Introduction
Osteoclasts are cells of the macrophage lineage and
are characterized by unique bone resorption activity (1–5).
Since osteoclasts are terminal cells and possess no proliferation
ability, they can only be derived from the differentiation of
macrophages (1,3). Previous studies indicated that in the
presence of receptor activator of nuclear factor κB ligand (RANKL)
and macrophage colony-stimulating factor (M-CSF), osteoclasts are
able to be formed by the differentiation of several
monocyte/macrophage precursors (1,6–8).
Osteoclast differentiation and bone resorption is mainly regulated
by osteoprotegerin (OPG)/RANKL/receptor activator of nuclear factor
κB (RANK) system (9–11), in which RANKL and OPG are secreted
by osteoblasts (4,12). RANKL binds to its receptor
activator RANK in the osteoclast precursors, thereby inducing the
differentiation of macrophages into osteoclasts. OPG is the decoy
receptor of RANKL and competes with RANK to bind RANKL, with
antagonist effects to RANK. It is through this mechanism that
osteoclast differentiation is inhibited (9,13).
In addition, OPG promotes the apoptosis of osteoclasts by
inhibiting the formation of the ruffled border in mature
osteoclasts (14–16). However, the effects of OPG on
osteoclast precursors in the process of osteoclast differentiation
remain to be elucidated.
In relevant studies of osteoclasts, RAW264.7 is the
most commonly used osteoclast precursor cell line (12,17,18).
Macrophages are the precursors of osteoclasts and also a type of
immune cell. Macrophages have important roles in bone remodeling
and the immune system (3,19). Numerous studies have focused on
elucidating the mechanisms underlying the differentiation of
macrophages into osteoclasts in the presence of RANKL and M-CSF
(6–10,20).
However, the changes of the RAW264.7 macrophages that do not
differentiate into osteoclasts have not been studied during
macrophage-osteoclast differentiation, to the best of our
knowledge.
Rho guanosine triphosphate (GTP)-ases are among the
molecular switches involved in signal transduction by membrane
receptors. Rho GTPases contain high-affinity sites that bind to GTP
and guanosine diphosphate (GDP). They are activated by GTP binding
and are deactivated by GDP binding. Rho GTPases act as molecular
switches between the activated and the deactivated states. Rho
GTPases are associated with the regulation of various processes in
the organisms, including cell apoptosis (21,22).
The Rho GTPase family comprises 22 members (23), which are divided into a classical
type and an atypical type. Rac1 and RhoA are classical Rho GTPases,
while RhoV is an atypical Rho GTPase (24,25).
It has been reported that Rho GTPases, including Rac1 (26), RhoA (24.25) and RhoV (27), have important roles in cell
apoptosis.
In the present study, the changes of RAW264.7
macrophages that did not differentiate into osteoclasts and the
function of Rho GTPases in these changes were investigated.
Materials and methods
Morphological observations
RAW264.7 and BRL-3A cell lines were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China).
In order to observe the morphological alterations of
RAW264.7 macrophages during the differentiation into osteoclasts,
the whole process of osteoclast differentiation was studied.
RAW264.7 macrophages were seeded into six-well plates containing
α-minimum essential medium (MEM; Gibco Life Technologies, Carlsbad,
CA, USA) supplemented with 50 ng/ml M-CSF (Peprotech, Inc., Rocky
Hill, NJ, USA) and 60 ng/ml RANKL (Peprotech) at a cell density of
1562.5 cells/cm2 (identical cell density was used in all
experiments). The morphological changes of RAW264.7 were observed
by a Hoffman microscope (Leica DMI3000; Leica Microsystems GmbH,
Wetzlar, Germany) on days one to four.
To determine the cause of RAW264.7 macrophage
apoptosis, the BRL-3A cell line, which has no differentiation
capacity, was analyzed in order to distinguish between the
potentially toxic activity of RANKL and the differentiation induced
by RANKL. Subsequently, RAW264.7 and BRL-3A cells were plated with
α-MEM supplemented with 50 ng/ml M-CSF, 60 ng/ml RANKL and 50 ng/ml
M-CSF plus 60 ng/ml RANKL. No cytokines were added to cells in the
control group. The cells were cultured for four days. The
subsequent changes in the macrophages in each group were observed
using a Hoffman microscope.
Immunofluorescent staining
In order to further study the association between
differentiation and apoptosis, OPG was introduced to inhibit the
differentiation of RAW264.7 cells. The RAW264.7 cells were cultured
in α-MEM supplemented with 50 ng/ml M-CSF and 60 ng/ml RANKL or 50
ng/ml M-CSF, 60 ng/ml RANKL and 40 ng/ml OPG (Peprotech). The
former treatment was the inducer treatment group and the latter was
the OPG inhibition group. No cytokines were added to cells in the
control group. BRL-3A cells were cultured in α-MEM supplemented
with 50 ng/ml M-CSF and 60 ng/ml RANKL. All cells were cultured for
four days and were finally fixed in paraformaldehyde for 30 min.
Propidium iodide (PI; Sigma-Aldrich, St Louis, MO, USA) and Hoechst
33258 (Sigma-Aldrich) double staining was performed for 15 min,
respectively. Images were captured using a Leica DMI3000 inverted
fluorescence microscope.
