Introduction
Prostate cancer (PCa) is one of the most common
types of non-cutaneous malignant tumors in older males. According
to the World Health Organization, ~899,000 new cases of PCa are
diagnosed each year, and the worldwide mortality is ~258,000 per
year (1). In China, the incidence
of PCa and its associated mortality have significantly increased in
recent years, largely as a result of longer lifespans and altered
dietary habits. The androgen receptor is important in the
initiation and progression of PCa. While tumors are often initially
sensitive to androgen deprivation therapy (ADT), a significant
proportion rapidly develop resistance and become
androgen-independent (2,3). Thus, numerous studies have focused on
identifying novel therapeutic targets in androgen-independent
prostate cancer.
Glucose-regulated protein 78 (GRP78) is a heat shock
protein, which is also termed binding immunoglobulin heavy chain
protein (BiP). It is a multifunctional Ca2+-binding
protein that participates in protein folding, transportation and
degradation. It has also been shown to be involved in cancer cell
survival (4,5). Previous studies in ovarian cancer,
breast cancer and lung cancer have shown that high levels of GRP78
expression in tumors correlate with reduced cell differentiation
and a poorer prognosis (6–8). Other studies have demonstrated that
silencing GRP78 using RNA interference, induces apoptosis and
inhibits migration in hepatoma cells (9,10).
These findings suggest that GRP78/BiP may be a potential
therapeutic target in malignant tumors. However, little is
currently known regarding the importance of GRP78 in the
development of PCa.
RNA interference (RNAi) is an effective method by
which to silence genes of interest. As a result, it is an
indispensable tool in experimental and clinical research. Certain
small interfering RNAs (siRNAs) have demonstrated potential
therapeutic value in multiple types of tumor, including colon,
renal, liver and bladder cancers (11–14).
Conventional RNAi methods rely on siRNAs consisting of 19–21 bp
that form symmetrical duplexes. However, a number of unintended
nonspecific effects triggered by siRNA structures have been
demonstrated (15,16). Recent studies have indicated that
gene silencing mediated by asymmetrical siRNAs (asiRNAs), which are
siRNA duplexes with shortened sense strands, is more effective and
shows greater specificity than when conventional symmetric siRNA is
used (17–19).
In the present study, a series of GRP78-specific
asiRNAs ranging from 14–17 bp in duplex region length were designed
and their activity in the human androgen-independent prostate
cancer cell line PC-3 was investigated. The aim was to evaluate the
effect of these molecules on the expression of GRP78, and the
ensuing effects of this on the apoptosis and migration of PC-3
cells.
Materials and methods
Cell culture
PC-3 and DU145 human prostate cancer cells (China
Center for Type Culture Collection, Wuhan, China) were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; Gibco Life Technologies,
Carlsbad, CA, USA). Non-malignant BPH1 human prostate cells (China
Center for Type Culture Collection) were cultured in RPMI-1640
medium (Gibco Life Technologies) supplemented with 10% fetal bovine
serum (FBS; Gibco Life Technologies), 100 mg/ml streptomycin and
100 U/ml penicillin (Goodbio Technology Co., Ltd., Wuhan, China).
The cells were maintained in a cell incubator (Forma Scientific,
Inc., Cincinnati, OH, USA) at 37°C under a humidified 5%
CO2 atmosphere for 48 h.
Design and synthesis of siRNA and
asiRNAs
The 19 nucleotide (nt) target sequence for GRP78 was
selected from GenBank (accession no. NM_005347.4; nt sequence,
1293-1311). This sequence has previously been shown to downregulate
