Introduction
Gestational trophoblastic disease (GTD) includes a
number of pregnancy disorders that arise from placental
trophoblastic tissue (1). GTD
disorders include, complete and invasive hydatidiform moles,
placental site trophoblastic and epithelioid trophoblastic tumors,
and choriocarcinoma, which is the most malignant disease, with a
tendency to spread to lungs, liver and/or brain (2). The majority of cases of
choriocarcinoma occur secondary to a complete mole; however,
choriocarcinoma may also occur following any normal or abnormal
pregnancy, including partial molar pregnancy, term pregnancy,
induced or spontaneous abortion, premature delivery and stillbirth
(3). Chemotherapy has notably
improved the prognosis of patients with choriocarcinoma in the
previous decades; however, ~25% of gestational trophoblastic tumors
are resistant to chemotherapy, or relapse following initial
chemotherapy, which may lead to mortality and requires treatment
with salvage combination chemotherapy (4,5).
During the development of GTD, cell apoptosis serves a role in the
progression of a hydatidiform mole into persistent GTD (6–10)
and may also influence the chemo-resistance of choriocarcinoma
(11). Therefore, novel medicines
that are able to induce trophoblastic cell apoptosis may
potentially improve efficacy of treatment of choriocarcinoma.
Apoptosis is the primary mechanism of cell death.
Generally, there are two main pathways by which apoptosis may be
triggered, including the extrinsic receptor-mediated pathway and
the intrinsic mitochondrial pathway (12). The extrinsic apoptotic pathway
relies on the binding of a death ligand to its membrane receptor on
the extracellular domain, including binding of tumor necrosis
factor receptor superfamily member 6 (Fas) ligand binding to Fas
receptor, and the subsequent formation of a death-inducing
signaling complex, leading to the activation of pro-caspase-8 and
promotion of cell death (12–14).
In the intrinsic pathway, mitochondria serve a role in response to
internal stimuli that result in an increase in mitochondrial outer
membrane permeability (MOMP) (15). Alterations in MOMP lead to release
of proteins from inside the mitochondria to the cytoplasm, and
these proteins activate the caspase cascade (typically caspase-9)
and other apoptotic responses, including the cleavage of
poly(ADP-ribose) polymerase (PARP)-1 (16,17).
MOMP is primarily controlled by anti-apoptotic proteins, including
apoptosis regulator B cell lymphoma (Bcl)-2, Bcl-2-like protein 1,
induced myeloid leukemia cell differentiation protein-1,
Bcl-2-related protein A1, Bcl-2-like protein 10 and Bcl-2-like
protein 2, and pro-apoptotic proteins, including Bcl-2 associated
X, apoptosis regulator (Bax), Bcl-2 homologous antagonist/killer,
BH3-interacting domain death agonist, Bcl-2-like protein 11 and
Bcl-2-associated agonist of cell death (18). Therefore, activation of caspase-3
and cleavage of PARP are markers of apoptosis, whereas the
activation of caspase-9 and reduction of the ratio of Bcl-2 to Bax
are considered indicators of activation of mitochondrial apoptotic
pathway.
It has recently been proposed that widely accessible
and safe dietary ingredients may demonstrate antitumor effects
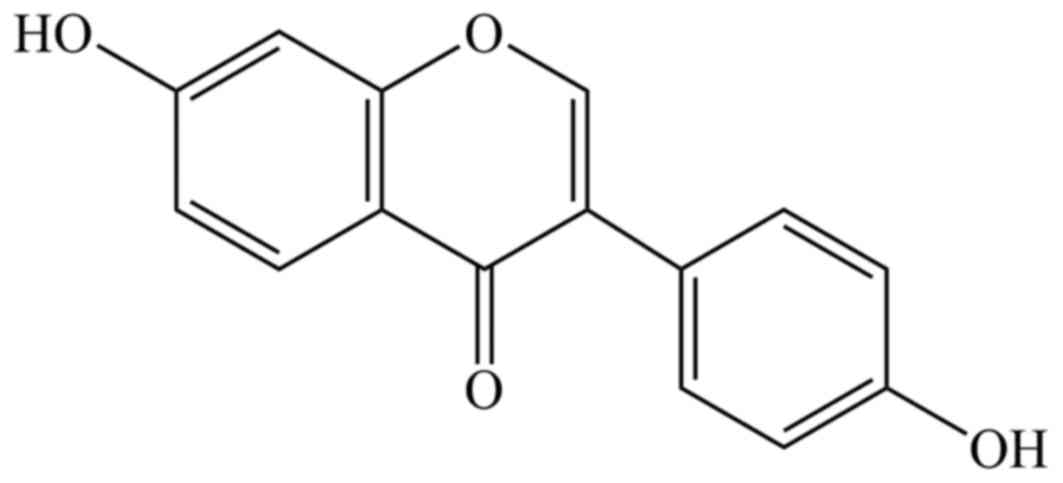
(19). Daidzein (Fig. 1) is classified as isoflavone, and
is one of the most commonly ingested and most extensively studied
types of phytoestrogen, which is abundant in nuts, fruits, soybeans
and soy-based products (20).
Previously, daidzein has garnered interest due to its antitumor
activity exerted via induction of apoptosis (21). Daidzein induces apoptosis via the
mitochondrial apoptotic pathway in a number of cancer types,
including breast cancer, gastric carcinoma and hepatic cancer by
altering the Bcl-2/Bax ratio and activating the caspase cascade
(22–24). Additionally, derivatives of
daidzein have been demonstrated to influence apoptosis in colon
adenocarcinoma and hepatocellular carcinoma cells (25,26).
