Introduction
Dendrobium is a large genus of orchids, and
Dendrobium candidum Wall. ex Lindl. (D. candidum) is
a functional analog of Dendrobium moniliforme (L.) Sw.
(1,2). It is a traditional Chinese medicinal
herb that is used either raw or processed for healthcare products
in China (3). D. candidum
contains water-soluble polysaccharides, phenanthrenes and numerous
amino acids. High contents of chrysotoxen and erianin may have
inhibitory activities in liver cancer and ehrlich ascites carcinoma
cells (4).
Metastasis is defined as the spread of cancer cells
from one organ or area to another adjacent organ or location
(5) and it is considered that
malignant tumor cells have the capacity to metastasize. Cancer can
occur in cells of a tissue that are genetically mutated in a
progressive manner, resulting in cancer stem cells possessing a
malignant phenotype (6). Metastasis
is the leading cause of mortality among cancer patients, and
involves the spread of cancer from a primary site and formation of
new tumors in distant organs (7).
Matrix metalloproteinases (MMPs) function in numerous physiological
and pathological processes, including embryonic development,
morphogenesis, reproduction, tissue remodeling, arthritis,
cardiovascular disease and metastasis (8). MMP activity is inhibited by specific
endogenous tissue inhibitors of metalloproteinases (TIMPs)
(9). To prevent the majority of
cancer types, improved treatments against metastasis are needed
(10).
D. candidum has been previously shown to
exhibit strong in vitro anticancer effects on HeLa S3 human
cervical carcinoma cells and HepG2 liver cancer cells (9). In the present study, the
anti-metastatic effects of D. candidum were investigated in
mice injected with 26-M3.1 colon carcinoma cells, and the molecular
mechanisms underlying the anti-metastatic effects of the D.
candidum were studied. The in vivo anti-metastatic
effects were determined by tumor count, cytokine levels, and mRNA
and protein expression experiments. The association between the
anticancer activities and functional components of D.
candidum was additionally explored.
Materials and methods
Preparations of D. candidum
D. candidum was purchased from Shanghai
Pharmacy Co., Ltd. (Shanghai, China). The D. candidum was
stored at −80°C and freeze-dried to produce a powder. A 20-fold
volume of boiling water was added to the powdered sample and
extracted twice by stirring overnight. The aqueous extract was
evaporated and concentrated using a rotary evaporator (N-1100;
Eyela, Tokyo, Japan).
Anti-metastatic effects of D. candidum in
mice bearing 26 M3.1 cells
The following experiment was performed according to
the methods of a previous study (11). 26-M3.1 colon carcinoma cells were
obtained from Professor Yoon (Department of Food and Nutrition,
Yuhan University, Bucheon, South Korea). The metastatic cells were
cultured in Eagle’s minimum essential medium (Gibco-BRL, Carlsbad,
CA, USA) supplemented with 7.5% FBS (fetal bovine serum), a vitamin
solution, sodium pyruvate, non-essential amino acids and
L-glutamine (Gibco-BRL) by 5% CO2 at 37°C. The
6-week-old female Balb/c mice (Experimental Animal Center of
Chongqing Medical University, Chongqing, China) were induced lung
metastasis by injecting colon 26-M3.1 cells. The experimental mice
were divided three groups, there were 20 mice in each group. The
control group of mice was without any treatment for 2 weeks. The
D. candidum group mice were treated with D. candidum
aqueous extract solutions (200 and 400 mg/kg b.w.) by gavage for 2
weeks. After 2 weeks, all the mice were intravenously inoculated
with 26-M3.1 cells at the concentration of
2.5×104/mouse. Two days later, the mice were sacrificed
and the lungs of 10 mice in each group were fixed in Bouin’s
solution (saturated picric acid: formalin: acetic acid; 15:5:1,
v/v/v) (12). Then the rates of
metastasis were determined by counting tumor colonies in the photos
(Canon D550; Canon, Tokyo, Japan). Inhibitory rate = [(lung tumor
number of control mice - lung tumor number of D. candidum treated
mice)/lung tumor number of control mice] × 100%. The other lungs of
10 mice in each group were tested for reverse transcription
(RT)-PCR and western blot assays. The protocol for these
experiments was approved by the Animal Ethics Committee of
Chongqing Medical University.
Analysis of IL-6, IL-12, TNF-α and IFN-γ
cytokines in serum by ELISA
For the serum cytokine assay, blood from the
inferior vena cava was collected in a tube and centrifuged at 1100
× g and 4°C for 10 min. The serum was aspirated and assayed as
described below. Concentrations of cytokines of IL-6, IL-12, TNF-α
and IFN-γ in serum were measured using mouse IL-6, IL-12, TNF-α and
IFN-γ ELISA kits according to the manufacturer’s instructions
(Biolegend, San Diego, CA, USA). Briefly, biotinylated antibody
reagent was added to 96-well plates, then supernatants of
homogenized serum were added and the plates were incubated at 37°C
in CO2 for 2 h. After washing with phosphate-buffered
saline (PBS), streptavidin-horseradish peroxidase (HRP) solution
was added and the plate was incubated for 30 min at room
temperature. The absorbance was measured at 450 nm using a
microplate reader (iMark; Bio-Rad, Hercules, CA, USA) (13).
