Introduction
Glioblastoma is a highly aggressive brain tumor that
is commonly resistant to existing chemotherapeutic agents (1). Temozolomide (TMZ) is a DNA alkylating
agent and has been shown to prolong the survival of patients with
glioblastoma, when used in combination with surgery and radiation
therapy (2,3). However, due to the primary and
acquired drug resistance, the survival benefit of TMZ chemotherapy
remains limited. Elevated expression of
O6-methylguanine-DNA-methyltransferase (MGMT), a DNA
repair enzyme that removes TMZ-generated methyl groups at the
O6 position of guanine, represents a major mechanism of
TMZ resistance (4). The
transcription factor nuclear factor-κB (NF-κB), which is
constitutively activated in human glioblastoma, is important in the
regulation of MGMT expression (5).
The majority of NF-κB exists as a heterodimer of p65/p50 proteins
that are sequestered in the cytoplasm by inhibitor of κB (IκB)
proteins. Phosphorylation and degradation of IκBα results in the
liberation of the p65/p50 heterodimer for its translocation to the
nucleus where it transactivates target genes (6). Evidence indicates that
phosphorylation of p65 at serine 536 is involved in the nuclear
import and transcriptional activity of p65 (7). Inhibition of NF-κB signaling has been
found to reverse TMZ resistance by downregulation of MGMT in T98 G
glioblastoma cells (8). Therefore,
the combination with NF-κB inhibitors may provide a promising
strategy to improve the efficacy of TMZ chemotherapy against
glioblastoma.
Curcumin (diferuloylmethane), a hydrophobic
polyphenol molecule extracted from turmeric (Curcuma longa
Linn), has a wide range of pharmacological activities, including
anti-inflammatory, antioxidant and anticancer properties (9). Curcumin exerts a suppressive effect
on NF-κB activity in various biological contexts (10,11).
For instance, curcumin has been found to enhance the
chemosensitization of colorectal cancer cells to conventional
chemotherapeutic agents, such as 5-fluorouracil through inhibition
of NF-κB and Src protein kinase signaling pathways (10). In malignant gliomas, curcumin also
exhibits chemosensitizing activity through suppression of AP-1 and
NF-κB signaling pathways (12). It
is thus suggested that such activity of suppressing NF-κB signaling
may be exploited to overcome TMZ resistance. Indeed, curcumin was
reported to potentiate TMZ cytotoxicity against glioblastoma cells
(13). However, the mechanisms
underlying curcumin-mediated chemosensitization are not fully
understood.
MicroRNAs (miRNAs) are a class of endogenous
non-coding regulatory RNAs of ~22 nucleotides. They are important
in tumorigenesis, acting as oncogenes or tumor suppressor genes
(14). A single miRNA can
simultaneously regulate a large number of target genes and thus
affect various biological processes, such as cell proliferation,
differentiation, migration, invasion and apoptosis (14,15).
miR-146a is known to be a negative regulator of the NF-κB signaling
pathway (16,17). Celastrol, a plant triterpene, has
been shown to induce the apoptosis of gastric cancer cells via
miR-146a-mediated inhibition of NF-κB activity (16). Notably, diflourinated-curcumin
(CDF) is capable of switching on suppressor miRNAs including
miR-146a in pancreatic cancer, consequently leading to reduced
tumor growth and aggressiveness (18). These findings suggest that the
induction of miR-146a and suppression of NF-κB signaling could
partially account for the enhanced curcumin-induced TMZ
cytotoxicity in glioblastoma.
Therefore, in the present study, the cytotoxic
effects of curcumin and TMZ, alone or in combination in human
glioblastoma cells were investigated and the involvement of
miR-146a and NF-κB signaling were explored in curcumin-mediated
chemosensitization.
Materials and methods
Cell culture and drug treatment
U-87 MG human glioblastoma cells were obtained from
the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China) and maintained in high glucose Dulbecco's modified Eagle's
medium supplemented with 10% fetal bovine serum (), 100 U/ml
penicillin, and 100 µg/ml streptomycin (all from Invitrogen
Life Technologies, Carlsbad, CA, USA). The cells were seeded at a
density of 2×105 cells/well onto 12-well plates. After
incubation overnight to allow cell attachment, curcumin
(Sigma-Aldrich, St. Louis, MO, USA) and TMZ (Suzhou Bichal
Biological Technology, Suzhou, China) at different concentrations
were added alone or in combination to the cell cultures. The
control cells were treated with 0.5% dimethyl sulfoxide (DMSO;
Sigma-Aldrich). After incubation for 72 h, cells were harvested for
assessment of cell proliferation and apoptosis. If not stated
otherwise, 20 µM curcumin and 100 µM TMZ were used.
For inhibition of NF-κB signaling, cells were pretreated with 100
µM pyrrolidine dithiocarbamate (PDTC; Sigma-Aldrich)
(19) for 1 h prior to TMZ
treatment.