Jeschke et al (27)
observed a significant concentration-dependent decrease in
production of human chorionic gonadotropin in trophoblast cells
treated with daidzein. Furthermore, the authors previously
demonstrated the anti-proliferation activity of daidzein in
choriocarcinoma (28). However,
few studies regarding the effect of daidzein on apoptosis have been
published.
Therefore, the present study aimed to determine
whether daidzein may induce choriocarcinoma cell apoptosis via the
mitochondrial apoptotic pathway.
Materials and methods
Cell culture
Human choriocarcinoma cell lines JAR and JEG-3 were
obtained from American Type Culture Collection (Manassas, VA, USA)
and were cultured in Dulbecco's modified Eagle's medium (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Thermo Fisher Scientific, Inc.) in an
atmosphere of 5% CO2 at 37°C. Daidzein (Abcam,
Cambridge, UK) was dissolved in dimethyl sulfoxide to a
concentration of 100 mM and stored at −20°C. Daidzein was added
into the culture medium at a concentration of 0, 25, 50 or 100 µM
or 48 h prior to the following experiments.
MTT assay
Cell viability was examined using an MTT assay
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) following treatment
of JAR and JEG-3 cells with 12.5, 25, 50, 100, 200 and 400 µM
daidzein for 48 h. The cells were subsequently incubated with 20 µl
MTT solution (0.5 mg/ml) at 37°C for 4 h. The medium was carefully
discarded and dimethyl sulfoxide (150 µl) was added to dissolve the
formazan crystals. The absorbance was measured at a wavelength of
490 nm using a universal microplate reader (model ELx800; BioTek
Instruments, Inc., Winooski, VT, USA).
Cell apoptosis analysis
An Annexin-V-FITC apoptosis detection kit (BD
Biosciences, San Jose, CA, USA) was used to determine the apoptotic
rate. JAR and JEG-3 cells at 60–80% confluence were trypsinized,
washed with cold PBS twice and resuspended in 100 µl binding
buffer. Cell suspensions were incubated with Annexin-V (20 µg/ml)
and propidium iodide (PI; 50 µg/ml) for 15 min at room temperature
in the dark and detected using a FACSCalibur flow cytometer (BD
Biosciences) and analyzed with FlowJo 7.6.1 software (FlowJo LCC,
Ashland, OR, USA). Double negative cells were considered viable,
early apoptotic cells were Annexin V positive and PI negative,
double positive cells were considered late apoptotic, while Annexin
V negative and PI positive cells were necrotic.
Fluorescence microscopy
Cells were fixed in 4% paraformaldehyde for 15 min
at room temperature, washed with precooled PBS, permeabilized with
0.1% Triton X-100 for 15 min and blocked in 1% bovine serum albumin
for 1 h at room temperature. Cells were subsequently incubated in
DAPI solution (1 µg/ml) for 5 min at room temperature in the dark,
and washed again with PBS. Slides were analyzed and images were
captured using fluorescent microscopy with an Olympus BX51
microscope at ×400 magnification (Olympus Corporation, Tokyo,
Japan). A total of 100 cells were randomly selected and the number
of cells with apoptotic nuclear morphology were counted; 3 sets of
100 cells were analyzed in each group.
Western blot analysis
Cells were washed once with cold PBS and lysed in
radioimmunoprecipitation assay buffer (50 mM Tris, pH 8.0; 150 mM
NaCl; 0.1% SDS; 1% NP-40; and 0.5% sodium deoxycholate) containing
protease inhibitors. The protein concentration was determined using
an enhanced bicinchoninic acid protein assay kit. Protein samples
(20 µg/lane) were separated by 8–12% SDS-PAGE and blotted onto
nitrocellulose membranes. The membranes were blocked with 5%
skimmed milk at room temperature for 1 h and incubated with primary
antibodies against β-actin (cat. no. ab6276; Abcam; 1:2,000),
caspase-3 (cat. no. 9662; Cell signaling Technology, Inc., Danvers,
MA, USA; 1:1,000), caspase-9 (cat. no. 9502; Cell signaling
Technology, Inc.; 1:1,000), PARP (cat. no. 9532; Cell signaling
Technology, Inc.; 1:1,000), Bcl-2 (cat. no. sc-7382; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; 1:500), Bax (cat. no.
sc-7480; Santa Cruz Biotechnology, Inc.; 1:500) at 4°C overnight.
Subsequently, cells were washed in Tris buffered saline containing
0.1% Tween 20, and incubated for 1 h with horseradish
peroxidase-conjugated goat anti-rabbit and goat anti-mouse
secondary antibodies (cat. nos. KC-RB-035 and KC-MM-035; Aksomics,
Inc., Shanghai, China; 1:5,000) at room temperature. Protein bands
were visualized with a Molecular Imager ChemiDoc XRS System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) using an enhanced
chemiluminescence reagent (EMD Millipore, Billerica, MA, USA).