DNA Ladder experiment
In order to make the present study more rigorous,
the DNA Ladder experiment was used to validate the association
between differentiation and apoptosis. The RAW264.7 cells were
cultured in α-MEM supplemented with 50 ng/ml M-CSF and 60 ng/ml
RANKL or supplemented with 50 ng/ml M-CSF, 60 ng/ml RANK and 40
ng/ml OPG. The former was the inducer treatment group, and the
latter was the OPG inhibition group. No cytokines were added to
cells in the control group. All cells were cultured for four days.
DNA extraction was performed using a Wizard Genomic DNA
Purification kit (Promega Corporation, Madison, WI, USA). The
extracted DNA was separated by 2% agarose gel electrophoresis for
50 min at 90 V. DNA bands were detected by a gel imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Fluorescence quantitative polymerase
chain reaction (qPCR) analysis
In order to study the roles of RhoA, Rac1 and RhoV
in the process of RAW264.7 macrophage apoptosis caused by
osteoclast differentiation, fluorescence qPCR was used to detect
the mRNA expression levels of each.
The mRNA expression levels of RhoA, Rac1 and RhoV
were detected during the process of osteoclast differentiation. The
RAW264.7 macrophages were cultured by the method described for
observing the morphological changes of macrophages during
differentiation. mRNA extraction was performed on days 1–4 using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA).
Subsequently, reverse transcription was performed using the
PrimeScript RT reagent kit with genomic DNA eraser (Takara Bio,
Inc., Otsu, Japan). A real-time PCR system (Applied Biosystems
7500, Life Technologies, Foster City, CA, USA) was used to detect
mRNA expression levels of Rac1, RhoA and RhoV. The primers were
designed using Primer Premier 5 from published gene sequences
(http://www.ncbi.nlm.nih.gov/) and shown
in Table I.
 | Table IPrimers used in quantitative
polymerase chain reaction analyses. |
Table I
Primers used in quantitative
polymerase chain reaction analyses.
| Gene | Accession no. | Upstream | Downstream |
|---|
| RhoA | NM_016802 |
CAAGGACCAGTTCCCAGAGG |
CGCAGGCGGTCATAATCTTC |
| Rac1 | NM_009007 |
GCCTGCTCATCAGTTACACG |
GACGCAATCTGTCATAATCTTC |
| RhoV | NM_145530 |
GCAGCCTCATCGTCAGCTACAC |
GAAGCAAGCCAGAAAGACATCG |
| GAPDH | GU214026 |
ATGGTGAAGGTCGGTGTG |
TGAAGGGGTCGTTGATGG |
The expression of mRNA of RhoA, Rac1 and RhoV in
undifferentiated, differentiated and differentiation-inhibited
RAW264.7 macrophages was detected. RAW264.7 cells were seeded into
a six-well plate using the method described in the DNA Ladder
experiment. The cells were cultured for four days, following which
mRNA extraction and reverse transcription were performed. The mRNA
expression levels of Rac1, RhoA and RhoV were detected using
qPCR.
Statistical analysis