miRNA oligonucleotides and
transfection
Locked nucleic acid (LNA)-based anti-human miR-146a
and universal LNA-based negative control were purchased from Exiqon
(Vedbaek, Denmark), and human pre-miR-146a and pre-miR negative
control oligonucleotides were purchased from Ambion (Austin, TX,
USA). For depletion of endogenous miR-146a, U-87 MG cells were
transiently transfected with LNA-anti-miR-146a or LNA inhibitor
control, using Lipofectamine™ 2000 (Invitrogen Life Technologies)
according to the manufacturer's instructions. For overexpression of
miR-146a, pre-miR-146a or negative control oligonucleotides were
transfected into U-87 MG cells using Lipofectamine 2000. The final
concentration of each oligonucleotide was 50 nM. At 24 h post
transfection, the cells were exposed to curcumin and/or TMZ.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using the mirVana
miRNA Isolation kit (Ambion®, Austin, TX, USA). The
level of mature miR-146a was determined using Taqman miRNA assays
(Applied Biosystems, Foster City, CA, USA). Briefly, cDNA was
synthesized with a miRNA-specific stem-loop primer, and
quantitative PCR was performed using the 7900HT Fast Real-Time PCR
system (Applied Biosystems). Cycling conditions were as follows:
95°C for 10 min, followed by 40 cycles at 95°C for 15 sec, and 60°C
for 1 min. The primers for miR-146a were as follows: Forward:
5′-GCCCTGAGAACTGAATTCCATG-3′ and reverse:
5′-GTGCAGGGTCCGAGGTAACCCA-3′. The relative amount miR-146a
normalized to the U6 small nuclear RNA level was calculated using
the comparative cycle threshold (ΔΔCt) method (20). Each assay was conducted in
triplicate.
Cell proliferation assay
U-87 MG cells were seeded in triplicate in 96-well
microplates at a density of 6×103 cells/well. After drug
treatment, cells were subjected to cell viability analysis using
the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide
(MTT) method. MTT solution (5 mg/ml; Sigma Aldrich) was added to
the cell cultures and incubated at 37°C for 4 h. Following the
removal of the MTT solution, DMSO solution was added to dissolve
formazan crystals. The absorbance was measured at a wavelength of
570 nm using a Bio-Rad 96-well plate reader (model 550, Bio-Rad,
Hercules, CA, USA). The experiments were repeated three times.
Results were expressed as the percentage relative to DMSO-treated
control cells (defined as 100%).
Apoptosis analysis by flow cytometry
Following drug treatment, cells were harvested by
treatment with trypsin (Invitrogen Life Technologies), washed three
times, and resuspended in phosphate-buffered saline (PBS). Cell
apoptosis was determined using the Annexin V Apoptosis kit (Becton
Dickinson, San Diego, CA, USA). A single cell suspension (100
µl; 2×106 cells/ml) was stained with fluorescein
isothiocyanate-conjugated Annexin V and propidium iodide (PI)
solution (20 µg/ml) and incubated for 15 min in the dark.
Apoptotic cells (Annexin V-positive) were analyzed by flow
cytometry (A BD LSR II Flow Cytometer; Becton Dickinson). Each
assay was performed in triplicate and repeated three times.
Western blot analysis
Whole cell lysates were prepared in lysis buffer [10
mM Tris/HCl, pH 6.8; 10% glycerol, 2% sodium dodecyl sulfate (SDS),
1% Triton X-100 and 1% Nonidert P40] containing 1 mM
phenylmethanesulfonyl fluoride and complete protease inhibitors
(Roche, Mannheim Germany). Samples of the lysates containing ~50
µg protein were separated by SDS-polyacrylamide gel
electrophoresis and transferred to polyvinylidene fluoride
membranes (Millipore, Bedford, MA, USA). Subsequent to incubation
for 1 h in blocking solution containing 5% fat-free milk, membranes
were incubated at 4°C overnight with primary antibodies. Primary
antibodies used were as follows: Rabbit anti-human IκBα polyclonal
antibody (cat. #9242), mouse anti-human phospho-IκBα (Ser32/36)
monoclonal antibody (cat. no. 9246), rabbit anti-human NF-κB p65
polyclonal antibody (cat. no. 8242), rabbit anti-human
phospho-NF-κB p65 (Ser536) polyclonal antibody (cat. #3031), and
mouse anti-human β-actin monoclonal antibody (cat. no. 3700). These
antibodies were purchased from Cell Signaling Technology Inc.
(Beverly, MA, USA). All antibodies except β-actin (1:3,000
dilution) were diluted 1:500 before use. Membranes were washed and
incubated with appropriate horseradish peroxidase-conjugated
secondary antibodies (sc-2030 and sc-2005; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) for 1 h. Immunoreactive bands
were visualized with an enhanced chemiluminescent detection system
(Amersham Biosciences, Piscataway, NJ, USA) according to the
manufacturer's instructions. Densitometry was performed using
Quantity One software (version 4.6.8; Bio-Rad).
NF-κB luciferase activity assay
The reporter plasmid pNF-κB-Luc containing four
copies of NF-κB enhancer elements and a firefly luciferase gene
were purchased from Beyotime Institute of Biotechnology (Haimen,
China) and the pRL-TK Renilla luciferase control reporter
vector from Promega Corporation (Madison, WI, USA). U-87 MG cells
were seeded at a density of 2×105 cells/well onto
12-well plates and transiently transfected with pNF-κB-luc (0.5
µg) and pRL-TK (0.02 µg) together with pre-miR-146a
or control oligonucleotides. After transfection (24 h), the cells
were treated with 100 µM TMZ for 72 h and harvested. The
firefly and Renilla luciferase activities were measured in
the cellular lysates using the dual-luciferase reporter assay kit
(Promega Corporation), according to the manufacturer's
instructions. Firefly luciferase activity was normalized to the
Renilla luciferase activity, which served as an internal
control for transfection efficiency.
Statistical analysis
Data are presented as the mean ± standard deviation.