RT-PCR
RT-PCR was performed according to the methods
descirbed in a previous study (11). The RNA of lung tissue was treated
with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) for extraction. After total RNA was digested with RNase-free
DNase (Roche, Diagnostics Basel, Switzerland) for 15 min at 37°C,
then the digested RNA was purified by RNeasy kit (Qiagen, Hilden,
Germany). The cDNA was synthesized from 2 μg of total RNA at 37°C
for l h with AMV reverse transcriptase (GE Healthcare, Little
Chalfont, Buckinghamshire, UK) (2).
Sequences of Bax, Bcl-2, MMPs and TIMPs were specifically amplified
(Table I) by thermal cycler
(Eppendorf, Hamburg, Germany). The PCR products were separated in
1.0% agarose gels and visualized with ethidium bromide
staining.
 | Table IPrimer sequences used for quantitative
polymerase chain reaction. |
Table I
Primer sequences used for quantitative
polymerase chain reaction.
| Gene name | Sequence |
|---|
| Bax |
| Forward |
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ |
| Reverse |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| Bcl-2 |
| Forward |
5′-CTCGTCGCTACCGTCGTGACTTGG-3′ |
| Reverse |
5′-CAGATGCCGGTTCAGGTACTCAGTC-3′ |
| MMP-2 |
| Forward |
5′-CTTCTTCAAGGACCGGTTCA-3′ |
| Reverse |
5′-GCTGGCTGAGTACCAGTA-3′ |
| MMP-9 |
| Forward |
5′-TGGGCTACGTGACCTATGAC-3′ |
| Reverse |
5′-GCCCAGCCCACCTCCACTCC-3′ |
| TIMP-1 |
| Forward |
5′-GTCAGTGAGAAGCAAGTCGA-3′ |
| Reverse |
5′-ATGTTCTTCTCTGTGACCCA-3′ |
| TIMP-2 |
| Forward |
5′-TGGGGACACCAGAAGTCAAC-3′ |
| Reverse |
5′-TTTTCAGAGCCTTGGAGGAG-3′ |
| GAPDH |
| Forward |
5′-CGGAGTCAACGGATTTGGTC-3′ |
| Reverse |
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Protein extraction and western blot
analysis in the gastric tissue
Total lung tissue protein was obtained with
radioimmunoprecipitation assay buffer as described (12). Protein concentrations were
determined with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA,
USA). For the western blot analysis, aliquots of the lysate
containing 30–50 μg protein were separated by SDS-PAGE and then
electrotransferred onto a nitrocellulose membrane (Schleicher and
Schuell, Keene, NH, USA). The membranes were subjected to
immunoblot analysis and the proteins were visualized by an enhanced
chemiluminescence (ECL) method (Amersham ECL GST Western Blotting
Detection kit, product code: RPN1237; GE Healthcare) then
transferred onto a polyvinylidene fluoride membrane (GE
Healthcare), prior to blocking with 5% non-fat milk and
hybridization with primary antibodies (diluted 1:1,000). The
antibodies against Bax, Bcl-2, MMPs and TIMPs were obtained from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The membranes
were then incubated with a horseradish peroxidase-conjugated
secondary antibody (Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The blots were washed three times with PBS-Tween and
then developed by ECL using GE Healthcare ECL prime western
blotting detection reagent (GE Healthcare).
Component analysis by nuclear magnetic
resonance (NMR)
Dried D. candidum was refluxed and extracted
three times with 10 times amount of ethyl acetate. The ethyl
acetate extract was obtained after 1 h for every reflux extraction
and decompression concentrating extraction. The total ethyl acetate
extract was extracted by anhydrous ethanol three times. The ethanol
extract thus produced was resuspended in water before extraction by
petroleum ether, chloroform and butanol, respectively, as follows.
Firstly, the ethyl acetate extract was treated by gradient elution
in a silica gel column with a petroleum ether-ethyl acetate system.
Secondly, the chloroform extract was treated by gradient elution in
a silica gel column with a petroleum chloroform-methanol system.
Finally, the butanol extract was agitated by water ultrasonic,
filtered and then eluted by an HP2MGL macroporous resin column with
water, 10% ethanol, 30% ethanol and 60% ethanol, respectively.
Following elution, the different solvents had eluted various
compounds, and their composition could be identified by NMR (Varian
INOVO 400; Varian Inc., Palo Alto, CA, USA). The NMR was set at an
1H frequency of 300 MHz, temperature of 25°C, pulse
length of 8 μsec and spin speed of 20 Hz, and scanned 64 times. The
1H-NMR spectra were recorded using a standard
high-resolution magicangle spinning probe with magic-angle
gradient.
Statistical analysis
Data are presented as the means ± standard
deviation. Differences between the mean values for individual
groups were assessed by one-way analysis of variance with Duncan’s
multiple range test. P<0.05 was considered to indicate a
statistically significant difference. SAS, version 9.1 (SAS
Institute, Inc., Cary, NC, USA) was used for statistical
analyses.
Results
In vivo anti-metastatic effects of D.
candidum
26-M3.1 colon carcinoma cells were used to evaluate
the anti-metastatic effects of D. candidum in vivo.