GRP78 expression (10). The
symmetrical siRNA (19bp duplex region), designed according to the
target sequence, was designated as siGRP78. A series of asiRNAs
with 5′ sense strands shortened by 2 nt to 5 nt were designed and
designated asiGRP78-1, asiGRP78-2, asiGRP78-3 and asiGRP78-4
(Fig. 2A). These asiRNAs comprised
a duplex region ranging from 14 to 17bp and their antisense
sequence was identical to that of the siRNA. All siRNAs and asiRNAs
were synthesized and purified by Invitrogen Life Technologies
(Carlsbad, CA, USA). Two negative control siRNAs [with and without
a carboxyfluorescein (FAM) label] were also synthesized by
Invitrogen Life Technologies. The FAM-labeled siRNA was used to
measure transfection efficiency, and the non-FAM-labeled siRNA was
used for all other experiments.
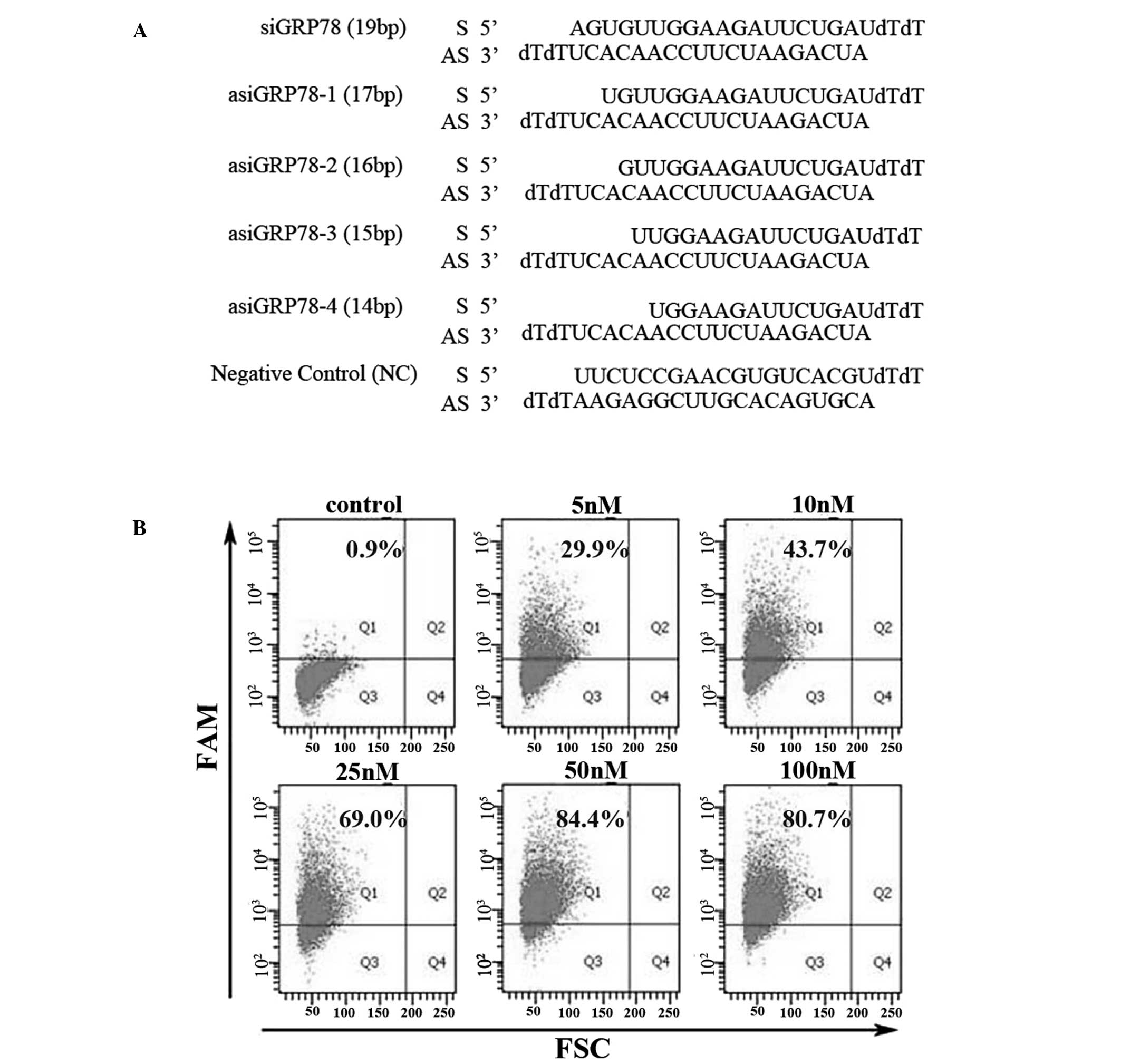 | Figure 2Design of GRP78-specific asiRNAs and
assessment of transfection efficiency. (A) Sequences of
GRP78-specific siRNA (siGRP78), GRP78-specific asiRNAs (asiGRP78-1,
asiGRP78-2, asiGRP78-3 and asiGRP78-4) and the negative control
siRNA. (B) PC-3 cells were transfected for 6 h with varying
concentrations (0, 5, 10, 25, 50 and 100 nM) of FAM-labeled siRNA.
Transfection efficiencies (Q1 region) were detected by flow
cytometry. siRNA, small interfering RNA; asiRNA, asymmetric siRNA;
GRP78, glucose-regulated protein 78; FAM, carboxyfluorescein; FSC,
forward scatter. |
Cell transfection and transfection
efficiency assay
PC-3 cell transfection was performed using the
INTERFERin® in vitro siRNA transfection reagent
(Polyplus-Transfection SA, Brant, France) according to the
manufacturer’s instructions for reverse transfection. Briefly,
siRNA or asiRNA were diluted in 200 μl of Opti-MEM (Gibco Life
Technologies, Carlsbad, CA, USA), and 12 μl of INTERFERin
transfection reagent was mixed with the diluted siRNA/asiRNA. This
mixture was incubated for 10 min at room temperature. It was then
transferred into 6-well culture plates and mixed with 1.95 ml of
antibiotic-free serum-free DMEM. PC-3 cells were immediately seeded
onto the plates. The transfected cells were maintained in an
incubator at 37°C. FBS (245 μl per well) was added into the medium
at 10% final concentration, 8 h following transfection. To compare
the efficiency of siRNA- and asiRNA-mediated silencing of GRP78,
all transfections were maintained for 48 h.
To assess transfection efficiency, cells transfected
with FAM-labeled siRNA were collected at 6 h post-transfection,
rinsed three times with phosphate-buffered saline (PBS; Goodbio
Technology Co., Ltd.) and resuspended in PBS. The cell suspensions
were analyzed by flow cytometry (BD Biosciences, Franklin Lakes,
NJ, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) and quantitative PCR (qPCR)
Total RNA was isolated using an AxyPrep total RNA
Miniprep Kit (Axygen Scientific, Inc., Union City, CA, USA) and was
reverse transcribed into cDNA using the ReverTra Ace®
First Strand cDNA Synthesis kit (Toyobo Co., Ltd., Osaka, Japan)
according to the Manufacturer’s instructions. The first strand cDNA
samples were amplified by RT-PCR using Taq PCR Mastermix (Tiangen
Biotech Co., Ltd., Beijing, China) or by qPCR using Thunderbird™
SYBR® qPCR Mix (Toyobo Co., Ltd.). All primer sequences
and the sizes of the PCR products are listed in Table I. For RT-PCR, the products were
electrophoresed on 1.5% agarose gels (Goodbio Technology Co.,
Ltd.), stained with Goldview (Goodbio Technology Co., Ltd.), and
visualized under ultraviolet light. β-Actin was used as a loading
control. For qPCR, the relative GRP78 mRNA expression levels were
calculated relative to β-actin according to the 2−ΔΔCt
method.
 | Table IPrimers for RT-PCR and qPCR. |
Table I
Primers for RT-PCR and qPCR.
