsen
AL, van der Put NM, Heil SG, Trijbels FJ and Blom HJ: Effect of the
methylenetetrahydrofolate reductase 677C-->T mutation on the
relations among folate intake and plasma folate and homocysteine
concentrations in a general population sample. Am J Clin Nutr.
77:687–693. 2003.
|
|
13
|
Cortese C and Motti C: MTHFR gene
polymorphism, homocysteine and cardiovascular disease. Public
Health Nutr. 4:493–497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huemer M, Vonblon K, Födinger M, Krumpholz
R, Hubmann M, Ulmer H and Simma B: Total homocysteine, folate, and
cobalamin, and their relation to genetic polymorphisms, lifestyle
and body mass index in healthy children and adolescents. Pediatr
Res. 60:764–769. 2006. View Article : Google Scholar
|
|
15
|
Rady PL, Szucs S, Grady J, Hudnall SD,
Kellner LH, Nitowsky H, Tyring SK and Matalon RK: Genetic
polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and
methionine synthase reductase (MTRR) in ethnic populations in
Texas; a report of a novel MTHFR polymorphic site, G1793A. Am J Med
Genet. 107:162–168. 2002. View Article : Google Scholar
|
|
16
|
Wakutani Y, Kowa H, Kusumi M, Nakaso K,
Yasui K, Isoe-Wada K, Yano H, Urakami K, Takeshima T and Nakashima
K: A haplotype of the methylenetetrahydrofolate reductase gene is
protective against late-onset Alzheimer’s disease. Neurobiol Aging.
25:291–294. 2004.PubMed/NCBI
|
|
17
|
Guenther BD, Sheppard CA, Tran P, Rozen R,
Matthews RG and Ludwig ML: The structure and properties of
methylenetetrahydrofolate reductase from Escherichia coli
suggest how folate ameliorates human hyperhomocysteinemia. Nat
Struct Biol. 6:359–365. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Isotalo PA, Wells GA and Donnelly JG:
Neonatal and fetal methylenetetrahydrofolate reductase genetic
polymorphisms: an examination of C677T and A1298C mutations. Am J
Hum Genet. 67:986–990. 2000. View
Article : Google Scholar
|
|
19
|
Weisberg IS, Jacques PF, Selhub J, Bostom
AG, Chen Z, Curtis Ellison R, Eckfeldt JH and Rozen R: The
1298A-->C polymorphism in methylenetetrahydrofolate reductase
(MTHFR): in vitro expression and association with homocysteine.
Atherosclerosis. 156:409–415. 2001.
|
|
20
|
Böttiger AK, Hurtig-Wennlöf A, Sjöström M,
Yngve A and Nilsson TK: Association of total plasma homocysteine
with methylenetetrahydrofolate reductase genotypes 677C>T,
1298A>C, and 1793G>A and the corresponding haplotypes in
Swedish children and adolescents. Int J Mol Med. 19:659–665.
2007.
|
|
21
|
Henríquez P, Doreste J, Deulofeu R, Fiuza
MD and Serra-Majem L: Nutritional determinants of plasma total
homocysteine distribution in the Canary Islands. Eur J Clin Nutr.
61:111–118. 2007.PubMed/NCBI
|
|
22
|
Serra Majem L, Ribas Barba L, Armas
Navarro A, Alvarez León E and Sierra A: Equipo de investigación de
ENCA: Energy and nutrient intake and risk of inadequate intakes in
Canary Islands (1997–98). Arch Latinoam Nutr. 50(Suppl 1): 7–22.
2000.(In Spanish).
|
|
23
|
Börjel AK, Yngve A, Sjöström M and Nilsson
TK: Novel mutations in the 5′-UTR of the FOLR1 gene. Clin Chem Lab
Med. 44:161–167. 2006.
|
|
24
|
Zittan E, Preis M, Asmir I, Cassel A,
Lindenfeld N, Alroy S, Halon DA, Lewis BS, Shiran A, Schliamser JE
and Flugelman MY: High frequency of vitamin B12 deficiency in
asymptomatic individuals homozygous to MTHFR C677T mutation is
associated with endothelial dysfunction and homocysteinemia. Am J
Physiol Heart Circ Physiol. 293:H860–H865. 2007. View Article : Google Scholar
|
|
25
|
Martínez-Frías ML, Bermejo E,
Rodríguez-Pinilla E, Scala I, Andria G and Botto L: Frequency of
the mutation 677C-T of methylenetetrahydrofolate reductase gene on
a sample of 652 Spanish liveborn infants. Med Clin (Barc).
122:361–364. 2004.(In Spanish).
|
|
26
|
Martín I, Obrador A, Gibert MJ, Hernanz A,
Fuster A, Pintos C, Garcia A and Tur J: Folate status and a new
repletion cut-off value in a group of healthy Majorcan females.