In the present study, each experiment was conducted
in triplicate. The mRNA expression level results were analyzed by
comparison of their 2−ΔΔCt values. Results are
represented statistically as the mean ± standard deviation.
Significance was assessed using one-way analysis of variance
(ANOVA). The results were compared between groups using ANOVA and
Fisher’s least significant difference post-hoc tests following
appropriate transformation to normalized data and equalized
variance where necessary. Statistical analysis was performed using
Statistical Analysis System (SAS) 9.1.3 (SAS Institute, Inc., Cary,
NC, USA); P<0.05 and P<0.01 were considered to indicate a
statistically significant difference between values. In the qPCR
experiments for osteoclast differentiation, comparisons were made
among the expression levels of Racl, RhoA and RhoV at different
stages of differentiation. In the qPCR experiment observing the
effects of OPG on undifferentiated RAW264.7, comparisons were made
between the expression of Racl, RhoA and RhoV within the inducer
group and the control group, along with that between the inducer
treatment group and OPG inhibition group.
Results
Process of osteoclast
differentiation
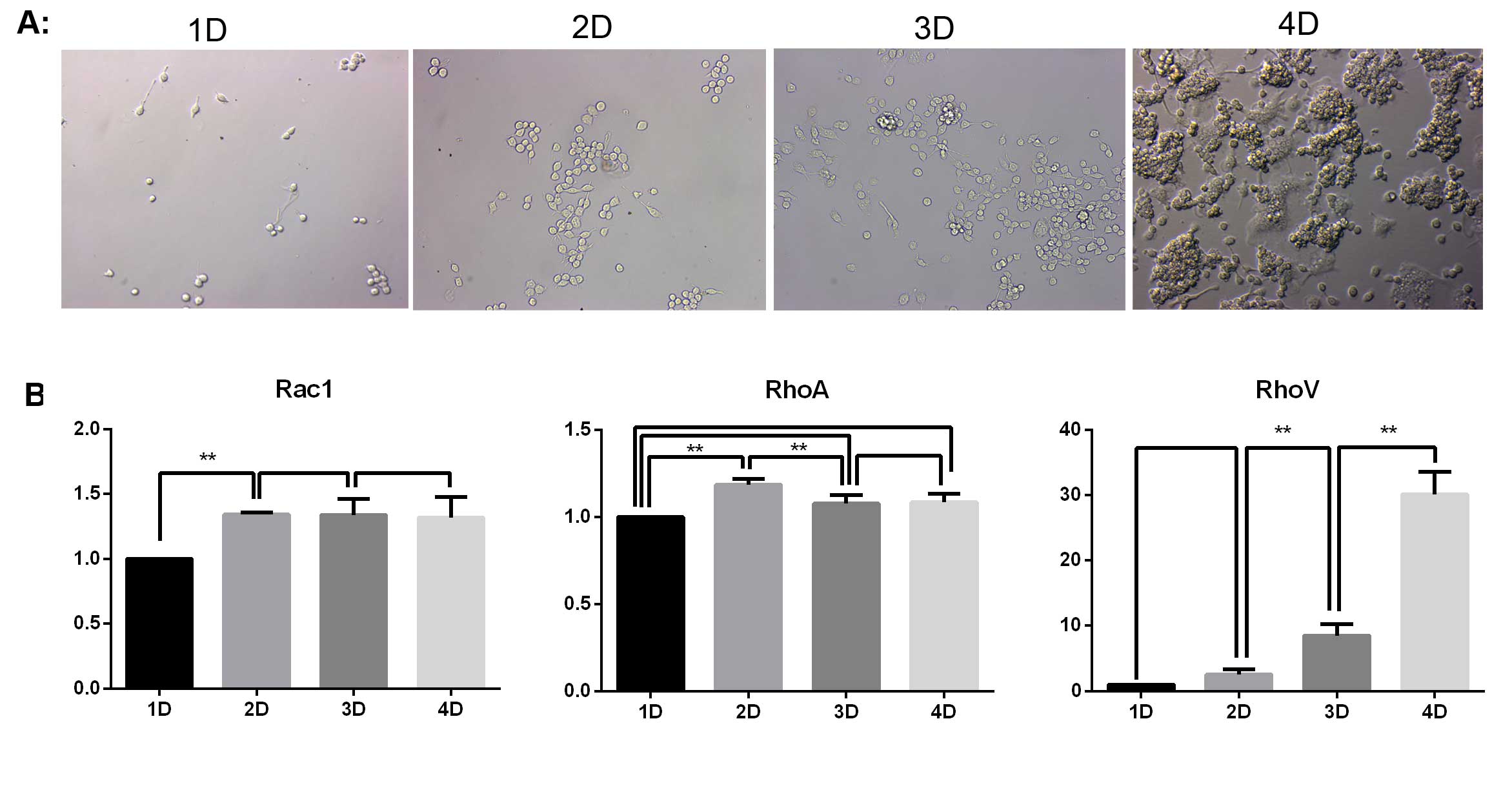
The various changes of the cells in the induction
group were observed on days 1–4 using a Hoffman microscope. As
indicated in Fig. 1A, the number
of RAW264.7 macrophages increased with the extension of induction
time. No osteoclasts were produced in the first three days. On day
four, a large number of osteoclasts had developed. Simultaneously,
there was a notable level of apoptosis of RAW264.7 cells.
In order to investigate the roles of Rac1, RhoA and
RhoV in the apoptosis of RAW264.7 cells that did not differentiate
into osteoclasts, the mRNA expression of Rac1, RhoA and RhoV was
detected by qPCR. As indicated in Fig.
1B, the mRNA expression of Rac1 and RhoA was maintained at a
constant level following day two, where a slight upregulation was
observed. The mRNA expression of RhoV was continually upregulated
with the extension of the induction treatment. The mRNA expression
of RhoV on day four was almost 30 times that of the expression
levels on day one.
Effects of M-CSF, RANKL and the
combination of the two inducers on cell differentiation and
apoptosis
The effects of M-CSF and RANKL alone and the
combined use of the two inducers on the cell differentiation and
apoptosis were investigated by observing changes in the cells of
each group following four days of culture using a Hoffman
microscope. The BRL-3A liver cells were used as the control to
discriminate between the apoptosis-inducing effect of the inducers
and the apoptosis caused by the differentiation of RAW264.7
cells.
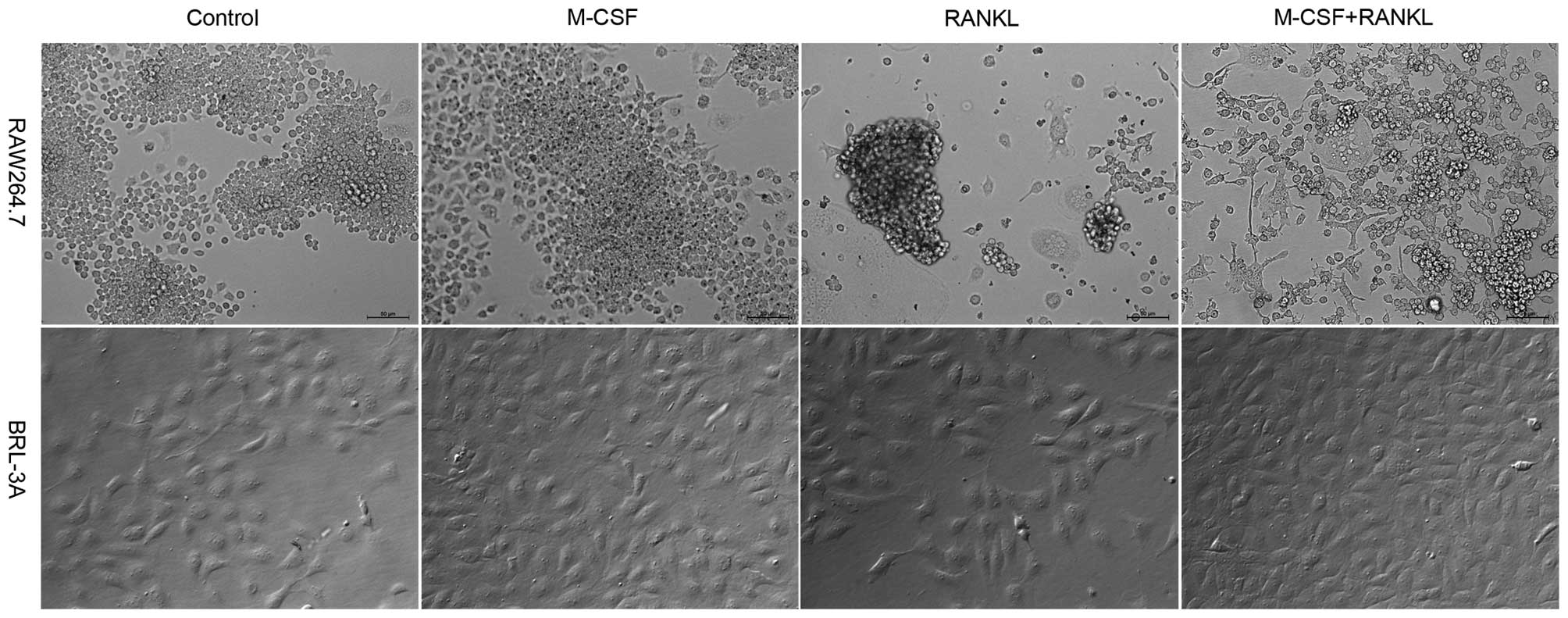
As indicated in Fig.