| Primer name | Sequence
(5′-3′) | Product size
(bp) |
|---|
GRP78
(RT-PCR) | F:
GTTCTTCAATGGCAAGGAACCATC
R: CCATCCTTTCGATTTCTTCAGGTG | 494 |
GRP78
(qPCR) | F:
GGAGGACAAGAAGGAGGACG
R: CAGGAGTGAAGGCGACATAGG | 152 |
β-actin
(RT-PCR and qPCR) | F:
GCAAAGACCTGTACGCCAAC
R: GTACTTGCGCTCAGGAGGAG | 143 |
Western blot analysis
Cells were harvested, rinsed and lysed with lysis
buffer supplemented with phenylmethylsulfonylfluoride (Beyotime
Institute of Biotechnology, Shanghai, China). Cell lysates were
kept on ice for 30 min and centrifuged at 12,000 × g for 15 min to
obtain lysate proteins. The protein concentration was measured
using a bicinchoninic acid protein assay kit (Goodbio Technology
Co., Ltd.,) and equal amounts of protein were resolved on 12%
SDS-PAGE gels prior to transfer to a polyvinylidene difluoride
membrane. Samples were blocked in 5% skimmed milk and the membranes
were incubated with primary antibody overnight at 4°C. The
following primary antibodies were used: Anti-GRP78/Bip rabbit
polyclonal antibody (1:1,000; catalog number, 11587-1-AP),
anti-E-cadherin rabbit polyclonal antibody (1:1,000, catalog
number, 20874-1-AP) and anti-caspase 3 rabbit polyclonal antibody
(1:1,000; catalog number, 19677-1-AP) (ProteinTech Group, Inc.,
Chicago, IL, USA); anti-vimentin rabbit polyclonal antibody (1:500;
catalog number, S0859; Epitomics, Burlingame, CA, USA); anti-AKT
rabbit monoclonal antibody (1:1,000; catalog number, 4691s) and
anti-pAKT (ser473) rabbit monoclonal antibody (1:200; catalog
number, 4058s) (Cell Signaling Technology, Inc., Danvers, MA, USA);
and anti-caspase 9 rabbit polyclonal antibody (1:500; catalog
number, sc-8355) and anti-β-actin rabbit polyclonal antibody
(1:1,500; catalog number, sc-130656) (Santa Cruz Biotechnology,
Inc., Dallas, TX. USA). Following incubation with primary
antibodies, the membranes were probed with horseradish
peroxidase-conjugated goat anti-rabbit polyclonal secondary
antibodies (1:5,000; catalog number, sc-2004; Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h, and the reactive
bands were detected using the BeyoECL plus (Beyotime Institute of
Biotechnology) reagent. Relative protein expression levels were
calculated based on the relative optical densities of the protein
bands with Quantity One 4.62 software (Bio-Rad Laboratories,
Hercules, CA, USA).
Apoptosis assay
Transfected cells were harvested at 48 h
post-transfection, rinsed twice with PBS and resuspended in 500 μl
binding buffer. The cell suspensions were immediately incubated for
15 min with 5 μl propidium iodide (PI) and 5 μl fluorescein
isothiocyanate (FITC)-labeled Annexin V (KeyGen, Nanjing, China) at
room temperature. Samples were analyzed on a flow cytometer (BD
Biosciences) within 1 h of the end of incubation.
Cell migration assay
Cell migration assays were performed using 6.5-mm
Transwell® chambers with 8.0-μm-pore polycarbonate
membranes (Corning Life Sciences, Tewksbury, MA, USA) in 24-well
culture plates. To perform serum starvation, complete medium in a
six-well plate was removed and replaced with serum-free DMEM 24 h
following transfection. The serum-starved cells were harvested 12 h
later and resuspended in DMEM with 0.1% bovine serum albumin
(Goodbio Technology Co., Ltd.). Equal numbers of cells were seeded
in the upper chamber. DMEM with 10% FBS was added to the lower
chamber. Following 12 h of incubation, the cells in the upper
chamber were removed and the cells on the lower surface of the
Transwell® membrane were stained with crystal violet
(Goodbio Technology Co., Ltd.) and photographed by an Olympus BX51
microscope (Olympus Corporation, Tokyo, Japan). To evaluate the
inhibitory rate of migration, crystal violet-stained cells from
each group were dissolved in 100 μl 10% ethylic acid. The optical
density (OD) of the solution was measured at 570 nm using a
microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The
inhibitory rate was determined using the following equation:
Inhibition rate (%) = [1-(OD of treatment group/OD of mock group)]
× 100. The mock group was transfected in the absence of RNA.
Statistical analysis
All statistical analyses were performed using SPSS
statistical 19.0 software (IBM SPSS, Armonk, NY, USA). One-way
analysis of was used to compare the results of three or more
groups, and Student’s t-test was used to compare differences
between two groups. Data are expressed as the mean ± standard
deviation. P<0.05 were considered to indicate a statistically
significant difference.
Results
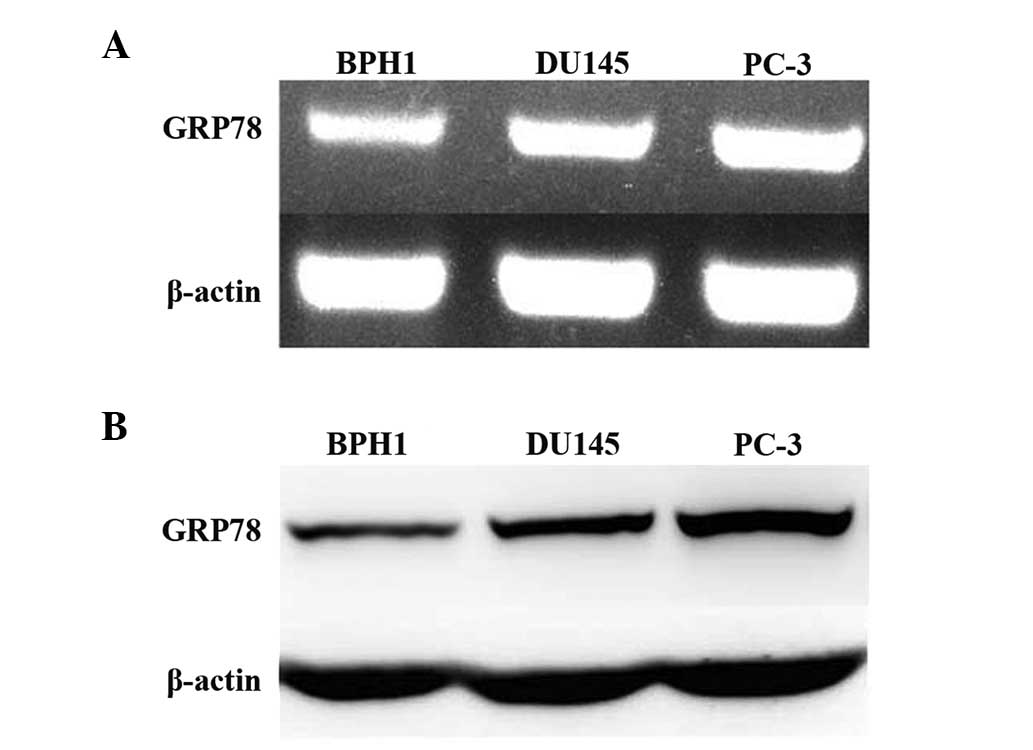
Grp78 expression is elevated in prostate
cancer cell lines
It has previously been reported that the expression
of GRP78 is upregulated in human prostate cancer tissues (20). In the current study the expression
of GRP78 in three prostate cell lines, including two cancerous
(PC-3 and DU145) cell lines and one non-malignant (BPH1) cell line
was measured. RT-PCR results showed that GRP78 mRNA expression was
higher in PC-3 and DU145 cells than it was in BPH1 cells. The
expression of GRP78 was highest in PC-3 cells (Fig. 1A). Western blot analysis showed
that GRP78 protein expression correlated with the mRNA expression
profile in all three cell lines (Fig.