The difference among the means of multiple groups was analyzed by
one-way analysis of variance followed by Tukey's test. All
statistical calculations were performed using SPSS.11 software
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Combination of curcumin and TMZ enhances
the cytotoxic effects on U-87 MG cells
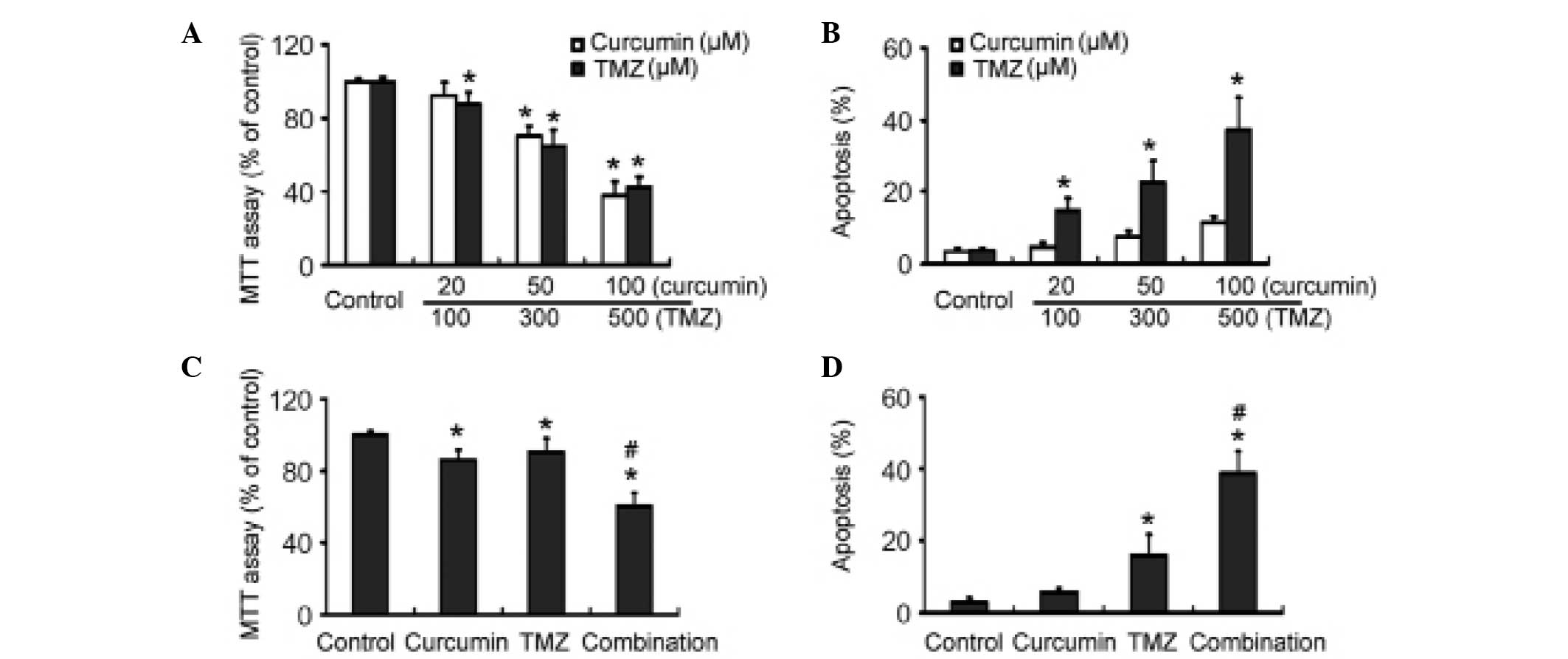
The MTT assay revealed that exposure to curcumin and
TMZ alone for 72 h resulted in a significant dose-dependent
inhibition of U-87 MG cell viability, as compared with control
cells (Fig. 1A). Flow cytometric
analysis revealed that treatment with TMZ caused significant
apoptosis of U-87 MG cells (Fig.
1B). Curcumin at 100 µM also promoted apoptosis in U-87
MG cells. TMZ induced apoptosis in a concentration-dependent
manner. When treated with 500 µM TMZ for 72 h, the cells
exhibited an ~10-fold higher apoptosis rate than control cells
(37.2±8.9% vs. 3.8±0.6%, respectively; P<0.05). Notably, the
combination of suboptimal doses of curcumin (20 µM) and TMZ
(100 µM) showed significantly (P<0.05) greater
growth-suppressive effects against U-87 MG cells than each alone
(Fig. 1C). Moreover, TMZ-induced
apoptosis was significantly (P<0.05) enhanced by its combination
with 20 µM curcumin (Fig.
1D).
Curcumin induces miR-146a expression in
U-87 MG cells
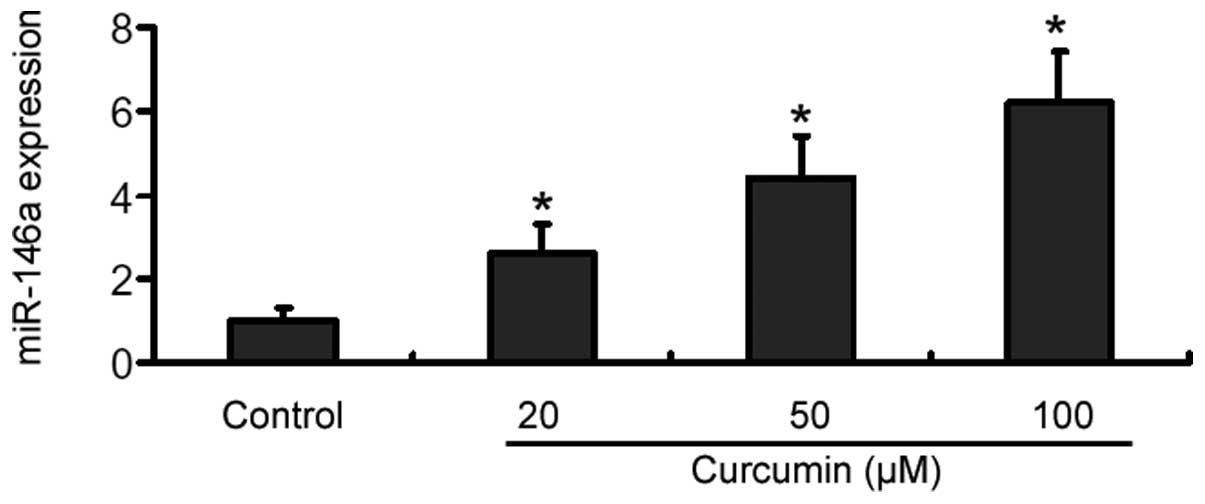
RT-qPCR analysis showed that treatment with curcumin
for 48 h caused a significant (P<0.05) elevation in the miR-146a
level in U-87 MG cells, compared with control cells (Fig. 2). Moreover, such elevation occurred
in a dose-dependent manner, with ~4- and 6-fold increase at 20 and
100 µM curcumin, respectively (Fig. 2). However, the miR-146a level
remained unchanged in TMZ-treated U-87 MG cells (data not
shown).