2, following four-day culture in α-MEM supplemented with RANKL
or supplemented with RANKL and M-CSF, RAW264.7 cells in the two
groups demonstrated a scattered distribution. A large amount of
osteoclasts were produced; however, a significant percentage of
RAW264.7 macrophages demonstrated apoptosis indicated by detached
or floating cells. This result was consistent with that observed on
day four in Fig. 1A. In the
control group and the M-CSF treatment group, the cells grew in
aggregates, no osteoclasts were produced and no apoptosis of
RAW264.7 cells was observed. BRL-3A cells have no differentiation
ability. In all four groups of BRL-3A cells, cell growth was normal
and no apoptosis was observed as in the case of RAW264.7. It was
also found that the cell count in the four groups receiving M-CSF
treatment was higher than that in the groups without M-CSF
treatment. This is because M-CSF promotes the division and
proliferation of macrophages.
Detection of cell apoptosis in the
control, inducer treatment and OPG inhibition groups by DNA
Ladder
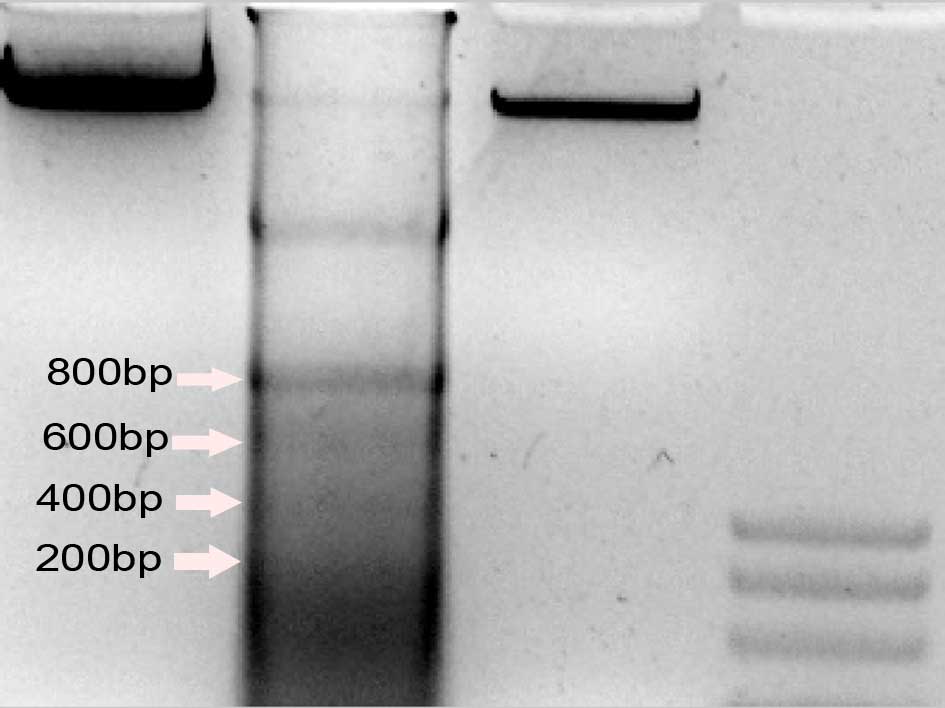
The 200 bp DNA ladder is a marker that
differentiates cell necrosis from cell apoptosis. DNA fragmentation
was detected in the control, M-CSF+RANKL and OPG+M-CSF+RANKL groups
to reveal the effects of osteoclast differentiation on the
apoptosis of RAW264.7 macrophages. The DNA Ladder was produced in
the M-CSF+RANKL group, but not in the OPG+M-CSF+RANKL or control
groups (Fig. 3).
Detection of RAW264.7 cell apoptosis by
Hoechst 33258 and PI double staining
PI and Hoechst 33258 bind to nuclear DNA. PI is
unable to pass through the cell membrane of live cells, while
Hoechst 33258 is a membrane-permeable fluorescent dye. For those
cells in necrosis or late-stage apoptosis, the cell membrane is
damaged and therefore, the respective cell is stained red by PI.