Protein bands were quantified using ImageLab software (version 4.1;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was carried out using SPSS software package
(version 19.0; IBM Corp., Armonk, NY, USA). Data were expressed as
the mean ± standard deviation. Differences between two groups were
performed using Student's t-test and multiple comparisons were
performed using one-way analysis of variance, followed by Dunnett's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Daidzein reduces viability of
choriocarcinoma cells
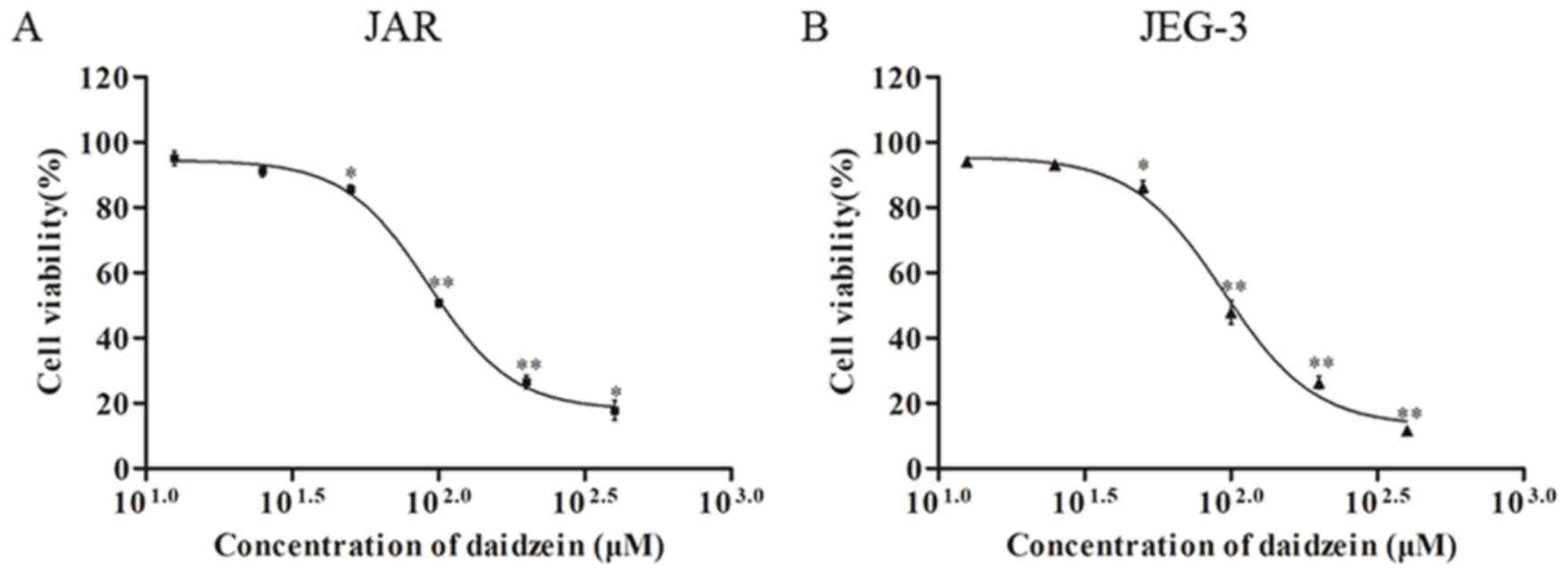
The effect of daidzein on viability of
choriocarcinoma cell lines JAR and JEG-3 was investigated using the
MTT assay. The two cell lines were treated with daidzein at
concentrations of 12.5, 25, 50, 100, 200 and 400 µM. The results
demonstrated that daidzein induced a dose-dependent decrease in
viability of JAR (Fig. 2A) and
JEG-3 (Fig. 2B) cells when the
concentration was higher than 25 µM, with an IC50 of
~100 µM.
Treatment with daidzein induces
apoptosis
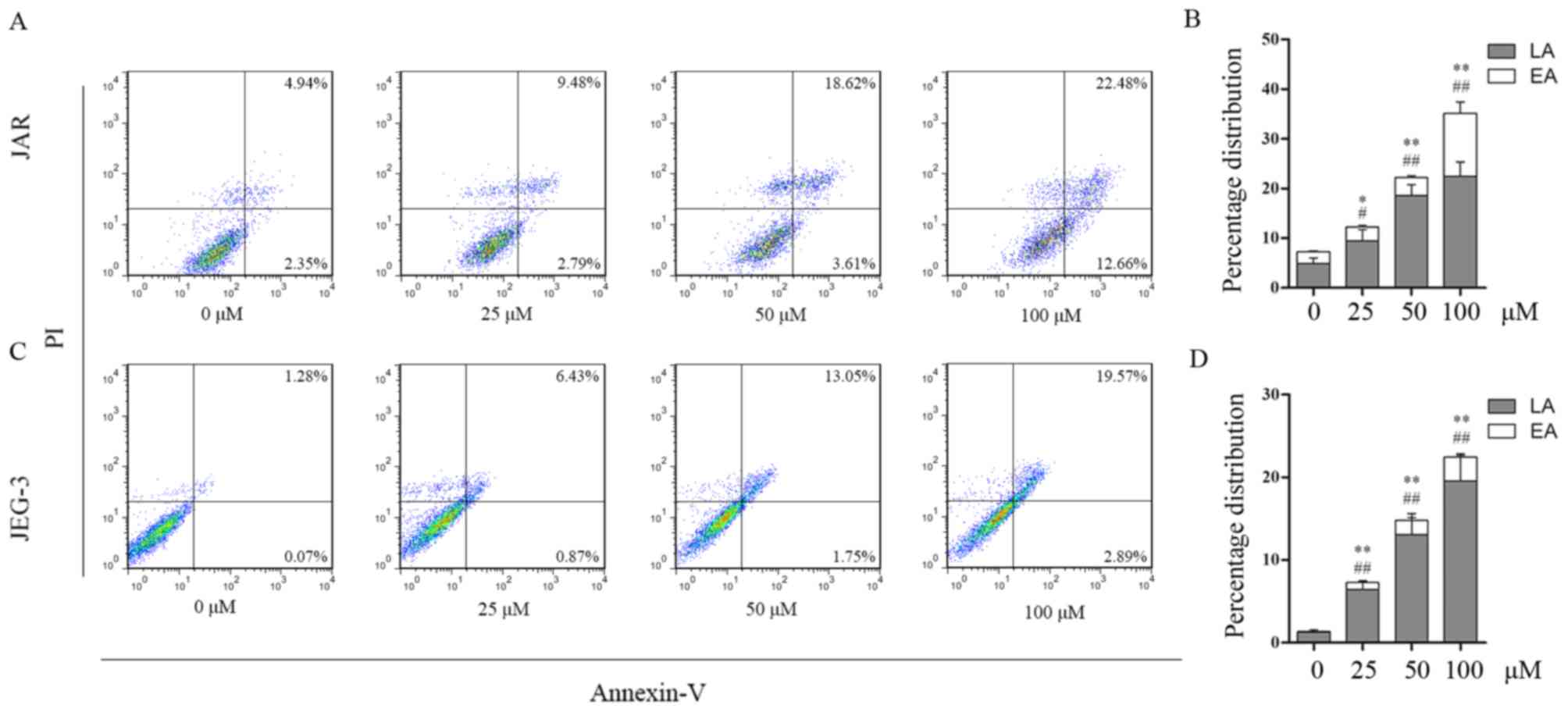
To determine whether apoptosis contributed to the
reduction in viability of daidzein-treated cells,
fluorescence-activated cell sorting analysis was performed to
detect apoptosis. The percentages of early and late apoptotic cells
increased following treatment with daidzein. In JAR cells, early