1B). PC-3 cells were selected as a model of
androgen-independent prostate cancer and used for subsequent
experiments.
Optimization of transfection
efficiency
To optimize siRNA and asiRNA transfection
efficiency, PC-3 cells were transfected with FAM-labeled siRNA at
final concentrations of 0, 5, 10, 25, 50 and 100 nM for 6 h. The
transfection efficiency for each condition was assessed by flow
cytometry. At an siRNA concentration of 50 nM, the transfection
efficiency was 84.4%. Flow cytometry analysis also revealed there
was no improvement in transfection efficiency with siRNA
concentrations >50 nM (Fig.
2B). Therefore, a final siRNA (asiRNA) concentration of 50 nM
for all of the siRNA or asiRNA transfection groups was used in all
subsequent experiments.
Silencing GRP78 gene expression by siRNA
and asiRNA
We compared the efficiency of siRNA- and
asiRNA-mediated silencing of GRP78. As shown in Fig. 3A, qPCR data demonstrated that GRP78
expression was reduced in the siRNA- and asiRNA-transfected groups.
The relative levels of GRP78 mRNA were decreased by ~77% in the
15bp asiRNA group and by ~63% in the siRNA group. GRP78 expression
was decreased by ~23, 52 and 58% in cells transfected with a 14, a
16 and a 17 bp asiRNA, respectively. The gene-silencing efficiency
of the 15bp asiRNA (asiGRP78-3) was the highest among all the
groups and was significantly higher than that of the siRNA
(siGRP78) groups (P=0.024).
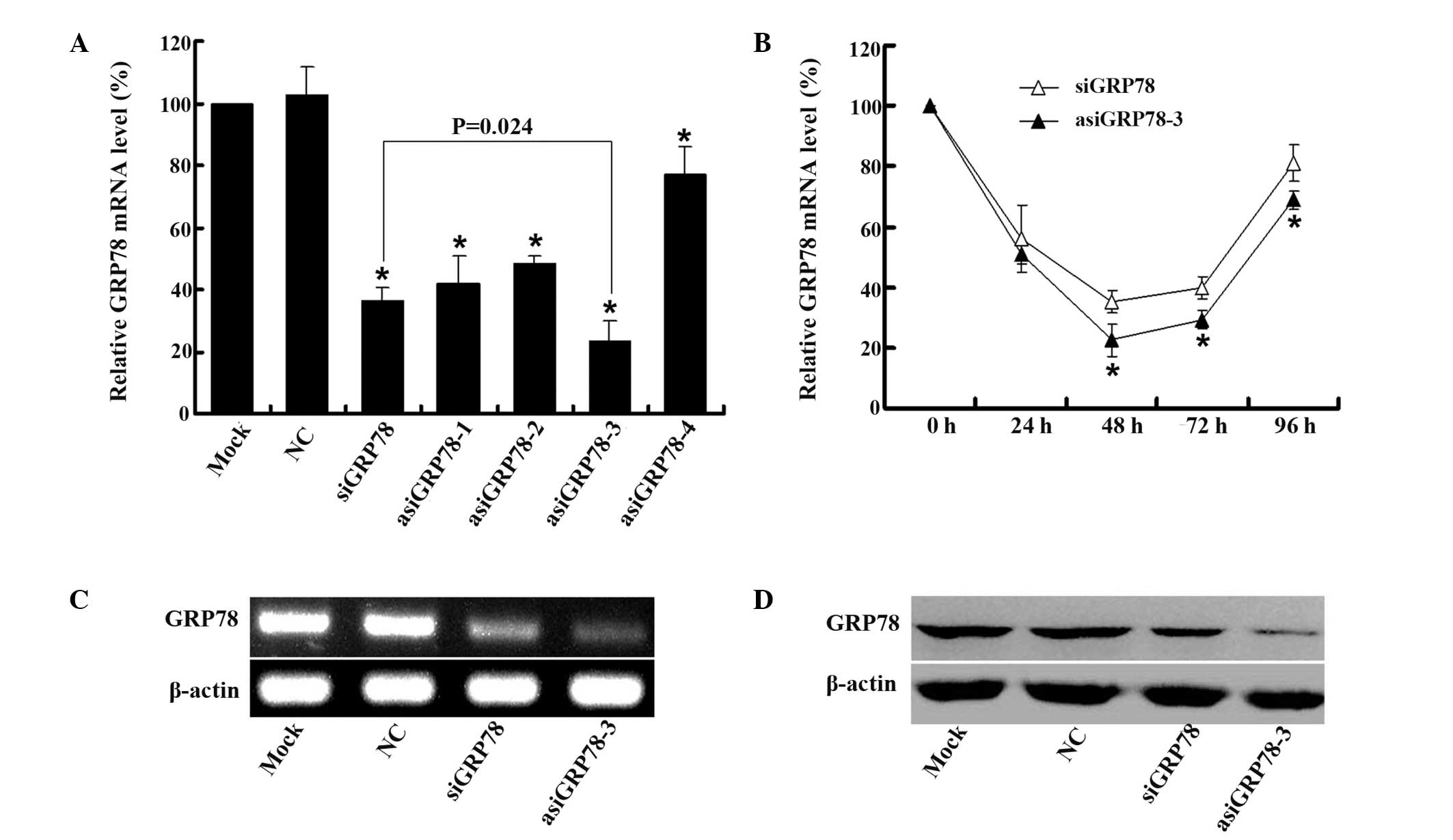 | Figure 3Effect of siRNA and asiRNAs on
silencing GRP78 expression. (A) PC-3 cells were transfected with 50
nM of siRNA or asiRNAs for 48 h. The mock group was transfected in
the absence of RNA. GRP78 mRNA expression levels relative to
β-actin were determined by qPCR. The levels were calculated as a
percentage change relative to the mock group and the data are
presented as the mean ± standard deviation of three independent
experiments. *P<0.01 compared with NC. (B) PC-3 cells
were transfected with 50 nM siGRP78 or asiGRP78-3 for 0, 24, 48, 72
and 96 h. The relative GRP78 mRNA expression levels were determined
by qPCR and were measured as the percentage change relative to the
initial values at time 0 h. Data are presented as the mean ±
standard deviation of three independent experiments.