miR-146a mediates the increase in
TMZ-induced apoptosis by curcumin
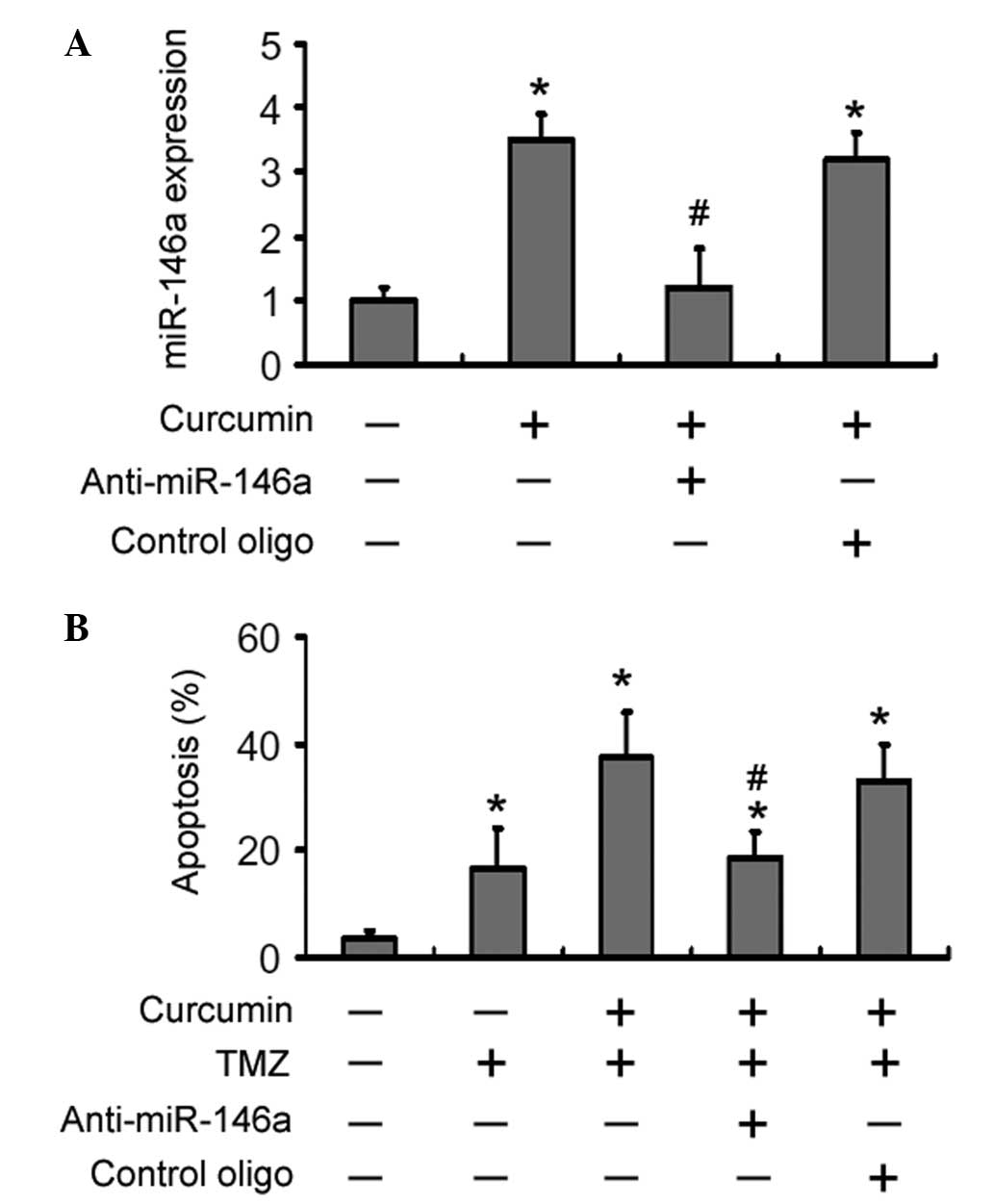
Having identified the upregulation of miR-146a by
curcumin, the role of miR-146a in curcumin-mediated
chemosensitization was next determined. Pre-transfection with
LNA-anti-miR-146a almost completely inhibited the upregulation of
miR-146a by curcumin (P<0.05 vs. transfection with control
oligonucleotides; Fig. 3A).
Notably, depletion of miR-146a reduced the curcumin-mediated
increase in of TMZ-induced apoptosis by ~50% (Fig. 3B). However, apoptosis was not
observed in U-87 MG cells transfected with either LNA-anti-miR-146a
or control oligonucleotide (data not shown).