apoptotic cells accounted for 2.79, 3.61, 12.66% of the cell
population following treatment with 25, 50 and 100 µM,
respectively, whereas the percentage of early apoptotic cells was
2.35% in the control (0 µM) group (25 µM, P<0.05 vs. control; 50
and 100 µM, P<0.01 vs. control). The percentages of late
apoptotic cells following treatment with 0, 25, 50 and 100 µM
daidzein were 4.94, 9.48 18.62 and 22.48% respectively (25 µM,
P<0.05 vs. control; 50 and 100 µM, P<0.01 vs. control;
Fig. 3A and B). The percentage of
early apoptotic JEG-3 cells increased from 0.07% at 0 µM to 0.87,
1.75 and 2.89% at 25, 50 and 100 µM daidzein, respectively
(P<0.01), while the percentage of late apoptotic cells increased
from 1.28% at 0 µM to 6.43, 13.05 and 19.57% at 25, 50 and 100 µM,
respectively (P<0.01; Fig. 3C and
D). The aforementioned results indicated that daidzein induced
choriocarcinoma cell apoptosis in a dose-dependent manner. This
effect may be due to the larger proportion of late apoptotic
cells.
Daidzein alters the morphology of
choriocarcinoma cells
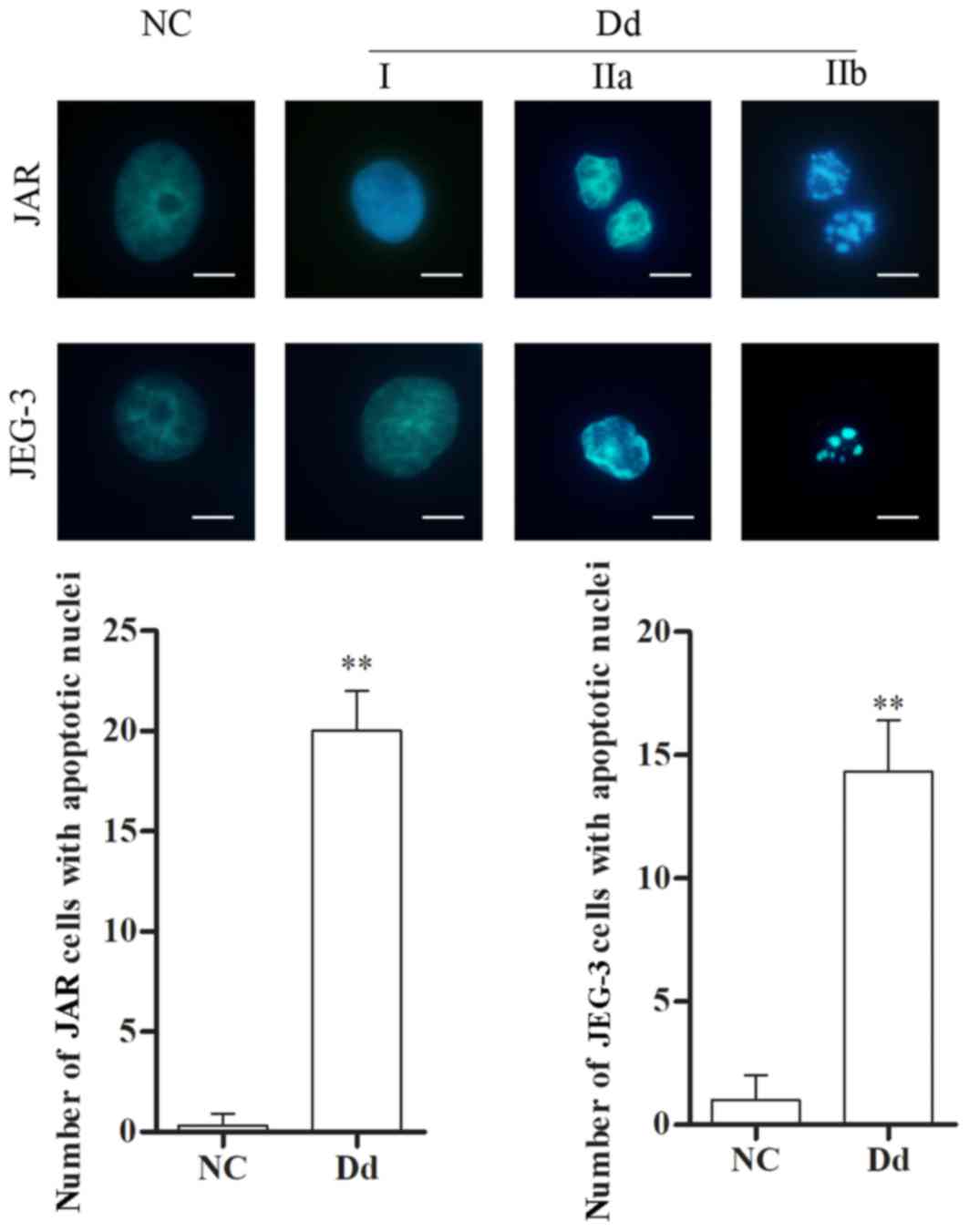
Morphological alterations are another manifestation
of cell apoptosis. Therefore, following treatment with 0 and 100 µM
daidzein for 48 h, morphological characteristics of JAR and JEG-3
cells were examined by fluorescence microscopy following DAPI
staining. In control cells (0 µM daidzein), the nuclei appeared