Prophylactic inhibition of tumor metastasis by D. candidum
was evaluated by using an experimental mouse metastasis model
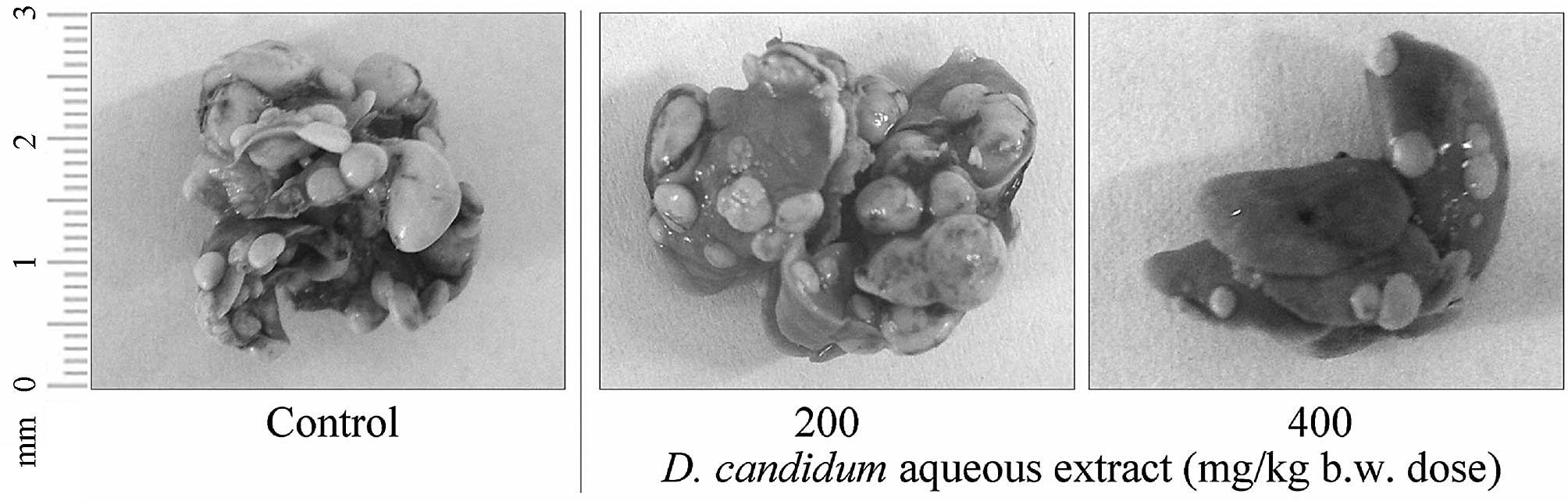
(Fig. 1). D.
candidum-treated mice had significantly fewer lung metastatic
colonies as compared with control mice (number of metastatic
tumors, 62±6; P<0.05). A dose of 400 mg/kg b.w. D.
candidum was the most effective at inhibiting lung metastasis.
This concentration (inhibitory rate, 64.5%; number of metastatic
tumors, 22±3) inhibited tumor formation and lung metastasis to a
greater degree as compared with a dose of 200 mg/kg solution
(inhibitory rate, 46.8%; number of metastatic tumors, 33±4).
IL-6, IL-12, TNF-α and IFN-γ serum levels
in mice
The control mice showed the highest serum levels of
IL-6, IL-12, TNF-α and IFN-γ. These levels were significantly
decreased in D. candidum-treated mice (P<0.05, Table II). A higher concentration of 400
mg/kg b.w. D. candidum was more effective as compared with
the 200 mg/kg b.w. dose in promoting a decrease in cytokine serum
levels.
 | Table IISerum IL-6, IL-12, TNF-α, and IFN-γ
levels of mice bearing 26-M3.1 cells treated with D.
candidum. |
Table II
Serum IL-6, IL-12, TNF-α, and IFN-γ
levels of mice bearing 26-M3.1 cells treated with D.
candidum.
| Treatment | IL-6 | IL-12 | TNF-α | IFN-γ |
|---|
| Control
(untreated) | 235.7±33.8a | 712.1±55.8a | 84.1±12.0a | 80.5±7.7a |
| D. candidum
(mg/kg) |
| 200 | 172.7±21.6b | 571.7±42.9b | 61.2±4.9b | 63.8±5.9b |
| 400 | 113.0±26.1c | 338.6±39.7c | 50.6±6.6c | 44.8±6.2c |
Apoptosis-related gene expression of Bax
and Bcl-2 in the lung
To determine which apoptotic pathways were induced
by D. candidum, the mice were treated with 200 and 400 mg/kg
b.w. dose, and the lung tissues were dissected and analyzed for
apoptosis-related gene expression by RT-PCR and western blotting.
