Introduction
At present, the standard first-line chemotherapy for
patients with advanced non-small cell lung cancer (NSCLC) is
platinum doublet chemotherapy using a third-generation anticancer
agent (1,2). Pemetrexed (PEM) is a novel metabolic
antagonist capable of inhibiting multiple enzymes involved in
folate metabolism that has been clinically introduced as an
effective therapeutic agent in the treatment of NSCLC (3–5).
Previously, the results of two phase III studies indicated the
efficacy of PEM as a first-line therapy for advanced NSCLC
(6,7).
In the JMDB study, cisplatin (CDDP) plus PEM therapy was not
determined to be inferior to CDDP plus gemcitabine (GEM) therapy in
terms of the overall survival (OS), and the incidence of severe
adverse events was significantly lower following CDDP plus PEM
therapy (6). Furthermore, in a
subgroup analysis according to histological type, the OS of
patients with non-squamous NSCLC was significantly longer in the
CDDP plus PEM therapy group compared with the CDDP plus GEM group.
In addition, the effectiveness of PEM for the treatment of
non-squamous NSCLC was supported by two of the phase III studies
(JMEI and JMEN) (8,9). Thus, at present, CDDP plus PEM therapy
is used as a standard primary therapeutic strategy for patients
with advanced non-squamous NSCLC.
Vascular endothelial growth factor (VEGF) is
important in tumor neovascularization (10,11), with
a previous study reporting that increased VEGF expression levels
are associated with prognosis in NSCLC (12). Bevacizumab (BEV) is a humanized
monoclonal antibody to VEGF and its clinical efficacy in the
treatment of various types of cancer has been previously reported
(13–17).
A randomized phase II study conducted in the United
States investigated the time to progression in patients with NSCLC.
The study demonstrated a significant extension of the time to
progression following carboplatin (CBDCA) and paclitaxel (CP) with
BEV therapy compared with CP therapy alone, however, severe lung
bleeding occurred in a large number of the patients exhibiting
squamous cell carcinoma (18).
Subsequently, the Eastern Cooperative Oncology Group (ECOG)
conducted a phase III study (E4599) comparing CP therapy with CP
plus BEV therapy in patients with advanced non-squamous NSCLC
(13). In study E4599, the addition
of BEV to platinum doublet chemotherapy resulted in an extension in
the primary endpoint of OS [hazard ratio (HR), 0.79; 95%
confidential interval (CI), 0.67–0.92]. In the subsequent Avastin®
in Lung (AVAiL) study, which was primarily conducted in Europe, the
effects of adding 7.5 mg/kg BEV to CDDP plus GEM therapy was
evaluated, revealing a significant extension in the primary
endpoint of progression-free survival (PFS) (19). In accordance with these results, the
addition of BEV to platinum doublet chemotherapy has become a
standard treatment strategy for patients with advanced NSCLC,
excluding cases of squamous cell carcinoma.
In Japan, the randomized phase II JO19907 study was
conducted to clarify the efficacy of adding BEV to CP therapy for
Japanese patients. This study demonstrated a significant extension
of the PFS time following the administration of CP plus BEV (dose,
15 mg/kg) therapy compared with CP therapy alone (HR, 0.61; 95% CI,
0.42–0.89) (20). Therefore, the BEV
combined with platinum therapy is now considered to be a standard
chemotherapeutic regimen for the treatment of advanced non-squamous
NSCLC in Japan.
Furthermore, the phase III AVAPERL study reported a
comparison between maintenance therapy of uncombined BEV, and
combined BEV plus PEM following initial treatment with CDDP, PEM
and BEV (CPB) chemotherapy. This study reported that the PFS time
was significantly extended following maintenance therapy with BEV
plus PEM and that the patients exhibited a high tolerability to
this therapeutic regime (21).
However, the dose of BEV used in the AVAPERL study was 7.5 mg/kg (a
commonly used dose in Europe). This differs from the dose of 15
mg/kg used in the E4599 study (13),
which identified an extension in the OS period following the
addition of BEV to platinum doublet chemotherapy. Furthermore, in
the United States and Japan, 15 mg/kg is the approved and most
frequently used BEV dose (13,20).
The aim of the present study was to evaluate the
safety of CPB therapy with a BEV dose of 15 mg/kg. In addition, the
current study aimed to analyze the percentage of patients for whom
CPB therapy is applicable as the primary chemotherapeutic strategy
for advanced non-squamous NSCLC.
Patients and methods
Patients
A total of 31 consecutive patients with non-squamous
NSCLC were treated with CPB therapy (BEV dose, 15 mg/kg) as the
first-line chemotherapeutic strategy at the National Kyushu Cancer
Center (Fukuoka, Japan) between November 2009 and December 2011.