round, intact and uniformly stained, and few apoptotic cells were
observed. However, following treatment with daidzein, cell nuclei
exhibited different degrees of apoptosis, including: i) phase I,
where rippled or creased nuclei were observed, with mildly
condensed chromatin; ii) phase IIa, where chromatin was highly
condensed; and iii) phase IIb, where fragmented nuclei were
observed. The number of cells with apoptotic nuclear morphology
increased following treatment with daidzein, compared with the
untreated control (P<0.01 Fig.
4). The aforementioned results suggested that daidzein leads to
alterations in the nuclear morphology of choriocarcinoma cells,
thereby further indicating the pro-apoptotic effect of
daidzein.
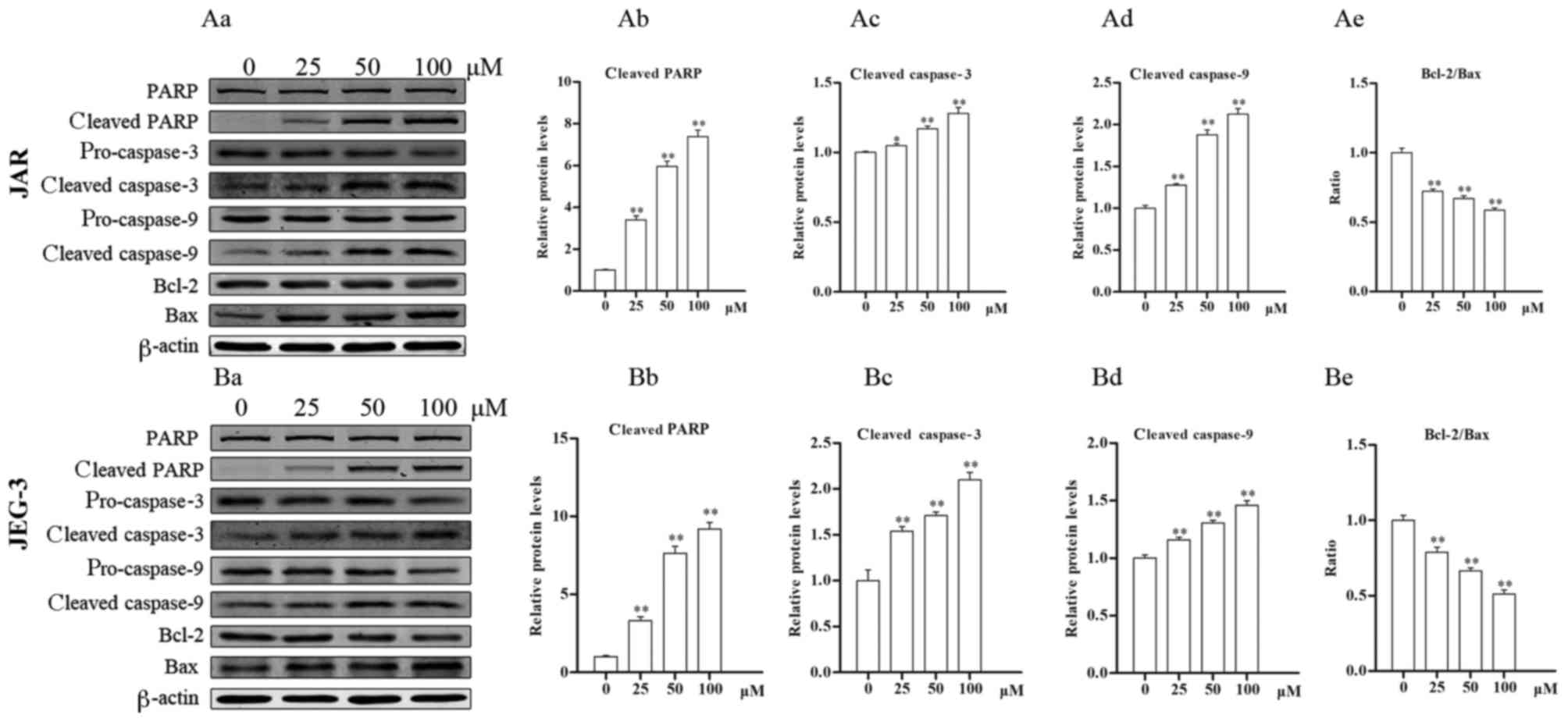
Daidzein affects the protein levels of
markers of apoptosis
The levels of apoptosis-associated proteins were
detected by western blotting. In JAR cells (Fig. 5Aa), levels of cleaved-PARP (all
P<0.05) and cleaved caspase-3 (25 µM, P<0.05 vs. control; 50
and 100 µM, P<0.01 vs. control) increased following treatment
with daidzein, compared with untreated cells (Fig. 5Ab and c), indicating the induction
of apoptosis. Furthermore, the levels of cleaved caspase-9
increased following treatment with daidzein, whereas the ratio of
Bcl-2 to Bax decreased, also in a dose-dependent manner (all
P<0.01; Fig. 5Ad and e).