The effects of the differentiation of RAW264.7 macrophages on their
apoptotic rate was further studied by PI and Hoechst 33258 double
staining.
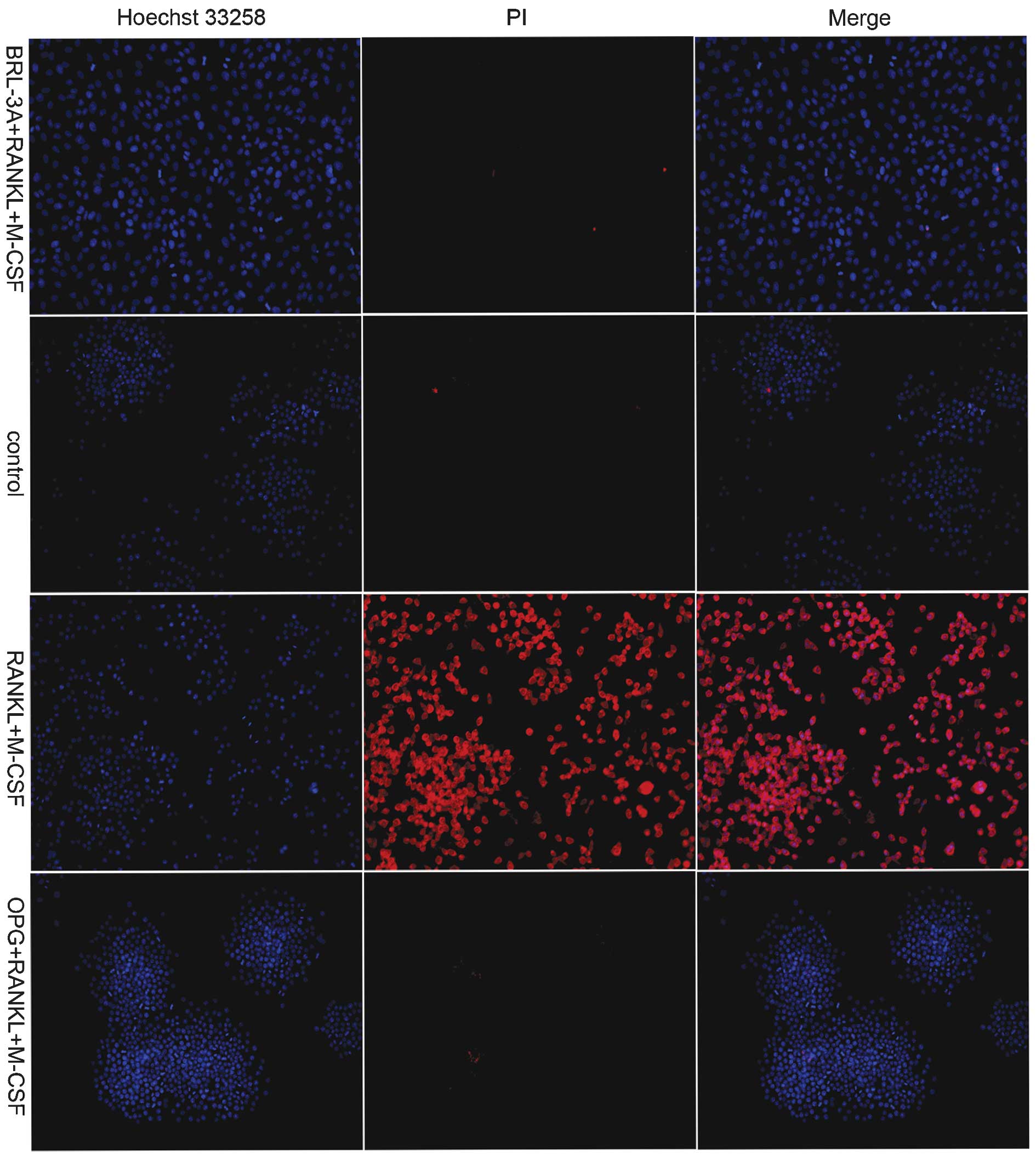
As exhibited in Fig.
4, the BRL-3A cells were not stained by PI in the M-CSF+RANKL
treatment group, which indicated that no apoptosis of BRL-3A cells
occurred in the presence of the inducers. This result was
consistent with that exhibited in Fig.
2. Following M-CSF and RANKL treatments alone, a large number
of RAW264.7 macrophages were stained red by PI. This indicated that
marked apoptosis had occurred in RAW264.7 cells following the
induction. Conversely, the cells in the control group and the OPG
inhibition group were not stained by PI. This indicated that no
apoptosis of the cells occurred in these groups, consistent with
the results of the DNA Ladder detection experiment (Fig. 3).