*P<0.05 compared with siGRP78. (C) PC-3 cells were
transfected with mock or NC siRNA, siGRP78 or asiGRP78-3 for 48 h.
GRP78 and β-actin mRNA levels were measured by RT-PCR. β-actin was
used as a loading control. (D) PC-3 cells were transfected with
mock or NC siRNA, siGRP78 or asiGRP78-3 for 48 h. GRP78 and β-actin
protein levels were detected by western blot analysis. β-actin was
used as the loading control. siRNA, small interfering RNA; asiRNA,
asymmetric siRNA; GRP78, glucose-regulated protein 78; NC, negative
control; qPCR, quantitative polymerase chain reaction; RT-PCR,
reverse transcription-PCR. |
The duration of gene silencing following
siRNA/asiRNA transfection was also investigated. PC-3 cells were
transfected with 50 nM asiGRP78-3 or siGRP78. Cells were harvested
at 0, 24, 48, 72 and 96 h following transfection. The relative
GRP78 mRNA levels were determined by qPCR. As shown in Fig. 3B, GRP78 expression in the
asiGRP78-3 group was significantly lower than that in the siGRP78
group at 48, 72 and 96 h (P<0.05). Thus, the duration of gene
silencing was greater in the asiGRP78-3 group.
Transfection of asiGRP78-3 into PC-3 cells resulted
in reduced expression of GRP78 at the mRNA and protein levels
(Figs. 3C and D). For this reason,
asiGRP78-3 (15 bp asiRNA) was selected for use in subsequent
experiments examining the potential antitumor effects of GRP78
downregulation.
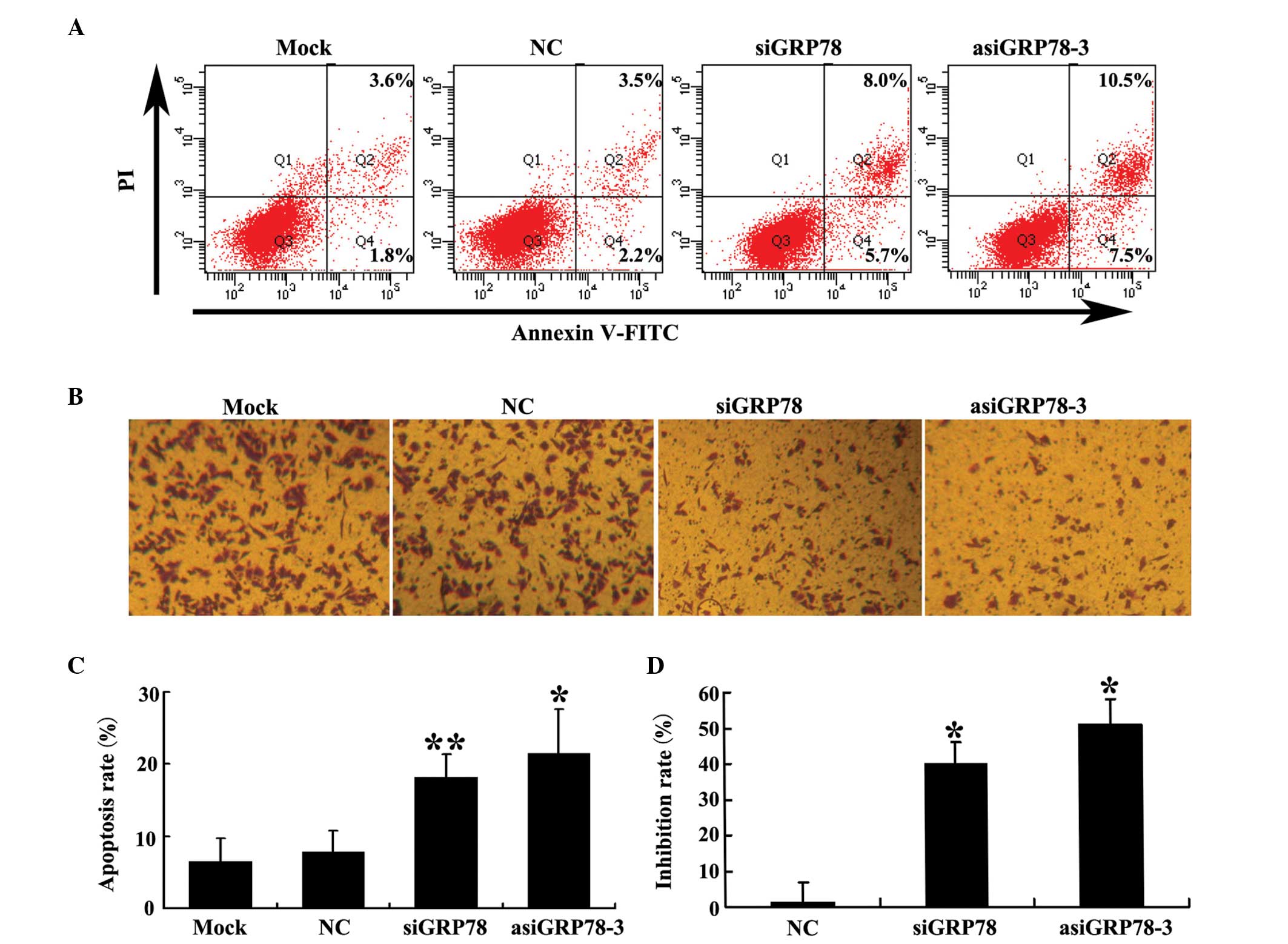
Silencing of GRP78 by asiRNA induces PC-3
cell apoptosis and inhibits PC-3 cell migration
The percentage of apoptotic transfected cells was
detected by flow cytometry using double staining for Annexin V-FITC
and PI 48 h following transfection. As shown in Fig. 4A and C, the percentage of apoptotic
cells was significantly higher in the GRP78-depleted groups than it
was in either the negative control (NC) group or the mock group.