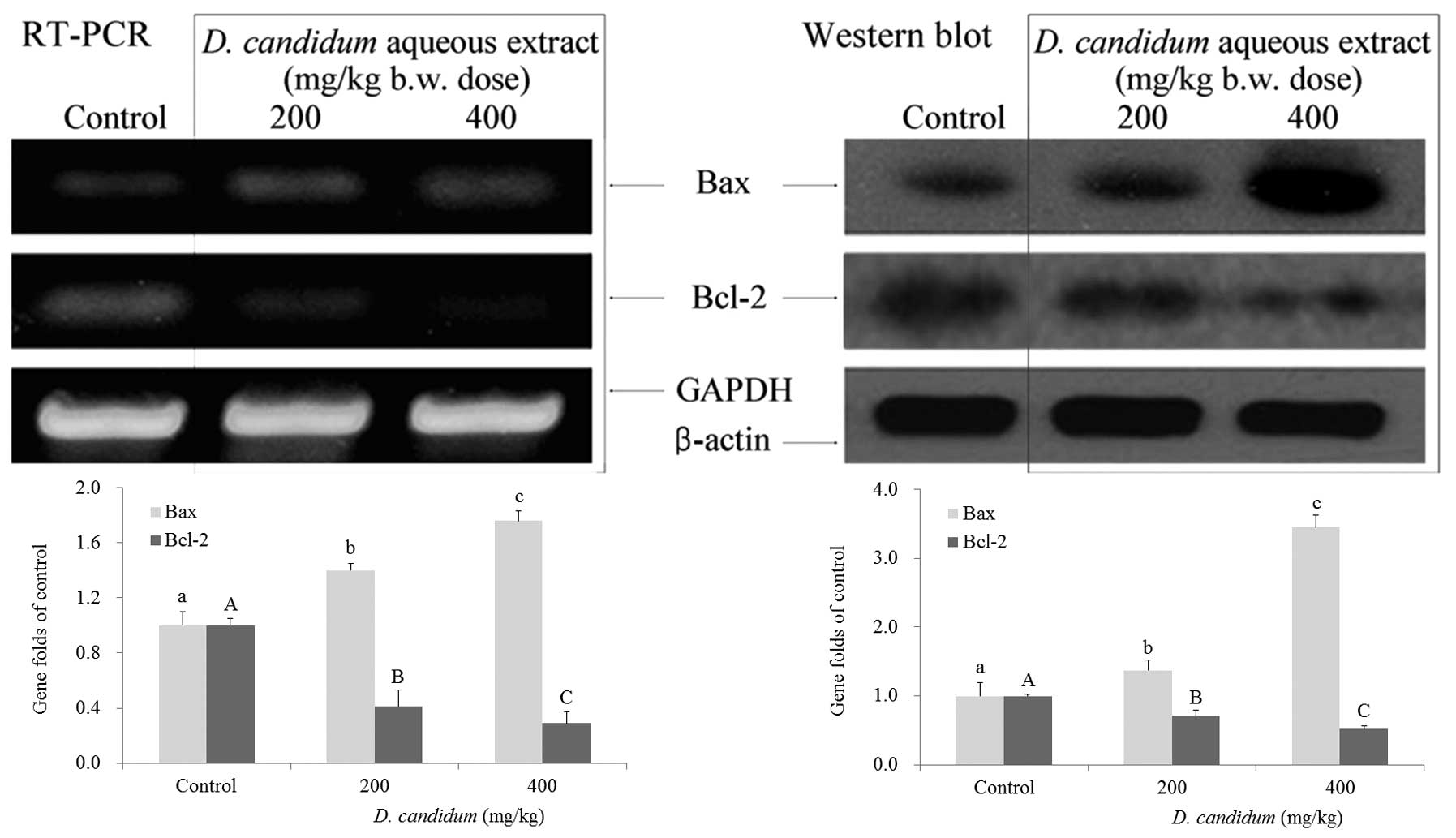
As shown in Fig. 2, in the presence
of D. candidum, there were significant differences
(P<0.05) in the expression of Bax and Bcl-2, with an increase in
Bax expression and a reduction in Bcl-2 expression, as determined
by RT-PCR. The Bax gene expression increased with D.
candidium treatment, in a dose dependent manner, and Bcl-2 gene
expression showed a crosscurrent when mice were treated with D.
candidium (P<0.05). The results suggested that D.
candidum induced apoptosis in 26-M3.1 cell-injected lung
metastatic mice through a Bax- and Bcl-2-dependent pathway. The
increased expression of Bax and decreased expression of Bcl-2
induced by 400 mg/kg D. candidum was more notable at the
mRNA expression level, as compared with the 200 mg/kg dose. From
these results, D. candidum showed good anticancer effects in
its ability to induce apoptosis, and these effects were observed in
a dose-dependent manner.
Metastasis-related gene expression of
MMPs and TIMPs in the lung
RT-PCR and western blot analysis was conducted to
investigate whether the inhibitory effects of D. candidum on
metastasis were due to gene regulation of metastatic mediators,
such as MMPs (MMP-2 and MMP-9) and TIMPs (TIMP-1 and TIMP-2). The
expression of MMPs and TIMPs was therefore analyzed in lung tissues
taken from control and D. candidum-treated mice. As shown in
Fig. 3, D. candidum
significantly decreased the mRNA and protein expression of MMP-2
and MMP-9 (P<0.05), and significantly increased the expression
of TIMP-1 and TIMP-2 (P<0.05). The most prominent
anti-metastatic effects were associated with the most marked
decrease in expression of MMP-2 and MMP-9, together with the most
marked increase in expression of TIMP-1 and TIMP-2. These results
suggested that the higher 400 mg/kg dose of D. candidum,
cultivated in the presence of sulfur, could elicit a stronger
anti-metastatic activity as compared with the lower 200 mg/kg
dose.