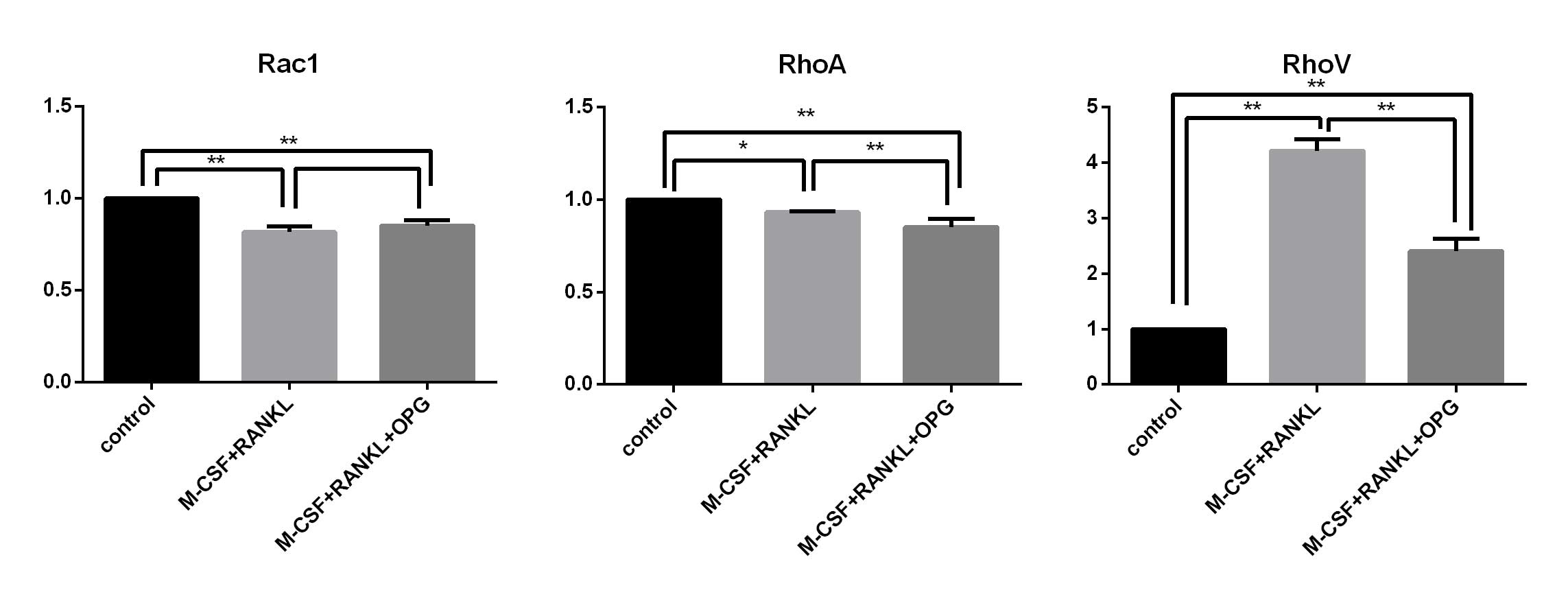
mRNA expression levels of Rac1, RhoA and
RhoV in the control, inducer treatment and OPG inhibition
groups
In order to investigate the roles of Rac1, RhoA and
RhoV in the process of RAW264.7 cell apoptosis caused by RAW264.7
differentiation, mRNA expression of Rac1, RhoA and RhoV was
detected using qPCR in the control, M-CSF+RANKL treatment and
OPG+M-CSF+RANKL groups (Fig.
5).
It was demonstrated that the mRNA expression of Rac1
and RhoA exhibited significant decreases in the inducer treatment
and OPG inhibition groups. Conversely, RhoV mRNA expression was
significantly upregulated in the inducer treatment group. Compared
with the inducer treatment group, RhoV mRNA expression was
significantly downregulated in the OPG inhibition group.
Discussion
In the present study, the effects of osteoclast
differentiation on RAW264.7 macrophages were investigated. With the
exception of a number of RAW264.7 macrophages which differentiated
into osteoclasts following RANKL and M-CSF induction, nearly all
RAW264.7 macrophages underwent apoptosis. It was also observed that
when OPG was used to inhibit the differentiation of RAW264.7
macrophages, the apoptosis of RAW264.7 macrophages was inhibited.
Furthermore, RhoV was the mediator of the apoptosis of RAW264.7
macrophages caused by their differentiation.
In bones, RANKL and M-CSF are produced by cells of
osteoblastic lineage. RANKL and M-CSF are able to activate a series
of complex reaction of macrophages and induce their differentiation
into osteoclasts. The oversecretion of RANKL and M-CSF leads to the
abnormal proliferation of osteoclasts and hence a variety of bone
diseases, including rheumatoid arthritis, osteoporosis and multiple
myeloma (2,28). There is a reduction in estrogen
levels in females in the menopausal period (29), which leads to an increase in RANKL
expression. Therefore, this results in excessive differentiation of
macrophages into osteoclasts (30,31).
According to existing studies, the probability of occurrence of
various diseases was increased during the menopausal period due to
decreased immunity (32–35). Unexpectedly, a large amount of
osteoclasts were produced on day four, under the induction of RANKL
and M-CSF. Simultaneously, a considerable amount of apoptosis of
RAW264.7 macrophages occurred. Subsequently, the effects exposure
to of M-CSF and/or RANKL on the apoptosis of RAW264.7 macrophages
were studied. It was found that the undifferentiated RAW264.7
macrophages underwent apoptosis in the two groups receiving RANKL
treatment. This process was accompanied by the production of large
quantities of osteoclasts. In order elucidate whether the cause of
the apoptosis of RAW264.7 macrophages was RANKL induction or
differentiation of RAW264.7, the BRL-3A cell line, which has no
differentiation capability, was used as a control group. The
results indicated that neither RANKL nor M-CSF induced the
apoptosis of BRL-3A cells. Furthermore, RANKL itself is a substance
produced in normal organisms. These combined results indicated that
the differentiation of RAW264.7 macrophages induced the observed
apoptosis. Whether the high levels of apoptosis of macrophages are
the direct reason for the low immunity associated with the
menopausal period remains to be elucidated by further
experiments.