Additionally, the percentage of cells in which apoptosis was
induced by asiGRP78-3 was higher than that induced by siGRP78.
The effect of GRP78 knockdown on PC-3 cell migration
using was investigated using Transwell® chambers.
Migration inhibition in each of the four groups were calculated as
described in the materials and methods section. As shown in
Fig. 4B and D, knockdown of GRP78
decreased the migratory capability of PC-3 cells compared with the
control group. This effect was greater in the asiGRP78-3 group than
it was in the siGRP78 group (P=0.044).
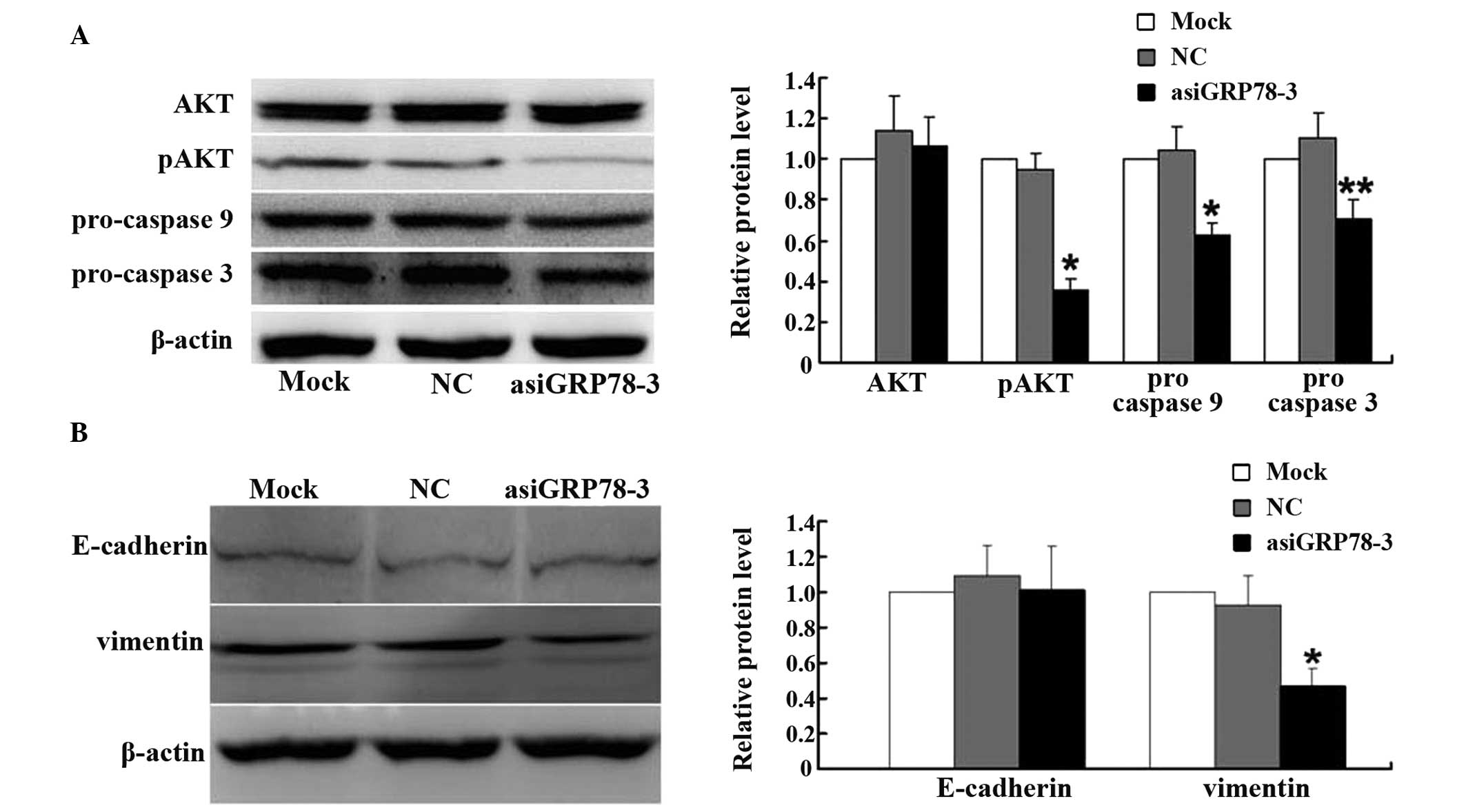
Apoptosis-related and migration-related
proteins are regulated by asiRNA
To further investigate how GRP78 may regulate
apoptosis, the expression of pro-caspase 9, pro-caspase 3, and
phosphorylated AKT (pAKT) in the GRP78-depleted groups and the
control groups were measured. At 48 h post-transfection, western
blot analysis showed reduced expression of all three of these
markers of apoptosis following GRP78 depletion (Fig. 5A).
To investigate the possible mechanisms by which
GRP78 may regulate cellular migration, the expression of E-cadherin
and vimentin by were measured western blotting. As shown in
Fig. 5B, vimentin expression was
significantly decreased in the asiGRP78-3 group compared with the
control group; however, the expression of E-cadherin was
unchanged.
Discussion
Primary treatment options for patients with prostate
cancer include surgery and ADT. In some cases, for example in
elderly patients, surgery may not be possible. In these cases, ADT
is the only available option. However, patients receiving ADT often
develop resistance to treatment after a period of time. Thus, the
identification of alternative therapies for prostate cancer is
critical.
Over the last decade, RNA interference has been
proven to be effective in the treatment of cancer. The majority of
conventional RNA interference methods use 19–25 bp symmetrical
oligonucleotides. However, Sun et al (19) first demonstrated the successful
silencing of a gene using asiRNA. They showed that asiRNA is
superior to symmetrical siRNA for the purpose of gene silencing. A
number of other studies have since confirmed this (18,21–23).
In addition to more efficient gene silencing, asiRNA typically
causes fewer unpredictable off-target effects and produces a longer
duration of knockdown compared with conventional symmetric siRNA
(18,19,21–23).