Similar results were determined in JEG-3 cells (Fig. 5Ba), where following treatment with
daidzein, activation of caspase-9, caspase-3 and PARP, and a
decrease in the Bcl-2/Bax ratio were observed (all P<0.01;
Fig. 5Bb-e). These data indicate
that daidzein induces choriocarcinoma cell apoptosis via the
mitochondrial apoptotic pathway.
Discussion
Choriocarcinoma is the most malignant form of
gestational trophoblastic neoplasia (29). Although chemotherapy has notably
improved the prognosis of patients with choriocarcinoma in the
previous decades, a substantial proportion of patients develop
drug-resistance or relapse, while certain patients succumb to brain
or/and liver metastases (30).
Disruption of cellular processes, including the induction of
apoptosis, are likely to be involved tumorigenic mechanism of GTD
(31). Therefore, development of
treatments inducing apoptosis of choriocarcinoma cells may aid in
improving the prognosis of patients with GTD.
Daidzein belongs to the family of isoflavones, and
is abundant in soybeans and soy-based products; and therefore, may
be easily obtained from daily dietary intake (32–34).
Known as a type of phytoestrogen, daidzein has been reported to
serve an estrogen-like function in hormone-dependent cells,
including prostate cells, breast cells, and Sertoli and Leydig
cells of the testes (35–37). Daidzein exhibits anti-tumor
activity, including a pro-apoptotic function in numerous tumor
types. Daidzein has been demonstrated to induce apoptosis in MCF-7
breast cancer cell xenografts in rodents (38). Han et al (39) demonstrated that daidzein increases
the levels of reactive oxygen species and induces a decrease in
mitochondrial membrane potential, leading to apoptosis induction in
the BEL-7402 hepatocellular carcinoma cell line. In bladder cancer
cells, daidzein also induces cell apoptosis (40).
In choriocarcinoma, daidzein regulates production of
human chorionic gonadotropin (27)
and, as demonstrated in the authors' previous study, also inhibits
cell proliferation (28). In the
present study, in vitro experiments were performed to
determine whether daidzein exhibits a pro-apoptotic effect on
choriocarcinoma cells.
It was demonstrated by flow cytometry that daidzein
increased the percentage of early and late apoptotic JAR and JEG-3
cells, with the greatest increase observed in late apoptotic cells.
The aforementioned results demonstrated a positive association
between concentration of daidzein and induction of apoptosis.
Immunofluorescence analysis of DAPI-stained cells indicated that
alterations in nuclear morphology occurred following treatment with
daidzein, with round, intact and uniformly-stained nuclei becoming
rippled or creased, condensed and fragmented, indicating different
degrees of apoptosis. Furthermore, the levels of cleaved-PARP and
cleaved-caspase-3 in these two cell lines significantly increased
following treatment with daidzein. These results suggested that
daidzein may induce choriocarcinoma cell apoptosis in a
dose-dependent manner.
Daidzein induces apoptosis via the extrinsic
receptor-mediated pathway, intrinsic mitochondrial pathway or
endoplasmic reticulum stress pathway, depending on the type of
tumor (21). For example, daidzein
induces tumor necrosis factor-related apoptosis inducing ligand
(TRAIL)-mediated apoptosis in prostate cancer cells (41), however activates the
mitochondria-mediated pathway in breast cancer, gastric carcinoma
and hepatic cancer (22–24). Vilela et al (42) reported that bio-transformed soybean
extract containing daidzein increases expression of TRAIL and its
receptor DR4 in melanoma, resulting in cell apoptosis. Equol, which
is a metabolite of daidzein, induces apoptosis in SMMC-7721 human
hepatocellular carcinoma cells through the intrinsic and
endoplasmic reticulum stress pathways (26). Caspase-9 and the Bcl-2/Bax ratio
are commonly used activity markers of the mitochondrial apoptotic
pathway (43–45). In the present study, western
blotting was used to detect these markers, revealing that the
cleavage of caspase-9 increased and the ratio of Bcl-2/Bax
decreased in both cell lines in a dose-dependent manner, which
indicated that daidzein-induced apoptosis was mediated via the
mitochondrial apoptotic pathway. This process is similar to that in
the human gastric carcinoma cell line BGC-823, as Tang et al
(23) demonstrated that daidzein
induces apoptosis via downregulation of Bcl-2/Bax and triggering of
the mitochondrial pathway.