OPG is also produced by cells of osteoblastic
lineage, and is able to inhibit the differentiation of macrophages
into osteoclasts (9,11). To verify the hypothesis that the
differentiation of RAW264.7 macrophages caused their apoptosis, OPG
was added into the RANKL+M-CSF induction treatment group in order
to inhibit the differentiation of RAW264.7 macrophages. PI and
Hoechst 33258 double staining and DNA Ladder experiments were
performed. In the inducer treatment group, a large amount of
undifferentiated RAW264.7 macrophages experienced apoptosis.
However, in the OPG inhibition group and the control group without
any cytokines, no apoptosis of RAW264.7 macrophages was observed.
These results indicated that the differentiation of RAW264.7
macrophages into osteoclasts itself induced apoptosis. By
inhibiting the differentiation of RAW264.7 macrophages, OPG
inhibited the differentiation-dependent RAW264.7 apoptosis.
Rho GTPases regulate diverse metabolic processes in
cells, including dynamic changes of the cytoskeleton, cell
adhesion, gene expression and cell apoptosis (21,26,36,37).
Rho GTPases, including RhoA, Rac1 and RhoV, have important roles in
cell apoptosis (24–27). The mRNA expression levels of RhoA,
Rac1 and RhoV during the process of osteoclast differentiation were
detected in order to investigate the effects of the inducers RANKL
and M-CSF, and the inhibitor OPG on the apoptotic rate of RAW264.7
macrophages. Of note, the apoptosis of undifferentiated RAW264.7
macrophages occurred on day four of the culture. Compared with day
one, the mRNA expression of RhoA and Rac1 on day four demonstrated
only minor upregulation and subsequently remained at this level. In
the qPCR experiment examining the effects of OPG on
undifferentiated RAW264.7, the mRNA expression levels of RhoA and
Rac1 showed significant downregulation in the inducer treatment
groups and OPG inhibition group compared with those in the control
group. This change was inverse to the apoptotic rate of the cells.
It was demonstrated that the apoptotic rate of RAW264.7 macrophages
induced by their differentiation did not necessarily involve the
action of RhoA and Rac1. However, on day four, when high levels of
apoptosis of RAW264.7 cells were present, the mRNA expression of
RhoV was significantly upregulated. In the Hoechst 33258 and PI
double staining experiment, and the DNA Ladder experiment, compared
with the control group and OPG inhibition group where no apoptosis
occurred, the cells in the inducer treatment group experienced
notable apoptosis. The mRNA expression of RhoV was also
significantly upregulated, corresponding to the apoptotic rate of
the cells. These results demonstrated that RhoV had an important
role in regulating the apoptosis of RAW264.7 macrophages caused by
their differentiation.
In the present study, the apoptotic rate of RAW264.7
macrophages in the presence of RANKL was investigated. RANKL
induced the differentiation of RAW264.7 macrophages into
osteoclasts. In conclusion, it was demonstrated that the
differentiation of RAW264.7 macrophages itself was the cause of the
high levels of apoptosis. The addition of OPG inhibited the
differentiation of RAW264.7 macrophages into osteoclasts, which
thereby inhibited the apoptosis of RAW264.7 macrophages.
Additionally, in the process of osteoclast differentiation, RhoV
mediated the RAW264.7 macrophage apoptosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 31172373, 31302154 and
31372495), the Specialized Research Fund for the Doctoral Program
of Higher Education (no. 20113250110003) and a project funded by
the Priority Academic Program Development of Jiangsu Higher
Education Institutions and the Graduate Innovation Project of
Jiangsu Province (no. CXZZ12_0917).
References
|
1
|
Husheem M, Nyman JK, Vääräniemi J,
Vaananen HK and Hentunen TA: Characterization of circulating human
osteoclast progenitors: development of in vitro resorption assay.
Calcif Tissue Int. 76:222–230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Väänänen HK and Laitala-Leinonen T:
Osteoclast lineage and function. Arch Biochem Biophys. 473:132–138.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teitelbaum SL: Bone resorption by
osteoclasts. Science. 289:1504–1508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Väänänen HK, Zhao H, Mulari M and Halleen
JM: The cell biology of osteoclast function. J Cell Sci.
113:377–381. 2000.PubMed/NCBI
|
|
6
|
Jansen ID, Vermeer JA, Bloemen V, Stap J
and Everts V: Osteoclast fusion and fission. Calcif Tissue Int.
90:515–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasuda H, Shima N, Nakagawa N, et al:
Osteoclast differentiation factor is a ligand for
osteoprotegerin/osteoclastogenesis-inhibitory factor and is
identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 95:3597–3602.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacey DL, Timms E, Tan HL, et al:
Osteoprotegerin ligand is a cytokine that regulates osteoclast
differentiation and activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hofbauer LC, Kühne CA and Viereck V: The
OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet
Neuronal Interact. 4:268–275. 2004.PubMed/NCBI
|
|
10
|
Khosla S: Minireview: the OPG/RANKL/RANK
system. Endocrinology. 142:5050–5055. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song R, Gu J, Liu X, et al: Inhibition of
osteoclast bone resorption activity through osteoprotegerin-induced
damage of the sealing zone. Int J Mol Med. 34:856–862.