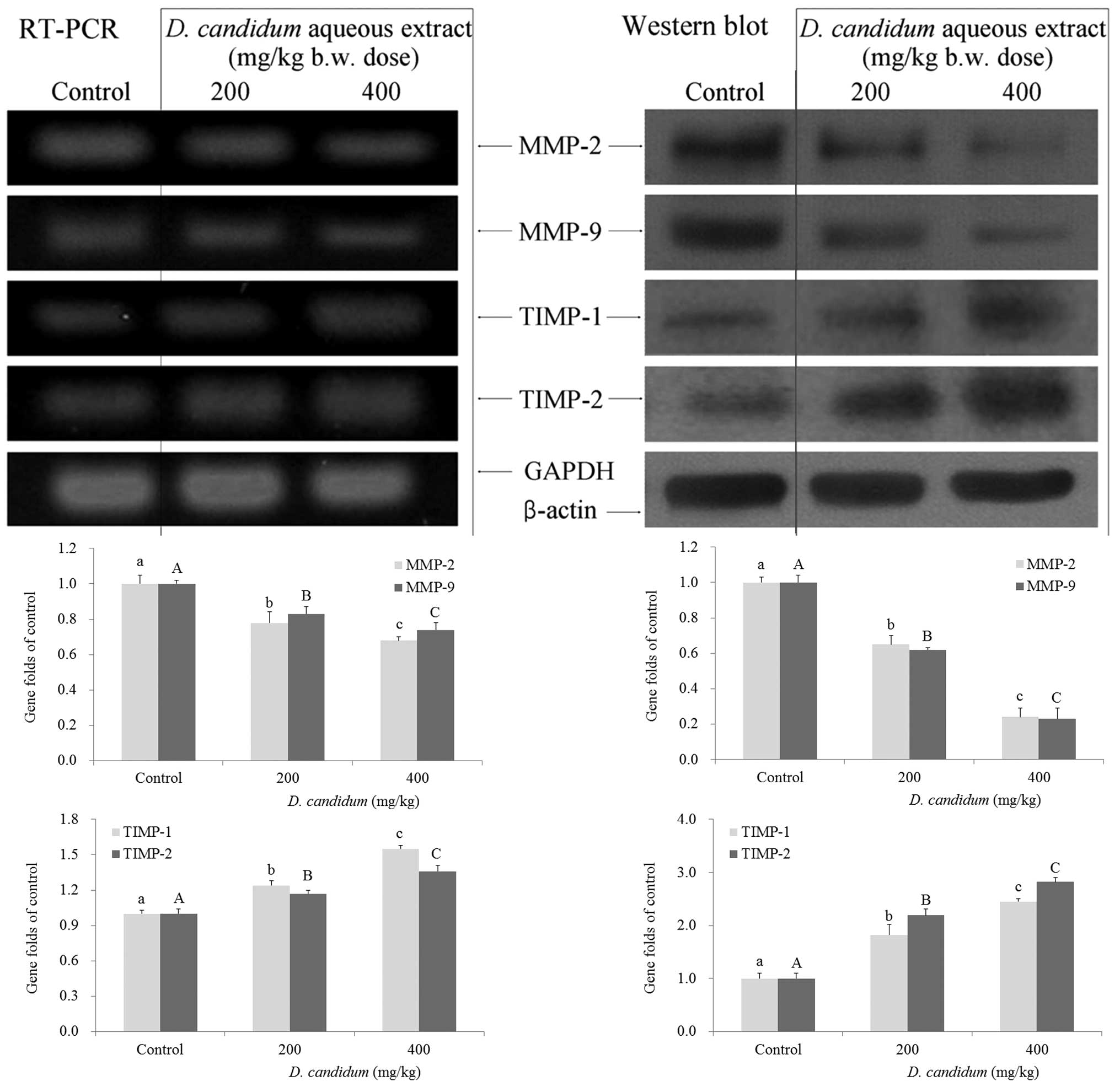 | Figure 3Effects of aqueous extract from D.
candidum on the mRNA (left) and protein (right) expression of
MMPs and TIMPs in mice. Fold ratio = gene expression/GAPDH ×
control numerical value (control fold ratio: 1).
a–c,A–CMean values with different letters over the bars
are significantly different (P<0.05) according to Duncan’s
multiple-range test. a,AP<0.05, vs. the control;
b,BP<0.05, vs. the 200 mg/kg D. candidum dose;
c,CP<0.05, vs. the 400 mg/kg D. candidum dose.