In conclusion, the present study demonstrated that
daidzein induced choriocarcinoma cell apoptosis in a dose-dependent
manner via the mitochondrial apoptotic pathway. These results
provide a novel insight into the potential application of daidzein
in the treatment of choriocarcinoma to improve therapeutic
efficiency.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81671491) and the
Youth Project Fund of the First Affiliated Hospital of Xi'an
Jiaotong University (grant no. 2015YK8).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WZ, RA and YX conceived and designed the study. WZ,
TL and RS developed the methodology and performed the experiments.
WZ and LY performed the statistical analysis. WZ and YX wrote the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brown J, Naumann RW, Seckl MJ and Schink
J: 15 years of progress in gestational trophoblastic disease:
Scoring, standardization, and salvage. Gynecol Oncol. 144:200–207.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berkowitz RS and Goldstein DP: Current
advances in the management of gestational trophoblastic disease.
Gynecol Oncol. 128:3–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ryu N, Ogawa M, Matsui H, Usui H and Shozu
M: The clinical characteristics and early detection of postpartum
choriocarcinoma. Int J Gynecol Cancer. 25:926–930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alazzam M, Tidy J, Osborne R, Coleman R,
Hancock BW and Lawrie TA: Chemotherapy for resistant or recurrent
gestational trophoblastic neoplasia. Cochrane Database Syst Rev.
12:CD0088912012.PubMed/NCBI
|
|
5
|
Essel KG, Bruegl A, Gershenson DM,
Ramondetta LM, Naumann RW and Brown J: Salvage chemotherapy for
gestational trophoblastic neoplasia: Utility or futility? Gynecol
Oncol. 146:74–80. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong SY, Ngan HY, Chan CC and Cheung AN:
Apoptosis in gestational trophoblastic disease is correlated with
clinical outcome and Bcl-2 expression but not Bax expression. Mod
Pathol. 12:1025–1033. 1999.PubMed/NCBI
|
|
7
|
Chiu PM, Ngan YS, Khoo US and Cheung AN:
Apoptotic activity in gestational trophoblastic disease correlates
with clinical outcome: Assessment by the caspase-related M30
CytoDeath antibody. Histopathology. 38:243–249. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fong PY, Xue WC, Ngan HY, Chan KY, Khoo
US, Tsao SW, Chiu PM, Man LS and Cheung AN: Mcl-1 expression in
gestational trophoblastic disease correlates with clinical outcome:
A differential expression study. Cancer. 103:268–276. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan
HY, Wong ES and Cheung AN: Downregulation of ASPP1 in gestational
trophoblastic disease: Correlation with hypermethylation, apoptotic
activity and clinical outcome. Mod Pathol. 24:522–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Braga A, Maesta I, Rocha Soares R, Elias
KM, Custódio Domingues MA, Barbisan LF and Berkowitz RS: Apoptotic
index for prediction of postmolar gestational trophoblastic
neoplasia. Am J Obstet Gynecol. 215:336.e1–336.e12. 2016.
View Article : Google Scholar
|
|
11
|
Wang TH and Wang HS: Gestational
trophoblastic diseases: Current trends and perspectives. J Formos
Med Assoc. 94:449–457. 1995.PubMed/NCBI
|
|
12
|
Patwardhan GA, Beverly LJ and Siskind LJ:
Sphingolipids and mitochondrial apoptosis. J Bioenerg Biomembr.
48:153–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang W, Chen Y, Li B and Gao S:
DBA-induced caspase-3-dependent apoptosis occurs through
mitochondrial translocation of cyt-c in the rat hippocampus. Mol
Biosyst. 13:1863–1873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang F, Yu X, Liu X, Zhou T, Nie T, Cheng
M, Liu H, Dai M and Zhang B: ABT-737 potentiates cisplatin-induced
apoptosis in human osteosarcoma cells via the mitochondrial
apoptotic pathway. Oncol Rep. 38:2301–2308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheikh BY, Sarker MMR, Kamarudin MNA and
Ismail A: Prophetic medicine as potential functional food elements
in the intervention of cancer: A review. Biomed Pharmacother.
95:614–648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liggins J, Mulligan A, Runswick S and
Bingham SA: Daidzein and genistein content of cereals. Eur J Clin
Nutr. 56:961–966. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Adjakly M, Ngollo M, Boiteux JP, Bignon
YJ, Guy L and Bernard-Gallon D: Genistein and daidzein: Different
molecular effects on prostate cancer. Anticancer Res. 33:39–44.
2013.PubMed/NCBI
|
|
22
|
Jin S, Zhang QY, Kang XM, Wang JX and Zhao
WH: Daidzein induces MCF-7 breast cancer cell apoptosis via the
mitochondrial pathway. Ann Oncol. 21:263–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang S, Hu J, Meng Q, Dong X, Wang K, Qi
Y, Chu C, Zhang X and Hou L: Daidzein induced apoptosis via
down-regulation of Bcl-2/Bax and triggering of the mitochondrial
pathway in BGC-823 cells. Cell Biochem Biophys. 65:197–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park HJ, Jeon YK, You DH and Nam MJ:
Daidzein causes cytochrome c-mediated apoptosis via the Bcl-2
family in human hepatic cancer cells. Food Chem Toxicol.