Forced expression of miR-146a sensitizes
U-87 MG cells to TMZ-induced apoptosis
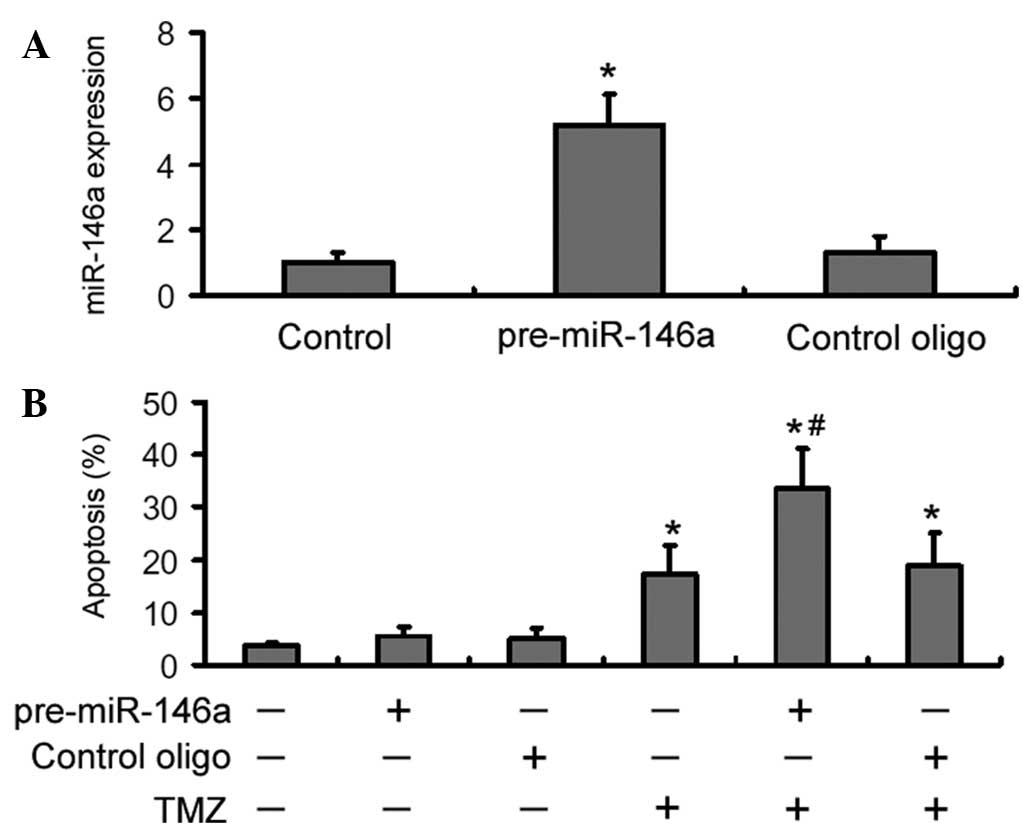
Next, it was analyzed whether over-expression of
miR-146a conferred chemosensitization to TMZ in U-87 MG cells.
RT-qPCR analysis showed that the miR-146a level was significantly
increased in pre-miR-146a-transfected U-87 MG cells than in control
oligonucleotide-transfected cells (Fig. 4A). Apoptosis analysis revealed that
overexpression of miR-146a significantly (P<0.05) enhanced
TMZ-induced apoptosis by ~2-fold, compared with the delivery of
control oligonucleotides (Fig.
4B). However, transfection of miR-146a alone did not
significantly alter cell apoptosis.
miR-146a suppresses NF-κB signaling in
U-87 MG cells exposed to TMZ
Finally, it was examined whether the chemo-sensitive
effects of miR-146a were associated with suppression of NF-κB
signaling. As shown in Fig. 5A,
miR-146a overexpression markedly inhibited the phosphorylation of
IκBα and p65 in TMZ-treated U-87 MG cells and increased the total
level of IκBα. However, the total level of NF-κB p65 remained
unchanged. The luciferase reporter assay demonstrated that the
delivery of pre-miR-146a led to a significant (P<0.05) decline
in the NF-κB transcriptional activity in control and TMZ-treated
cells (Fig. 5B). Notably,
pharmacological inhibition of NF-κB signaling significantly
increased TMZ-induced apoptosis in U-87 MG cells compared with
DMSO-treated cells (Fig. 5C),
which resembles the results obtained with overexpression of
miR-146a. However, PDTC alone failed to induce significant
apoptosis in U-87 MG cells.