D. candidum, Dendrobium candidum; MMP, matrix
metalloproteinase; TIMP, tissue inhibitor of matrix
metalloproteinases; RT-PCR, quantitative polymerase chain
reaction. |
Content of the D. candidum leaf
Eleven compounds were isolated and identified from
the D. candidum leaf. Compound 1 was obtained as a clear
crystal, and the 1H-NMR spectrum of this compound was as
follows: δ 6.92 (2H, d), 6.62 (2H, d), 6.06 (2H, s), 6.03 (1H, s),
2.65 (4H, m). This compound was confirmed as dihydrogen
resveratrol. Compound 2 was obtained as a white powder, and the
1H-NMR spectrum of this compound was as follows: δ 6.98 (2H, d),
6.74 (2H, d), 6.62 (1H, s), 6.47 (1H, d), 4.83 (1H,d), 4.63 (1H,
d), 3.1–3.8 (12H), 3.73 (3H, s), 3.69 (3H, s), 2.74 (4H, m). This
compound was confirmed as dendromoniliside E. Compound 3 was
obtained as a black red needle, and the 1H-NMR spectrum
of this compound was as follows: δ 11.00 (1H, s), 8.15 (1H, d),
6.06 (2H, s), 8.07 (1H, d), 6.95 (1H, s), 6.83 (1H, s), 6.15 (1H,
s), 3.96 (3H, s), 3.93 (3H, s). This compound was confirmed as
denbinobin. Compound 4 was obtained as a colorless needle, and the
1H-NMR spectrum of this compound was as follows: δ 4.72
(2H, m), 3.85 (1H, d), 6.06 (2H, s), 2.53 (1H, d), 2.49 (1H, t),
2.39 (1H, dd), 2.21 (1H, dd), 1.64 (1H, m), 1.35 (3H, s), 1.03 (3H,
d), 0.95 (3H, d). This compound was confirmed as aduncin. Compound
5 was obtained as a white needle, and the 1H-NMR
spectrum of this compound was as follows: δ 8.25 (1H, s), 8.10 (1H,
s), 5.90 (1H, d), 4.66 (1H, dd), 3.5–4.2 (4H, m). This compound was
confirmed as adenosine. Compound 6 was obtained as a white powder,
and the 1H-NMR spectrum of this compound was as follows:
δ 7.95 (1H, d), 5.85 (1H, d), 5.66 (1H, d), 3.2–4.3 (5H, m). This
compound was confirmed as uridine. Compound 7 was obtained as a
clear crystal, and the 1H-NMR spectrum of this compound
was as follows: δ 10.60 (1H, s), 7.92 (1H, s), 6.45 (2H, s), 5.66
(1H, d), 3.4–4.4 (5H, m). This compound was confirmed as guanosine.
Compound 8 was obtained as a white powder, and the
1H-NMR spectrum of this compound was as follows: δ 7.65
(1H, d), 7.41 (2H, d), 6.85 (2H, d), 6.33 (1H, d), 4.17 (2H, t),
1.69 (2H,m), 1.25 (54H, m), 0.85 (3H, t). This compound was
confirmed as defuscin. Compound 9 was obtained as a white powder,
and the 1H-NMR spectrum of this compound was as follows:
δ 7.45 (2H, d), 6.82 (2H, d), 6.81 (1H, d), 5.83 (1H, d), 4.16 (2H,
t), 1.67 (2H, m), 1.23 (54H, m), 0.88 (3H, t). This compound was
confirmed as n-triacontyl cis-p-coumarate.
Compound 10 was obtained as a white powder, and the
1H-NMR spectrum of this compound was as follows: δ 2.35
(2H, t), 1.62 (2H, m), 1.25 (24H, m), 0.88 (3H, t). This compound
was confirmed as hexadecanoic acid. Compound 11 was obtained as a
white powder, and the 1H-NMR spectrum of this compound
was as follows: δ 3.85 (2H, t), 1.75 (2H, m), 1.45 (2H, m), 1.22
(54H, m), 0.85 (3H, t). This compound was confirmed as
hentriacontane.
Discussion
Although D. candidum has been used as a
traditional Chinese medicine, there has been little scientific
research regarding its mechanism of action. D. candidum
contains high concentrations of benzenes and their derivatives,
phenolic, lignans, lactone, flavonoids and 18 novel D.
candidum pigments (14). D.
candidum has been previously reported to have various
therapeutic effects on numerous pathological conditions, including
inflammation, immunity, hyperglycemia and cancer (15).
Lower levels of IL-6, IL-12, IFN-γ and TNF-α
cytokines are indicative of improved anticancer effects (16,17).
IL-6 is regarded as an important tumor-promoting factor in various
types of human cancer. An increased expression of IL-6 has been
found in patients with cancer, in serum and tumor tissue (18). IL-12 has been shown to contribute to
tumor eradication, through IFN-γ-dependent induction of the
anti-angiogenic factors interferon-inducible protein 10 and
monokine induced by gamma interferon (19). In addition, a previous study has
shown that drugs targeting TNF-α may be useful for the treatment of
cancers (13). In the present
study, it was observed that the levels of IL-6, IL-12, TNF-α and
IFN-γ in mice injected with 26-M3.1 cells were markedly decreased
following D. candidum treatment. Based on this study, D.
candidum showed a strong preventive effect on the development
of lung metastases.
Tumor cells are able to migrate to another site,
penetrate the vessel walls, continue to multiply and eventually
form another tumor. Colon 26-M3.1 carcinoma cells have been
previously used to evaluate anti-metastatic effects in vivo
(20). Based on in vivo data
from previous studies, 26-M3.1 colon carcinoma cells were used to
examine the effects of D. candidum on metastasis in mice.
The results further proved the activity of D. candidum, and
the observed anticancer effects occurred in a dose-dependent
manner.