60:542–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lo YL: A potential daidzein derivative
enhances cytotoxicity of epirubicin on human colon adenocarcinoma
Caco-2 cells. Int J Mol Sci. 14:158–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang XL, Li M, Li J and Wang XL: Equol
induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells
through the intrinsic pathway and the endoplasmic reticulum stress
pathway. Anticancer Drugs. 25:633–640. 2014.PubMed/NCBI
|
|
27
|
Jeschke U, Briese V, Richter DU, Bruer G,
Plessow D, Waldschläger J, Mylonas I and Friese K: Effects of
phytoestrogens genistein and daidzein on production of human
chorionic gonadotropin in term trophoblast cells in vitro. Gynecol
Endocrinol. 21:180–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng W, Sun R, Yang L, Zeng X, Xue Y and
An R: Daidzein inhibits choriocarcinoma proliferation by arresting
cell cycle at G1 phase through suppressing ERK pathway in vitro and
in vivo. Oncol Rep. 38:2518–2524. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bruce S and Sorosky J: Gestational
trophoblastic disease. StatPearls. StatPearls Publishing StatPearls
Publishing LLC.; Treasure Island (FL): 2017
|
|
30
|
Seckl MJ, Sebire NJ and Berkowitz RS:
Gestational trophoblastic disease. Lancet. 376:717–729. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HW, Tsao SW and Cheung AN: Current
understandings of the molecular genetics of gestational
trophoblastic diseases. Placenta. 23:20–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laurenz R, Tumbalam P, Naeve S and Thelen
KD: Determination of isoflavone (genistein and daidzein)
concentration of soybean seed as affected by environment and
management inputs. J Sci Food Agric. 97:3342–3347. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin X, Sun J, Yu B, Wang Y, Sun WJ, Yang
J, Huang SH and Xie WL: Daidzein stimulates osteogenesis
facilitating proliferation, differentiation, and antiapoptosis in
human osteoblast-like MG-63 cells via estrogen receptor-dependent
MEK/ERK and PI3K/Akt activation. Nutr Res. 42:20–30. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhattarai K, Adhikari S, Fujitani M and
Kishida T: Dietary daidzein, but not genistein, has a
hypocholesterolemic effect in non-ovariectomized and ovariectomized
female Sprague-Dawley rats on a cholesterol-free diet. Biosci
Biotechnol Biochem. 81:1805–1813. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koo J, Cabarcas-Petroski S, Petrie JL,
Diette N, White RJ and Schramm L: Induction of proto-oncogene BRF2
in breast cancer cells by the dietary soybean isoflavone daidzein.
BMC Cancer. 15:9052015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu Y, Xu H, Li M, Gao Z, Huang J, Liu L,
Huang X and Li Y: Daidzein impairs Leydig cell testosterone
production and Sertoli cell function in neonatal mouse testes: An
in vitro study. Mol Med Rep. 14:5325–5333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Q, Feng H, Qluwakemi B, Wang J, Yao
S, Cheng G, Xu H, Qiu H, Zhu L and Yuan M: Phytoestrogens and risk
of prostate cancer: An updated meta-analysis of epidemiologic
studies. Int J Food Sci Nutr. 68:28–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu X, Suzuki N, Santosh Laxmi YR, Okamoto
Y and Shibutani S: Anti-breast cancer potential of daidzein in
rodents. Life Sci. 91:415–419. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Han BJ, Li W, Jiang GB, Lai SH, Zhang C,
Zeng CC and Liu YJ: Effects of daidzein in regards to cytotoxicity
in vitro, apoptosis, reactive oxygen species level, cell cycle
arrest and the expression of caspase and Bcl-2 family proteins.
Oncol Rep. 34:1115–1120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
He Y, Wu X, Cao Y, Hou Y, Chen H, Wu L, Lu
L, Zhu W and Gu Y: Daidzein exerts anti-tumor activity against
bladder cancer cells via inhibition of FGFR3 pathway. Neoplasma.
63:523–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Szliszka E and Krol W: Soy isoflavones
augment the effect of TRAIL-mediated apoptotic death in prostate
cancer cells. Oncol Rep. 26:533–541. 2011.PubMed/NCBI
|
|
42
|
Vilela FM, Syed DN, Chamcheu JC,
Calvo-Castro LA, Fortes VS, Fonseca MJ and Mukhtar H:
Biotransformed soybean extract (BSE) inhibits melanoma cell growth
and viability in vitro: Involvement of nuclear factor-kappa B
signaling. PLoS One. 9:e1032482014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Zhao L, Zhao X, Wang P, Liu Y and
Ruan J: Foxo1 attenuates NaF-induced apoptosis of LS8 cells through
the JNK and mitochondrial pathways. Biol Trace Elem Res.
181:104–111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shi XK, Bian XB, Huang T, Wen B, Zhao L,
Mu HX, Fatima S, Fan BM, Bian ZX, Huang LF and Lin CY: Azoxystrobin
induces apoptosis of human esophageal squamous cell carcinoma
KYSE-150 cells through triggering of the mitochondrial pathway.
Front Pharmacol. 8:2772017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu J, Cai Y, Li M, Zhang Y, Li H and Tan
Z: Oxymatrine promotes S-Phase arrest and inhibits cell
proliferation of human breast cancer cells in vitro through
Mitochondria-mediated apoptosis. Biol Pharm Bull. 40:1232–1239.
2017. View Article : Google Scholar : PubMed/NCBI
|