Clin Nutr. 22:53–58. 2003.PubMed/NCBI
|
|
27
|
Brattström L, Zhang Y, Hurtig M, Refsum H,
Ostensson S, Fransson L, Jonés K, Landgren F, Brudin L and Ueland
PM: A common methylenetetrahydrofolate reductase gene mutation and
longevity. Atherosclerosis. 141:315–319. 1998.PubMed/NCBI
|
|
28
|
Brown KS, Kluijtmans LA, Young IS, Murray
L, McMaster D, Woodside JV, Yarnell JW, Boreham CA, McNulty H,
Strain JJ, et al: The 5,10-methylenetetrahydrofolate reductase
C677T polymorphism interacts with smoking to increase homocysteine.
Atherosclerosis. 174:315–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chango A, Potier De Courcy G, Boisson F,
Guilland JC, Barbé F, Perrin MO, Christidès JP, Rabhi K, Pfister M,
Galan P, et al: 5,10-methylenetetrahydrofolate reductase common
mutations, folate status and plasma homocysteine in healthy French
adults of the Supplemalestation en Vitamines et Mineraux
Antioxydants (SU.VIMAX) cohort. Br J Nutr. 84:891–896. 2000.
|
|
30
|
Kluijtmans LA, Young IS, Boreham CA,
Murray L, McMaster D, McNulty H, Strain JJ, McPartlin J, Scott JM
and Whitehead AS: Genetic and nutritional factors contributing to
hyperhomocysteinemia in young adults. Blood. 101:2483–2488. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dimitrova KR, DeGroot K, Myers AK and Kim
YD: Estrogen and homocysteine. Cardiovasc Res. 53:577–588. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fischer LM, da Costa KA, Galanko J, Sha W,
Stephenson B, Vick J and Zeisel SH: Choline intake and genetic
polymorphisms influence choline metabolite concentrations in human
breast milk and plasma. Am J Clin Nutr. 92:336–346. 2010.
View Article : Google Scholar
|
|
33
|
Stead LM, Brosnan JT, Brosnan ME, Vance DE
and Jacobs RL: Is it time to reevaluate methyl balance in humans?
Am J Clin Nutr. 83:5–10. 2006.PubMed/NCBI
|
|
34
|
Yang QH, Botto LD, Gallagher M, Friedman
JM, Sanders CL, Koontz D, Nikolova S, Erickson JD and Steinberg K:
Prevalence and effects of gene-gene and gene-nutrient interactions
on serum folate and serum total homocysteine concentrations in the
United States: findings from the third National Health and
Nutrition Examination Survey DNA Bank. Am J Clin Nutr. 88:232–246.
2008.
|
|
35
|
Guéant-Rodriguez RM, Guéant JL, Debard R,
Thirion S, Hong LX, Bronowicki JP, Namour F, Chabi NW, Sanni A,
Anello G, et al: Prevalence of methylenetetrahydrofolate reductase
677T and 1298C alleles and folate status: a comparative study in
Mexican, West African, and European populations. Am J Clin Nutr.
83:701–707. 2006.PubMed/NCBI
|
|
36
|
D’Angelo A, Coppola A, Madonna P, Fermo I,
Pagano A, Mazzola G, Galli L and Cerbone AM: The role of vitamin
B12 in fasting hyperhomocysteinemia and its interaction with the
homozygous C677T mutation of the methylenetetrahydrofolate
reductase (MTHFR) gene. A case-control study of patients with
early-onset thrombotic events. Thromb Haemost. 83:563–570.
2000.
|
|
37
|
Hustad S, Midttun Ø, Schneede J, Vollset
SE, Grotmol T and Ueland PM: The methylenetetrahydrofolate
reductase 677C-->T polymorphism as a modulator of a B vitamin
network with major effects on homocysteine metabolism. Am J Hum
Genet. 80:846–855. 2007.
|
|
38
|
Sensoy N, Şoysal Y, Kahraman A, Doǧan N
and Imirzalioǧlu N: Modulator effects of the
methylenetetrahydrofolate reductase C677T polymorphism on response
to vitamin B12 therapy and homocysteine metabolism. DNA Cell Biol.
31:820–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arjona Mateos CR, Criado Velasco J and
Sánches Solís L: Enfermedades crónicas y consumo de fármacos en
mayores de 65 años. Medicina General. 47:684–695. 2002.(In
Spanish).
|
|
40
|
Gonzalez-Gross M, Sola R, Albers U,
Barrios L, Alder M, Castillo MJ and Pietrzik K: B-vitamins and
homocysteine in Spanish institutionalized elderly. Int J Vitam Nutr
Res. 77:22–33. 2007. View Article : Google Scholar : PubMed/NCBI
|