Apoptosis is a fundamental cell event, and
understanding its mechanisms of action will have a significant
effect on antitumor therapy. The Bcl-2 family, which includes
promoters (Bax and Bid) and inhibitors (Bcl-2 and Bcl-xL), is a key
regulator in mitochondria-mediated apoptosis (21). In the present study, the gene and
protein expression of Bax was increased, whereas the protein
expression of Bcl-2 was decreased following treatment with D.
candidum. Based on the gene expression results, D.
candidum showed strong activity in promoting apoptosis in
cancer.
MMPs comprise a family of zinc-dependent
endopeptidases that function in tumorigenesis and metastasis
(22). MMPs can cleave the majority
of all extracellular matrix (ECM) substrates. Degradation of the
ECM is a key event in tumor progression, invasion and metastasis
(23). Among the MMP family
members, MMP-2 and MMP-9 are molecules important for cancer
invasion, and have been shown to be highly expressed in cancer
cells (24). Inhibition of MMP
activity is useful for controlling tumorigenesis and metastasis
(25). TIMPs are naturally
occurring inhibitors of MMPs that prevent catalytic activity by
binding to activated MMPs, thereby blocking the degradation of the
ECM. Disturbances in the ratio between MMPs and TIMPs have been
observed during tumorigenesis (26). Maintaining the balance between MMPs
and TIMPs, or increasing TIMP activity, are useful methods by which
to control tumor metastasis (27).
In the present study, strong anti-metastatic effects were
correlated with a reduction of MMPs and an increase of TIMPs
following administration of D. candidum in mice. From the
results, D. candidum showed a strong anti-metastatic effect
and, therefore, may be a functional drug for cancer prevention.
Numerous compounds were isolated and identified by
NMR of the D. candidum leaf in the present study.
Resveratrol is an antioxidant that has been often recommended for
use as treatment in patients with colon cancer (28). Denbinobin is a biologically active
chemical that has been demonstrated to inhibit colon cancer growth
both in vitro and in vivo (29). Aduncin is a unique component that
has only been found in Dendrobium. Aduncin may have anticancer
effects, but its function requires further research (30). Adenosine serves as a physiological
regulator and acts as a cardio-, neuro- and chemo-protector, and as
an immunomodulator. Adenosine has been shown to exert anticancer
effects at certain concentration. When administered in combination
with chemotherapy, adenosine can enhance the chemotherapeutic index
and acts as a chemoprotective agent (31). The availability of uridine can alter
the sensitivity of tumor cells to antimetabolites. Adenine is
incorporated into polynucleotides and the two compounds have been
identified as important cancer-associated substances (32). Defuscin, n-triacontyl
cis-p-coumarate, hexadecanoic acid and hentriacontane
have also shown functional activities in human health (33,34).
Taken together, these compounds all exhibit anticancer activities,
and with the high content of functional compounds, this may explain
why D. candidum showed a functional effect in cancer
prevention. The synergy of these bioactive components may increase
the anticancer effects of D. candidum.
In summary, the present study found that D.
candidum has potent in vivo anti-metastatic activity,
particularly in the inhibition of in vivo tumor metastasis.
The analysis of cytokine levels, as well as mRNA and protein
expression, has provided a mechanistic basis for these functional
effects and a scientific basis for the development of D.
candidum in cancer therapy.
Acknowledgements
The present study was supported by the Program for
Innovation Team Building at Institutions of Higher Education in
Chongqing (grant no. KJTD201325).
References
|
1
|
Jones WE, Kuehnle AR and Arumuganathan K:
Nuclear DNA content of 26 orchids (Orchidaceae) genera with
emphasis on Dendrobium. Ann Bot. 82:189–194. 1998.
|
|
2
|
Wang Q, Sun P, Li G, Zhu K, Wang C and
Zhao X: Inhibitory effects of Dendrobium candidum Wall ex
Lindl. on azoxymethane- and dextran sulfate sodium-induced colon
carcinogenesis in C57BL/6 mice. Oncol Lett. 7:493–498. 2014.
|
|
3
|
Xiao F and Zhang JZ: First report of
Fusarium oxysporum causing wilt of Dendrobium
candidum in Zhejiang province, China. Plant Dis.
96:13772012.
|
|
4
|
Shao H, Zhang LQ, Li JM and Wei RC:
Advances in research of Dendrobium officinale. Chinese
Tradit Herbal Drugs. 35:109–111. 2004.
|
|
5
|
Chiang AC and Massagué J: Molecular basis
of metastasis. N Engl JMed. 359:2814–2823. 2008.
|
|
6
|
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer
J and Terzis AJ: Opinion: the origin of the cancer stem cell:
current controversies and new insights. Nat Rev Cancer. 5:899–904.
2005.
|
|
7
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998.
|
|
8
|
Itoh Y and Nagase H: Matrix
metalloproteinases in cancer. Essays Biochem. 38:21–36. 2002.
|
|
9
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors ofmetalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000.
|
|
10
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002.
|
|
11
|
Zhao X, Wang Q, Qian Y and Song JL:
Ilex kudingcha C.J. Tseng (Kudingcha) has in vitro
anticancer activities in MCF-7 human breast adenocarcinoma cells
and exerts anti-metastatic effects in vivo. Oncol Lett.