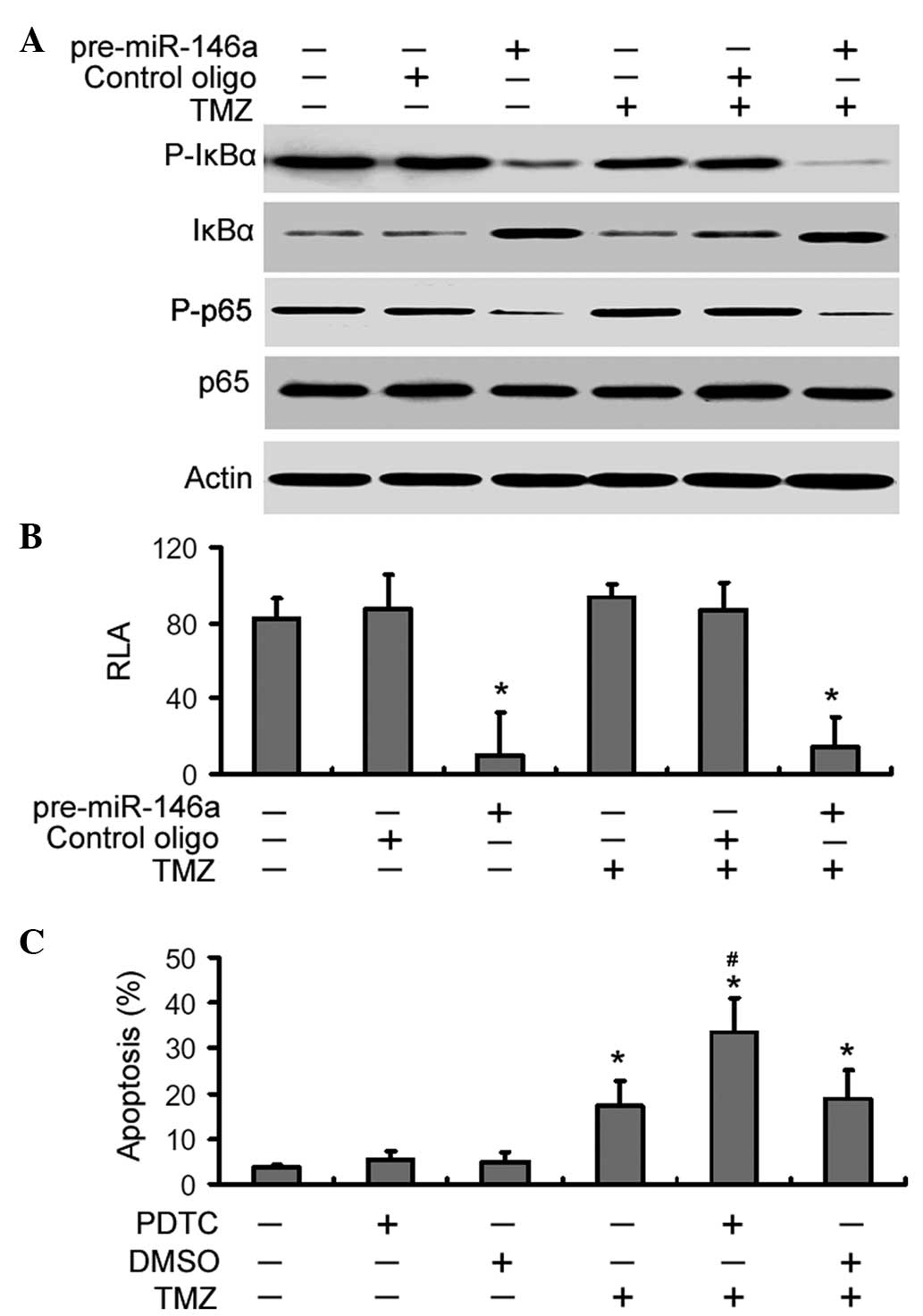 | Figure 5miR-146a suppresses NF-κB signaling in
U-87 MG cells exposed to TMZ. (A) Cells were transfected with
pre-miR-146a or control oligonucleotides (oligo) and treated with
or without TMZ. Western blot analysis of the protein levels of
total and phosphorylated IκBα and p65. Representative blots of
three independent experiments with similar results are shown. (B)
NF-κB luciferase reporter assay. Cells were co-transfected with
NF-κB luciferase reporter together with pre-miR-146a or control
oligonucleotides and RLA was measured as described in the Materials
and methods. Bar graphs show the mean ± SD of three independent
experiments. *P<0.05 vs. the control. (C) Effects of
pharmacological inhibition of NF-κB signaling on TMZ-induced
apoptosis. Cells were pre-treated with PDTC or DMSO prior to
exposure to TMZ and cell apoptosis was examined. Columns, mean
percentage of three independent experiments; bars, SD.
*P<0.05 vs. the control; #P<0.05 vs.
TMZ treatment alone. miR, microRNA; TMZ, temozolomide; IκBα,
inhibitor κBα; NF-κB, nuclear factor-κB; RLA, relative luciferase
act-vity; SD, standard deviation; PDTC, pyrrolidine
dithiocarbamate; DMSO, dimethyl sulfoxide. |
Discussion
Curcumin and its synthetic analogues have shown
anticancer properties in various types of cancer cell (21). By contrast, curcumin has been shown
to protect normal organs, such as the liver, kidney, oral mucosa
and heart from chemotherapy and radiotherapy-induced toxicity
(22). Given its safety and
tolerability (23), curcumin can
be used alone or in combination with other chemotherapeutic agents
in the treatment of neoplastic diseases. Preclinical studies have
demonstrated that curcumin is capable of suppressing tumor growth
in glioblastoma (24,25). Consistently, the present data
indicated a dose-dependent cytotoxic effect of curcumin on U-87 MG
cells. Moreover, when suboptimal doses of curcumin and TMZ were
combined, a significant increase in the suppression of cell
viability was observed. These results confirm the potentiation of
cytotoxicity by combination of curcumin and TMZ in glioblastoma
cells. It is noteworthy that curcumin at 20–100 µM did not
induce significant apoptosis in U-87 MG cells as determined by
Annexin-V/PI staining. Aoki et al (25) reported that curcumin causes G2/M
arrest and nonapoptotic autophagic cell death in malignant glioma
cells, which may provide an explanation for the growth-suppressive
effects of curcumin. Notably, curcumin showed the capacity to
promote TMZ-induced apoptosis, which partially explains the
enhanced cytotoxicity by combination of curcumin and TMZ.