2014.PubMed/NCBI
|
|
12
|
Singh PP, van der Kraan AG, Xu J,
Gillespie MT and Quinn JM: Membrane-bound receptor activator of
NFκB ligand (RANKL) activity displayed by osteoblasts is
differentially regulated by osteolytic factors. Biochem Biophys Res
Commun. 422:48–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang J, Chen TY, Qin S, Duan Y and Wang G:
Inhibitory effect of metformin on bone metastasis of cancer via
OPG/RANKL/RANK system. Med Hypotheses. 81:805–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiotani A, Takami M, Itoh K, Shibasaki Y
and Sasaki T: Regulation of osteoclast differentiation and function
by receptor activator of NFκB ligand and osteoprotegerin. Anat Rec.
268:137–146. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Z, Xu J, EL and Wang D: Ultrasound
enhances the healing of orthodontically induced root resorption in
rats. Angle Orthod. 82:48–55. 2012. View Article : Google Scholar
|
|
16
|
Fu YX, Gu JH, Zhang YR, Tong XS, Zhao HY,
Yuan Y, Liu XZ, Bian JC and Liu ZP: Influence of osteoprotegerin on
differentiation, activation, and apoptosis of Gaoyou duck embryo
osteoclasts in vitro. Poult Sci. 92:1613–1620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li CH, Zhao JX, Sun L, Yao ZQ, Deng XL,
Liu R and Liu XY: AG490 inhibits NFATc1 expression and STAT3
activation during RANKL induced osteoclastogenesis. Biochem Biophys
Res Commun. 435:533–539. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Robertson Remen KM, Gustafsson JÅ and
Andersson G: The liver X receptor promotes macrophage
differentiation and suppresses osteoclast formation in mouse
RAW264.7 promyelocytic leukemia cells exposed to bacterial
lipopolysaccharide. Biochem Biophys Res Commun. 430:375–380. 2013.
View Article : Google Scholar
|
|
19
|
Sinningen K, Rauner M, Goettsch C,
Al-Fakhri N, Schoppet M and Hofbauer LC: Monocytic expression of
osteoclast-associated receptor (OSCAR) is induced in
atherosclerotic mice and regulated by oxidized low-density
lipoprotein in vitro. Biochem Biophys Res Commun. 437:314–318.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song RL, Liu XZ, Zhu JQ, et al: New roles
of filopodia and podosomes in the differentiation and fusion
process of osteoclasts. Genet Mol Res. 13:4776–4787. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Coxon FP and Rogers MJ: The role of
prenylated small GTP-binding proteins in the regulation of
osteoclast function. Calcif Tissue Int. 72:80–84. 2003. View Article : Google Scholar
|
|
23
|
Vega FM and Ridley AJ: SnapShot: Rho
family GTPases. Cell. 129:14302007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakamura H, Hirata A, Tsuji T and Yamamoto
T: Role of osteoclast extracellular signal-regulated kinase (ERK)
in cell survival and maintenance of cell polarity. J Bone Miner
Res. 18:1198–1205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang N, Robaye B, Agrawal A, Skerry TM,
Boeynaems JM and Gartland A: Reduced bone turnover in mice lacking
the P2Y(13) receptor of ADP. Mol Endocrinol. 26:142–152. 2012.
View Article : Google Scholar
|
|
26
|
Fukuda A, Hikita A, Wakeyama H, Akiyama T,
Oda H, Nakamura K and Tanaka S: Regulation of osteoclast apoptosis
and motility by small GTPase binding protein Rac1. J Bone Miner
Res. 20:2245–2253. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shepelev MV, Chernoff J and Korobko IV:
Rho family GTPase Chp/RhoV induces PC12 apoptotic cell death via
JNK activation. Small GTPases. 2:17–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chamoux E, Houde N, L’Eriger K and Roux S:
Osteoprotegerin decreases human osteoclast apoptosis by inhibiting
the TRAIL pathway. J Cell Physiol. 216:536–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schindler AE: Climacteric symptoms and
hormones. Gynecol Endocrinol. 22:151–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blair HC, Robinson LJ and Zaidi M:
Osteoclast signalling pathways. Biochem Biophys Res Commun.
328:728–738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Syed F and Khosla S: Mechanisms of sex
steroid effects on bone. Biochem Biophys Res Commun. 328:688–696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stolberg M: From the ‘climacteric disease’
to the ‘male climacteric’ The historical origins of a modern
concept. Maturitas. 58:111–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rollenhagen C and Asin SN: Enhanced HIV-1
replication in ex vivo ectocervical tissues from post-menopausal
women correlates with increased inflammatory responses. Mucosal
Immunol. 4:671–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Breuil V, Ticchioni M, Testa J, et al:
Immune changes in post-menopausal osteoporosis: the Immunos study.
Osteoporos Int. 21:805–814. 2010. View Article : Google Scholar
|
|
35
|
Ambrogini E, Toraldo G and Marcocci C:
Post-menopausal osteoporosis: is it an autoimmune disease? J
Endocrinol Invest. 28:43–47. 2005.
|
|
36
|
Li F, Jiang Q, Shi KJ, Luo H, Yang Y and
Xu CM: RhoA modulates functional and physical interaction between
ROCK1 and Erk1/2 in selenite-induced apoptosis of leukaemia cells.
Cell Death Dis. 4:e7082013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ullah I, Lee HY, Kim MJ, Shah SA, Badshah
H, Kim TH, Chung HJ, Yang BC and Kim MO: Rho GTPase activating
protein 15 (arhGAP15) siRNA effect apoptosis-induced by ethanol in
bovine fibroblast cells. Pak J Pharm Sci. 26:605–610.
2013.PubMed/NCBI
|