5:1744–1748. 2013.
|
|
12
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
|
|
13
|
Chen LH, Song JL, Qian Y, Zhao X, Suo HY
and Li J: Increased preventive effect on colon carcinogenesis by
use of resistant starch (RS3) as the carrier for polysaccharide of
Larimichthys crocea swimming bladder. Int J Mol Sci.
15:817–829. 2014.
|
|
14
|
Li Y, Wang CL, Wang FF, Dong HL, Guo SX,
Yang JS and Xiao PG: Chemical constituents of Dendrobium
candidum. China J Chin Mater Med. 13:1715–1719. 2010.
|
|
15
|
Shao H, Zhang LQ, Li JM and Wei RC:
Inhibitory effects of water extracts from four species of
Dendrobiums on HelaS3 cells and HepG2 cells. J Anhui Agri Sci.
36:15968–15970. 2008.
|
|
16
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007.
|
|
17
|
Cahlin C, Körner A, Axelsson H, Wang WH,
Lundholm K and Svanberg E: Experimental cancer cachexia: the role
of host-derived cytokines interleukin (IL)-6, IL-12,
interferon-gamma, and tumor necrosis factor alpha evaluated in gene
knockout, tumor-bearing mice on C57 Bl background and
eicosanoid-dependent cachexia. Cancer Res. 60:5488–5493. 2000.
|
|
18
|
Kishimoto T, Akira S and Taga T:
Interleukin-6 and its receptor: a paradigm for cytokines. Science.
258:593–597. 1992.
|
|
19
|
Engel MA and Neurath MF: Anticancer
properties of the IL-12 family - focus on colorectal cancer. Curr
Med Chem. 17:3303–3308. 2010.
|
|
20
|
Ha ES, Hwang SH, Shin KS, Yu KW, Lee KH,
Choi JS, Park WM and Yoon TJ: Anti-metastatic activity of
glycoprotein fractionated from Acanthopanax senticosus, involvement
of NK-cell and macrophage activation. Arch Pharm Res. 27:217–224.
2004.
|
|
21
|
Zhao X, Song JL, Kim JD, Lee JS and Park
KY: Fermented Pu-erh tea increases in vitro anticancer activities
in HT-29 cells and has antiangiogenetic effects on HUVECs. J
Environ Pathol Toxicol Oncol. 32:275–288. 2013.
|
|
22
|
Sreenath T, Matrisian LM,
Stetler-Stevenson W, Gattoni-Celli S and Pozzatti RO: Expression of
matrix metalloproteinase genes in transformed rat cell lines of
high and low metastatic potential. Cancer Res. 52:4942–4947.
1992.
|
|
23
|
Chaudhary AK, Singh M, Bharti AC, Asotra
K, Sundaram S and Mehrotra R: Genetic polymorphisms of matrix
metalloproteinases and their inhibitors in potentially malignant
and malignant lesions of the head and neck. J Biomed Sci.
17:102010.
|
|
24
|
Zucker S and Vacirca J: Role of matrix
metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis
Rev. 23:101–117. 2004.
|
|
25
|
Duffy MJ and McCarthy K: Matrix
metalloproteinases in cancer: prognostic markers and targets for
therapy (review). Int J Oncol. 12:1343–1348. 1998.
|
|
26
|
Okazaki I, Noro T, Tsutsui N, Yamanouchi
E, Kuroda H, Nakano M, Yokomori H and Inagaki Y: Fibrogenesis and
carcinogenesis in nonalcoholic steatohepatitis (NASH): involvement
of matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinase (TIMPs). Cancers (Basel). 6:1220–1255. 2014.
|
|
27
|
Mysliwiec AG and Ornstein DL: Matrix
metalloproteinases in colorectal cancer. Clin Colorectal Cancer.
1:208–219. 2002.
|
|
28
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.
|
|
29
|
Yang KC, Uen YH, Suk FM, Liang YC, Wang
YJ, Ho YS, Li IH and Lin SY: Molecular mechanisms of
denbinobin-induced anti-tumorigenesis effect in colon cancer cells.
World J Gastroenterol. 11:3040–3045. 2005.
|
|
30
|
Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC,
Tong Y and Zhang KY: Review of research on Dendrobium, a prized
folk medicine. Appl Microbiol Biotechnol. 93:1795–1803. 2002.
|
|
31
|
Ohana G, Bar-Yehuda S, Barer F and Fishman
P: Differential effect of adenosine on tumor and normal cell
growth: focus on the A3 adenosine receptor. J Cell Physiol.
186:19–23. 2001.
|
|
32
|
Graff S, Engelman M, Gillespie HB and
Graff AM: Guanine in cancer. Cancer Res. 11:388–392. 1951.
|
|
33
|
Benoit SC, Kemp CJ, Elias CF, et al:
Palmitic acid mediates hypothalamic insulin resistance by altering
PKC-theta subcellular localization in rodents. J Clin Invest.
119:2577–2589. 2009.
|
|
34
|
Liang Q, Liang ZS, Wang JR and Xu WH:
Essential oil composition of Salvia miltiorrhiza flower.
Food Chem. 113:592–594. 2009.
|