A number of miRNAs have been identified to modulate
radio- and chemoresistance of gliomas (26). For instance, miR-21 is implicated
in the acquisition of TMZ resistance in glioblastoma cells and its
inhibition leads to a significant increase in TMZ-induced apoptosis
(27). miR-195, miR-455-3p, and
miR-10a* are also implicated in acquired TMZ resistance
in glioblastoma cells (28). In
this study, it was demonstrated that miR-146a was upregulated by
curcumin but not TMZ in U-87 MG cells. The upregulation of miR-146a
by the CDF curcumin analogue has been previously described in
pancreatic cancer (18). It has
been documented that ectopic expression of miR-146a inhibits the
glioma development of a human glioblastoma cell line in an
orthotopic xenograft model (29).
However, manipulating miR-146a expression alone had no significant
effect on U-87 MG cell apoptosis, which suggests that the
inhibitory function of miR-146a on gliomas is largely attributable
to apoptosis-independent mechanisms. Our data further revealed that
miR-146a mediated the chemosensitizing activity of curcumin, as
evidenced by the finding that its depletion significantly
antagonized TMZ-induced apoptosis even in the presence of curcumin.
Moreover, miR-146a overexpression significantly enhanced
TMZ-induced apoptosis in U-87 MG cells. These results indicate that
miR-146a as a responsive miRNA to curcumin treatment is critical in
the modulation of the susceptibility of glioblastoma cells to TMZ.
The potential implication of miR-146a in acquired chemoresistance
is also documented in breast cancer cells (30). The present data expand the list of
candidate miRNAs as therapeutic targets for increasing the
chemosensitivity of glioblastoma cells.
The NF-κB pathway is implicated in the progression
of glioblastoma and targeting NF-κB may thus provide therapeutic
benefits in this disease (31). It
has previously been documented that treatment with
dehydroxymethylepoxyquinomicin (DHMEQ), a unique small molecule
inhibitor of NF-κB, induces G2/M arrest and apoptosis in
glioblastoma cells (32).
Targeting NF-κB via DHMEQ is capable of improving the sensitivity
of human glioblastoma cells to TMZ (33). miR-146a has been found to act as a
suppressor of NF-κB signaling in multiple biological contexts
(16,17). The present data indicated that
enforced expression of miR-146a significantly inhibited the
transcriptional activity of NF-κB in TMZ-treated U-87 MG cells, as
determined by a luciferase reporter assay. Moreover,
pharmacological inhibition of NF-κB signaling sensitized U-87 MG
cells to TMZ-induced apoptosis, which is similar to the findings
with overexpression of miR-146a. These results collectively point
towards a critical role of the miR-146a/NF-κB axis in the
regulation of TMZ cytotoxicity. Several targets of miR-146a, such
as caspase recruitment domain-containing protein 10 (CARD10), COP9
signalosome complex subunit 8 (COPS8 (17) and TNF receptor-associated factor 6
(34), have been identified.
Repressing CARD10 and COPS8 expression is predominantly responsible
for the miR-146a-induced suppression of NF-κB activation in gastric
cancer (17), while in the setting
of natural killer/T cell lymphoma, targeting TRAF6 is linked to the
downregulation of NF-κB activity by miR-146a (34). However, the direct target mediating
the inhibitory effects of miR-146a in glioblastoma remains to be
identified.
In conclusion, the data demonstrated that curcumin
sensitizes glioblastoma cells to TMZ-induced apoptosis, which is,
at least partially mediated through regulation of the
miR-146a/NF-κB axis. These findings warrant further exploration of
the potential clinical utility of curcumin for improving the
efficacy of TMZ chemotherapy in glioblastoma. The therapeutic
potential of manipulating the miR-146a/NF-κB axis in glioblastoma
also requires further investigation.
References
|
1
|
Lwin Z, MacFadden D, Al-Zahrani A, Atenafu
E, Miller BA, Sahgal A, Menard C, Laperriere N and Mason WP:
Glioblastoma management in the temozolomide era: have we improved
outcome? J Neurooncol. 115:303–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Julka PK, Sharma DN, Mallick S, Gandhi AK,
Joshi N and Rath GK: Postoperative treatment of glioblastoma
multiforme with radiation therapy plus concomitant and adjuvant
temozolomide: A mono-institutional experience of 215 patients. J
Cancer Res Ther. 9:381–386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alnaami IM, Al-Nuaimi SK, Senthilselvan A,
Murtha AD, Walling S, Mehta V and Gourishankar S: Effectiveness of
adjuvant temozolomide treatment in patients with glioblastoma.
Neurosciences (Riyadh). 18:349–355. 2013.
|
|
4
|
Bocangel DB, Finkelstein S, Schold SC,
Bhakat KK, Mitra S and Kokkinakis DM: Multifaceted resistance of
gliomas to temozolomide. Clin Cancer Res. 8:2725–2734.
2002.PubMed/NCBI
|
|
5
|
Lavon I, Fuchs D, Zrihan D, Efroni G,
Zelikovitch B, Fellig Y and Siegal T: Novel mechanism whereby
nuclear factor kappaB mediates DNA damage repair through regulation
of O (6)-methylguanine-DNA-methyltransferase. Cancer Res.
67:8952–8959. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baldwin AS Jr: The NF-kappaB and I kappaB
proteins: new discoveries and insights. Annu Rev Immunol.
14:649–683. 1996. View Article : Google Scholar
|
|
7
|
Mattioli I, Sebald A, Bucher C, et al:
Transient and selective NF-kappaB p65 serine 536 phosphorylation
induced by T cell costimulation is mediated by I kappaB kinase beta
and controls the kinetics of p65 nuclear import. J Immunol.
172:6336–6344. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang H, Lin H, Zhang X and Li J:
Resveratrol reverses temozolomide resistance by downregulation of
MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway.