CPB therapy was applied for patients who satisfied all the
following criteria: i) An age of ≥20 years; ii) pathologically or
cytologically diagnosed as exhibiting non-squamous NSCLC (including
cases of adenosquamous NSCLC with a significant adenocarcinoma
component); iii) disease for which CDDP treatment was considered to
be applicable; iv) clinical stage III or IV disease (including
stage IIIA, non-applicable for radical radiotherapy); v) evaluable
lesions (cases with no measurable lesions were acceptable); vi) no
severe disorders of the major organs (bone marrow, heart, lungs,
liver and kidneys); and vii) laboratory analysis data at the
commencement of treatment indicating a neutrophil count of
≥2,000/mm3 (normal range, 1,500–5,950/mm3), a
hemoglobin level of ≥9.0 g/dl (normal range, 13.5–17.0 g/dl), a
platelet count of ≥10.0×104/mm3 (normal
range, 12.0–35.0×104/mm3), a prothrombin
time-international normalized ratio of ≤1.5 (normal ratio, 1.0), an
aspartate aminotransferase (AST) level of ≤100 IU/l (normal range,
13–33 IU/l), an alanine aminotransferase (ALT) level of ≤100 IU/l
(normal range, 8–42 IU/l), a total bilirubin level of ≤1.5 mg/dl
(normal range, 0.3–1.2 mg/dl), a serum creatinine level of ≤1.2
mg/dl (normal range, 0.6–1.1 mg/dl), a creatine clearance rate
(calculated with the Cockcroft-Gault equation) of ≥45 ml/min
[normal range, 90–120 ml/min (male); 80–110 ml/min (female)], a
urinary protein level of ≤1+ (normal level, 0) and an
SpO2 level of ≥90% (normal range, 90–100%). CPB therapy
was not applied for patients with brain metastases, a history of
hemoptysis (≥2.5 cm3 blood per bleeding episode from the
respiratory tract) or the possibility of tumor invasion of the
large blood vessels based on diagnostic imaging. This study was
approved by the ethics committee of the National Kyushu Cancer
Center. Patients were provided with sufficient information
regarding the treatment strategy prior to entry into the current
study. Treatment was administered to all patients following the
receipt of written consent.
Treatment strategy and study
design
A total of 4–6 courses of CPB therapy were
administered at intervals of 3 weeks, with initial doses of 75
mg/m2 CDDP plus 500 mg/m2 PEM plus 15 mg/kg
BEV administered on day 1. To reduce the incidence of adverse
events caused by PEM, patients were administered folic acid and
vitamin B12 supplements beginning 7 days prior to the commencement
of therapy. After 4–6 courses of CPB therapy, patients free of
progressive disease received maintenance therapy with 15 mg/kg BEV
alone or combined with 500 mg/m2 PEM. The condition of
the individual patient was considered upon selection of one of the
two maintenance regimes. Maintenance therapy was continued at
intervals of 3 weeks until disease progression was noted.
Prior to commencing therapy with CPB, diagnostic
imaging with computed tomography (CT) scanning was performed to
yield baseline information. Diagnostic imaging, including CT
scanning, was repeated every sixth week from the commencement of
treatment until disease progression was noted. Adverse events were
evaluated in accordance with the National Cancer Institute Common
Terminology Criteria for Adverse Events, version 4.0 (22). Additionally, responses to the
treatment strategies were evaluated using the Response Evaluation
Criteria in Solid Tumors (RECIST), version 1.1 (23).
Statistical analysis
Survival curve calculations were performed using the
Kaplan-Meier method. PFS time was defined as the time from the
start of treatment to disease progression or mortality. OS time was
defined as the time from the start of treatment to mortality.
Follow-up concluded on April 30, 2013, for patients still receiving
treatment or commencing receipt of subsequent treatment
strategies.
Results
Patient characteristics
(Table I) summarizes
the background characteristics of the 31 patients that received CPB
therapy in the present study. The cohort consisted of 21 male and
10 female individuals, with a median age of 60 years (range, 38–76
years). The ECOG performance status (PS) was 0 in 15 cases and 1 in
16 cases, and the histological type was adenocarcinoma in 29 cases
and adenosquamous carcinoma in 2 cases. Furthermore, the clinical
stage was IIIA, IIIB and IV in 2, 3 and 26 cases, respectively.
Additionally, the EGFR mutation status was positive (exon 19
deletion) in 3 patients, wild-type in 22 patients and unknown in 6
patients.
 | Table I.Patient characteristicsa. |
Table I.
Patient characteristicsa.
| Characteristic | No. of patients
(n=31) |
|---|
| Gender |
|
| Male | 21 |
|
Female | 10 |
| ECOG performance
status |
|
| 0 | 15 |
| 1 | 16 |
| Histology |
|
|
Adenocarcinoma | 29 |
|
Adenosquamous | 2 |
| Stage |
|
|
IIIAb | 2 |
| IIIB | 3 |
| IV | 26 |
| EGFR mutation
status |
|
| Positive
(exon 19 deletion) | 3 |
|
Wild-type | 21 |
|
Unknown | 7 |
Treatment strategy
The median follow-up time for the 31 patients who
received CPB therapy was 1,057 days (range, 640–1288 days). A total
of ≥4 courses of CPB therapy were administered to 28/31 patients
(90.3%). Of the 3 patients who received fewer than 4 courses, 1
patient discontinued therapy after 1 course due to a reduction in
the ECOG PS, and the remaining 2 patients discontinued therapy due
to disease relapse after 3 courses. Maintenance therapy was
administered to 24/31 patients (77.4%), consisting of BEV in 15
cases (62.5%), PEM in 6 cases (25.0%) and combined PEM plus BEV in
3 cases (12.5%). The median number of courses of BEV, PEM and
PEM/BEV maintenance therapy was 5 (range, 1–21 courses), 3 (range,
1–9 courses) and 4 (range, 3–15 courses), respectively. The primary
reasons for the selection of PEM maintenance therapy among the 6
cases were hypertension (2 cases), gastric ulcers (1 case),
diverticulitis (1 case) and bloody sputum (1 case). The reasons for
the non-applicability of maintenance therapy in 7 cases were
disease progression (5 cases), patient decision (1 case) and
surgery after the initial therapy (1 case).