Thus, downregulation of gene expression using asiRNA has become
common practice. In the present study, silencing of GRP78 by asiRNA
and siRNA was compared. All of the asiRNAs that were tested led to
decreased levels of GRP78 at the mRNA level. asiGRP78-3 (15bp) was
particularly effective at downregulating the expression of GRP78
and was significantly more effective than the symmetric siRNA in
this regard. Furthermore, transfection of the asiRNA into PC-3
cells had a more marked effect on apoptosis and migration compared
with the conventional siRNA. Previous studies have shown that
asiRNA duplex regions range from 15–19 bp. The most appropriate
length is likely to depend on the specific sequence of the target
gene of interest and the design of the interfering RNA sequence.
Therefore, for different target genes, a series of asiRNAs with
different duplex region lengths should be designed and screened for
the one that exhibits the greatest knockdown effect (22).
GRP78 is hypothesized to promote tumor cell survival
through two mechanisms. It is important in the unfolded protein
response (UPR). Changes in the microenvironment (such as glucose
depletion or hypoxia) or the administration of anticancer drugs may
induce endoplasmic reticulum stress (ERS) leading to the
aggregation of misfolded proteins. ERS then activates the UPR in an
attempt to correct these misfolded proteins in order to relieve the
cellular stress. GRP78 is an endoplasmic reticulum chaperone that
assists in the proper folding of proteins. Cancer cells tend to
produce large amounts of variant proteins, which require high
levels of GRP78 for proper folding (24). In addition, when it is bound to
α2-macroglobulin, T-cadherin or Cripto, GRP78 activates either the
phosphoinositide 3-kinase/AKT pathway or the mitogen-activated
protein kinase pathway, which promote tumor cell proliferation
(24–26). In previous studies, GRP78 has been
linked to various stages of tumor development (6–8).
GRP78 expression was also shown to be significantly higher in
prostate cancer tissues compared with that in the normal prostate.
Notably, this elevated GRP78 expression was correlated with
significantly decreased progression-free and overall survival
(20). In the current study it was
shown that GRP78 mRNA and protein levels are higher in the prostate
cancer cell lines PC-3 and DU145 than in the prostate hyperplasia
cell line BPH1 Thus, these findings are consistent with previous
reports.
Several groups have examined the effect of GRP78
inhibition on cancer cell biology. Blocking GRP78 using monoclonal
antibodies inhibits cell proliferation and induces apoptosis in
several types of tumor cells (4,27,28).
Others have reported that downregulating GRP78 expression using
RNAi induces apoptosis in lung, liver, cervical and colorectal
cancer cells (8,10,29,30).
Similarly, the present study showed that downregulation of GRP78 in
PC-3 cells using asiRNA significantly increases apoptosis. These
results indicate that GRP78 is important in the survival of
prostate cancer cells. To further investigate the mechanisms
underlying GRP78-mediated apoptosis, the expression levels of pAKT,
pro-caspase 9, and pro-caspase 3 were examined. Following GRP78
depletion, the levels of pAKT were reduced, which is consistent
with recent reports (31–33). AKT phosphorylation inhibits caspase
9 activation by phosphorylating pro-caspase 9 (34). Thus, downregulation of pAKT
activates pro-caspase 9 and pro-caspase 3 leading to an increase in
apoptosis (35). In the present
study, decreased levels of pro-caspase 9 and pro-caspase 3 were
also detected following GRP78 knockdown. Thus, inhibition of AKT
phosphorylation and the activation of caspase 9 and caspase 3 may
be a possible mechanism by which GRP78 downregulation induces
apoptosis in PC-3 cells.
Tumor metastasis is a complex, multi-step process
that involves, at least in part, the ability of cancer cells to
migrate. The current study found that downregulation of GRP78 with
asiRNA significantly decreased the migratory capacity of PC-3 cells
(by ~50%). Excluding the effects on apoptosis of GRP78 silencing in
these cells at the same time point (apoptosis rate ~20%), it is
likely that GRP78 downregulation also inhibits PC-3 cell migration.
Such findings are consistent with previous studies performed in
liver, lung, and head and neck cancers (8,9,36).
Finally, in the present study E-cadherin and vimentin protein
levels were examined, and it was shown that vimentin levels were
decreased following GRP78 knockdown. By contrast, E-cadherin levels
did not change. Vimentin is important role in cancer cell motility
(37). Thus, it is hypothesized
that the decreased migration of PC-3 cells following GRP78
silencing may be related to vimentin downregulation, although the
underlying mechanism requires further investigation.
In conclusion, the current study showed that
GRP78-specific asiRNA effectively downregulates GRP78 expression in
PC-3 cells. It provides evidence that GRP78 downregulation
increases PC-3 cell apoptosis and decreases cell migratory ability.
The use of GRP78-specific asiRNA may therefore be a novel
therapeutic approach for prostate cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81101944 and 81173608), the
Natural Science Foundation of Hubei (no. 2011CDB202) and the Seed
Foundation of HUST (no. 2011JC038).
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
asiRNA
|
asymmetric small interfering RNA
|
|
GRP78
|
glucose-regulated protein 78
|
|
NC
|
negative control
|
|
PCa
|
prostate cancer
|
|
PI
|
propidium iodide
|
|
RNAi
|
RNA interference
|
References
|
1
|
Semenas J, Allegrucci C, Boorjian SA,
Mongan NP and Persson JL: Overcoming drug resistance and treating
advanced prostate cancer. Curr Drug Targets. 13:1308–1323. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oh WK and Kantoff PW: Management of
hormone refractory prostate cancer: current standards and future
prospects. J Urol. 160:1220–1229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misra UK, Mowery Y, Kaczowka S and Pizzo
SV: Ligation of cancer cell surface GRP78 with antibodies directed
against its COOH-terminal domain up-regulates p53 activity and
promotes apoptosis. Mol Cancer Ther. 8:1350–1362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Misra UK, Wang F and Pizzo SV:
Transcription factor TFII-I causes transcriptional upregulation of
GRP78 synthesis in prostate cancer cells. J Cell Biochem.
106:381–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang LW, Lin CY, Lee CC, Liu TZ and Jeng
CJ: Overexpression of GRP78 is associated with malignant
transformation in epithelial ovarian tumors. Appl Immunohistochem
Mol Morphol. 20:381–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee E, Nichols P, Spicer D, Groshen S, Yu
MC and Lee AS: GRP78 as a novel predictor of responsiveness to
chemotherapy in breast cancer. Cancer Res. 66:7849–7853. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Q, Hua J, Wang Q, et al: Expressions
of GRP78 and Bax associate with differentiation, metastasis, and
apoptosis in non-small cell lung cancer. Mol Biol Rep.