Oncol Rep. 27:2050–2056. 2012.PubMed/NCBI
|
|
9
|
Gupta SC, Patchva S, Koh W and Aggarwal
BB: Discovery of curcumin, a component of golden spice and its
miraculous biological activities. Clin Exp Pharmacol Physiol.
39:283–299. 2012. View Article : Google Scholar :
|
|
10
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar
|
|
11
|
Orr WS, Denbo JW, Saab KR, et al: Curcumin
potentiates rhabdomyosarcoma radiosensitivity by suppressing NF-κB
activity. PLoS One. 8:e513092013. View Article : Google Scholar
|
|
12
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramachandran C, Nair SM, Escalon E and
Melnick SJ: Potentiation of etoposide and temozolomide cytotoxicity
by curcumin and turmeric force in brain tumor cell lines. J
Complement Integr Med9:. Article. 20:2012.
|
|
14
|
Fabbri M: MicroRNAs and cancer: towards a
personalized medicine. Curr Mol Med. 13:751–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Williams AE: Functional aspects of animal
microRNAs. Cell Mol Life Sci. 65:545–562. 2008. View Article : Google Scholar
|
|
16
|
Sha M, Ye J, Zhang LX, Luan ZY and Chen
YB: Celastrol induces apoptosis of gastric cancer cells by miR-146a
inhibition of NF-κB activity. Cancer Cell Int. 13:502013.
View Article : Google Scholar
|
|
17
|
Crone SG, Jacobsen A, Federspiel B,
Bardram L, Krogh A, Lund AH and Friis-Hansen L: microRNA-146a
inhibits G protein-coupled receptor-mediated activation of NF-κB by
targeting CARD10 and COPS8 in gastric cancer. Mol Cancer.
11:712012. View Article : Google Scholar
|
|
18
|
Bao B, Ali S, Banerjee S, et al: Curcumin
analogue CDF inhibits pancreatic tumor growth by switching on
suppressor microRNAs and attenuating EZH2 expression. Cancer Res.
72:335–345. 2012. View Article : Google Scholar
|
|
19
|
Park MH, Ahn BH, Hong YK and Min do S:
Overexpression of phospholipase D enhances matrix
metalloproteinase-2 expression and glioma cell invasion via protein
kinase C and protein kinase A/NF-kappaB/Sp1-mediated signaling
pathways. Carcinogenesis. 30:356–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
21
|
Agrawal DK and Mishra PK: Curcumin and its
analogues: potential anticancer agents. Med Res Rev. 30:818–860.
2010.
|
|
22
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nutr Cancer. 62:919–930. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chainani-Wu N: Safety and
anti-inflammatory activity of curcumin: a component of tumeric
(Curcuma longa). J Altern Complement Med. 9:161–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanotto-Filho A, Braganhol E, Edelweiss
MI, et al: The curry spice curcumin selectively inhibits cancer
cells growth in vitro and in preclinical model of glioblastoma. J
Nutr Biochem. 23:591–601. 2012. View Article : Google Scholar
|
|
25
|
Aoki H, Takada Y, Kondo S, Sawaya R,
Aggarwal BB and Kondo Y: Evidence that curcumin suppresses the
growth of malignant gliomas in vitro and in vivo through induction
of autophagy: role of Akt and extracellular signal-regulated kinase
signaling pathways. Mol Pharmacol. 72:29–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koshkin PA, Chistiakov DA and Chekhonin
VP: Role of microRNAs in mechanisms of glioblastoma resistance to
radio- and chemotherapy. Biochemistry (Mosc). 78:325–334. 2013.
View Article : Google Scholar
|
|
27
|
Wong ST, Zhang XQ, Zhuang JT, Chan HL, Li
CH and Leung GK: MicroRNA-21 inhibition enhances in vitro
chemosensitivity of temozolomide-resistant glioblastoma cells.
Anticancer Res. 32:2835–2841. 2012.PubMed/NCBI
|
|
28
|
Ujifuku K, Mitsutake N, Takakura S, et al:
miR-195, miR-455-3p and miR-10a (*) are implicated in acquired
temozolomide resistance in glioblastoma multiforme cells. Cancer
Lett. 296:241–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mei J, Bachoo R and Zhang CL:
MicroRNA-146a inhibits glioma development by targeting Notch1. Mol
Cell Biol. 31:3584–3592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pogribny IP, Filkowski JN, Tryndyak VP,
Golubov A, Shpyleva SI and Kovalchuk O: Alterations of microRNAs
and their targets are associated with acquired resistance of MCF-7
breast cancer cells to cisplatin. Int J Cancer. 127:1785–1794.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Atkinson GP, Nozell SE and Benveniste ET:
NF-kappaB and STAT3 signaling in glioma: targets for future
therapies. Expert Rev Neurother. 10:575–586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fukushima T, Kawaguchi M, Yorita K, et al:
Antitumor effect of dehydroxymethylepoxyquinomicin, a small
molecule inhibitor of nuclear factor-κB, on glioblastoma. Neuro
Oncol. 14:19–28. 2012. View Article : Google Scholar :
|
|
33
|
Brassesco MS, Roberto GM, Morales AG, et
al: Inhibition of NF-κB by dehydroxymethylepoxyquinomicin
suppresses invasion and synergistically potentiates temozolomide
and γ-radiation cytotoxicity in glioblastoma cells. Chemother Res
Pract. 2013:5930202013.
|
|
34
|
Paik JH, Jang JY, Jeon YK, et al:
MicroRNA-146a downregulates NFκB activity via targeting TRAF6 and
functions as a tumor suppressor having strong prognostic
implications in NK/T cell lymphoma. Clin Cancer Res. 17:4761–4771.
2011. View Article : Google Scholar : PubMed/NCBI
|