39:6753–6761. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Song H, Luo J, Liang J, Zhao S and
Su R: Knockdown of glucose-regulated protein 78 decreases the
invasion, metalloproteinase expression and ECM degradation in
hepatocellular carcinoma cells. J Exp Clin Cancer Res. 31:392012.
View Article : Google Scholar
|
|
10
|
Wang Q, Shu R, He H, et al: Co-silencing
of Birc5 (survivin) and Hspa5 (Grp78) induces apoptosis in hepatoma
cells more efficiently than single gene interference. Int J Oncol.
41:652–660. 2012.PubMed/NCBI
|
|
11
|
Prados J, Melguizo C, Roldan H, et al: RNA
Interference in the treatment of colon cancer. BioDrugs.
27:317–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi H, Deng JH, Wang Z, Cao KY, Zhou L and
Wan H: Knockdown of clusterin inhibits the growth and migration of
renal carcinoma cells and leads to differential gene expression.
Mol Med Rep. 8:35–40. 2013.PubMed/NCBI
|
|
13
|
Zhao Y, Jian W, Gao W, et al: RNAi
silencing of c-Myc inhibits cell migration, invasion, and
proliferation in HepG2 human hepatocellular carcinoma cell line:
c-Myc silencing in hepatocellular carcinoma cell. Cancer Cell Int.
13:232013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Z, Zhu Z, Pang Z, Xing Y, Wan F, Lan D
and Wang H: Short hairpin RNA targeting FOXQ1 inhibits invasion and
metastasis via the reversal of epithelial-mesenchymal transition in
bladder cancer. Int J Oncol. 42:1271–1278. 2013.PubMed/NCBI
|
|
15
|
Clark PR, Pober JS and Kluger MS:
Knockdown of TNFR1 by the sense strand of an ICAM-1 siRNA:
dissection of an off-target effect. Nucleic Acids Res.
36:1081–1097. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jackson AL and Linsley PS: Noise amidst
the silence: off-target effects of siRNAs? Trends Genet.
20:521–524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang CI, Yoo JW, Hong SW, et al:
Asymmetric shorter-duplex siRNA structures trigger efficient gene
silencing with reduced nonspecific effects. Mol Ther. 17:725–732.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jo SG, Hong SW, Yoo JW, Lee CH, Kim S and
Lee DK: Selection and optimization of asymmetric siRNA targeting
the human c-MET gene. Mol Cells. 32:543–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun X, Rogoff HA and Li CJ: Asymmetric RNA
duplexes mediate RNA interference in mammalian cells. Nat
Biotechnol. 26:1379–1382. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daneshmand S, Quek ML, Lin E, et al:
Glucose-regulated protein GRP78 is up-regulated in prostate cancer
and correlates with recurrence and survival. Hum Pathol.
38:1547–1552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yin Y, Chen X, Zhang CD, et al: Asymmetric
siRNA targeting the bcl-2 gene inhibits the proliferation of cancer
cells in vitro and in vivo. Int J Oncol. 42:253–260.
2013.PubMed/NCBI
|
|
23
|
Yuan Z, Wu X, Liu C, Xu G and Wu Z:
Asymmetric siRNA: new strategy to improve specificity and reduce
off-target gene expression. Hum Gene Ther. 23:521–532. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang LH and Zhang X: Roles of GRP78 in
physiology and cancer. J Cell Biochem. 110:1299–1305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Philippova M, Ivanov D, Joshi MB, et al:
Identification of proteins associating with
glycosylphosphatidylinositol-anchored T-cadherin on the surface of
vascular endothelial cells: role for Grp78/BiP in
T-cadherin-dependent cell survival. Mol Cell Biol. 28:4004–4017.
2008. View Article : Google Scholar
|
|
26
|
Shani G, Fischer WH, Justice NJ, Kelber
JA, Vale W and Gray PC: GRP78 and Cripto form a complex at the cell
surface and collaborate to inhibit transforming growth factor beta
signaling and enhance cell growth. Mol Cell Biol. 28:666–677. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen M and Petignat P: Purified
autoantibodies against glucose-regulated protein 78 (GRP78) promote
apoptosis and decrease invasiveness of ovarian cancer cells. Cancer
Lett. 309:104–109. 2011. View Article : Google Scholar
|
|
28
|
Rasche L, Duell J, Morgner C, et al: The
natural human IgM antibody PAT-SM6 induces apoptosis in primary
human multiple myeloma cells by targeting heat shock protein GRP78.
PLoS One. 8:e634142013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suzuki T, Lu J, Zahed M, Kita K and Suzuki
N: Reduction of GRP78 expression with siRNA activates unfolded
protein response leading to apoptosis in HeLa cells. Arch Biochem
Biophys. 468:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing X, Li Y, Liu H, Wang L and Sun L:
Glucose regulated protein 78 (GRP78) is overexpressed in colorectal
carcinoma and regulates colorectal carcinoma cell growth and
apoptosis. Acta Histochem. 113:777–782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang YJ, Huang YP, Li ZL and Chen CH:
GRP78 knockdown enhances apoptosis via the down-regulation of
oxidative stress and Akt pathway after epirubicin treatment in
colon cancer DLD-1 cells. PLoS One. 7:e351232012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu Y, Wey S, Wang M, et al: Pten null
prostate tumorigenesis and AKT activation are blocked by targeted
knockout of ER chaperone GRP78/BiP in prostate epithelium. Proc
Natl Acad Sci USA. 105:19444–19449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang LH, Yang XL, Zhang X, Cheng JX and
Zhang W: Association of elevated GRP78 expression with increased
astrocytoma malignancy via Akt and ERK pathways. Brain Res.
1371:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cardone MH, Roy N, Stennicke HR, et al:
Regulation of cell death protease caspase-9 by phosphorylation.
Science. 282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chiu CC, Lin CY, Lee LY, et al:
Glucose-regulated protein 78 regulates multiple malignant
phenotypes in head and neck cancer and may serve as a molecular
target of therapeutic intervention. Mol Cancer Ther. 7:2788–2797.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McInroy L and Määttä A: Down-regulation of
vimentin expression inhibits carcinoma cell migration and adhesion.
Biochem Biophys Res Commun. 360:109–114. 2007. View Article : Google Scholar : PubMed/NCBI
|