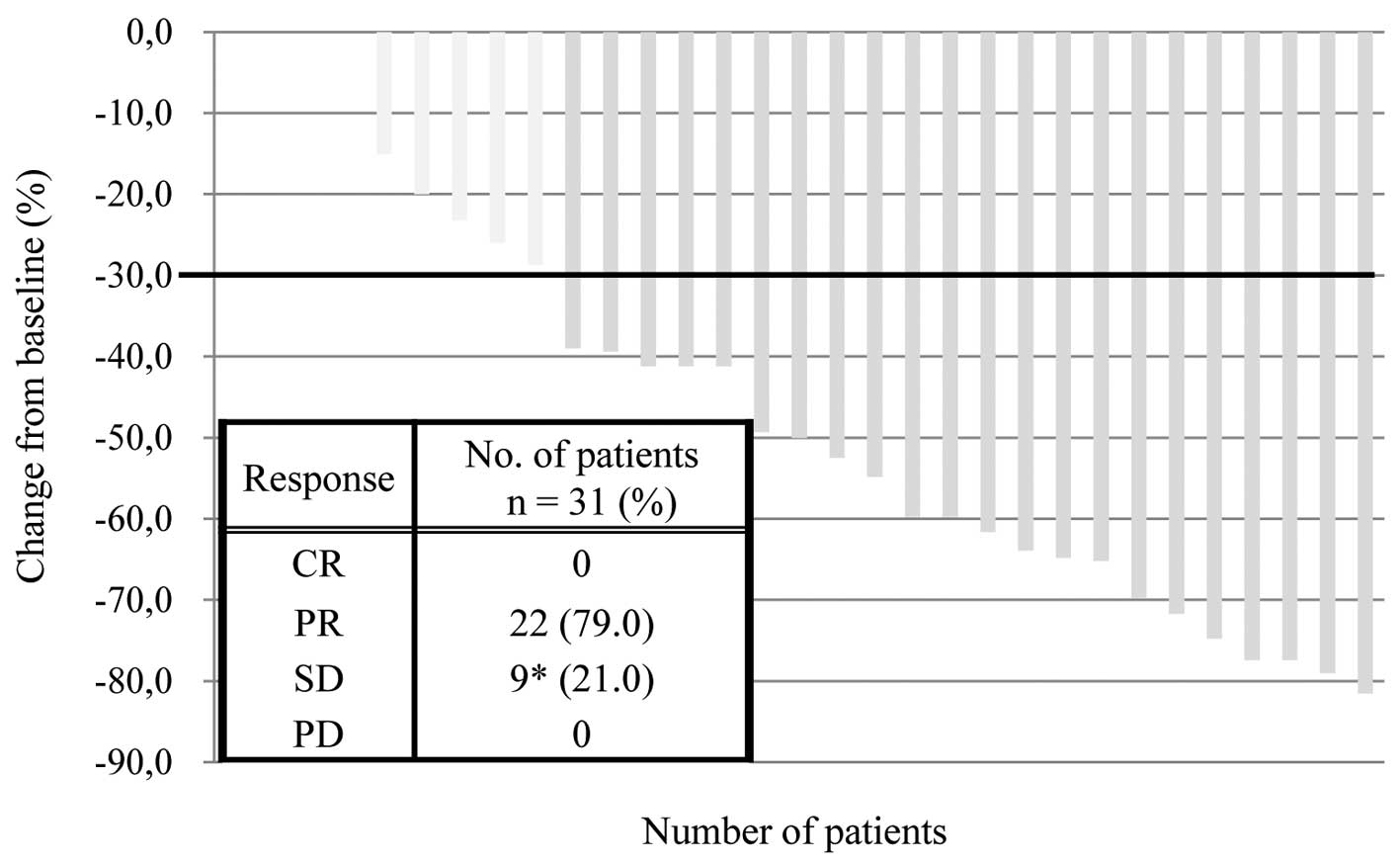
Response and survival analysis
Treatment efficacy was evaluated by each attending
physician in accordance with the RECIST criteria, version 1.1.
Among the 31 patients who received CPB therapy, the best overall
response was a partial response in 22 cases and stable disease in 9
cases. Furthermore, no cases of progressive disease or complete
response were observed; therefore, the response rate was 71.0% (95%
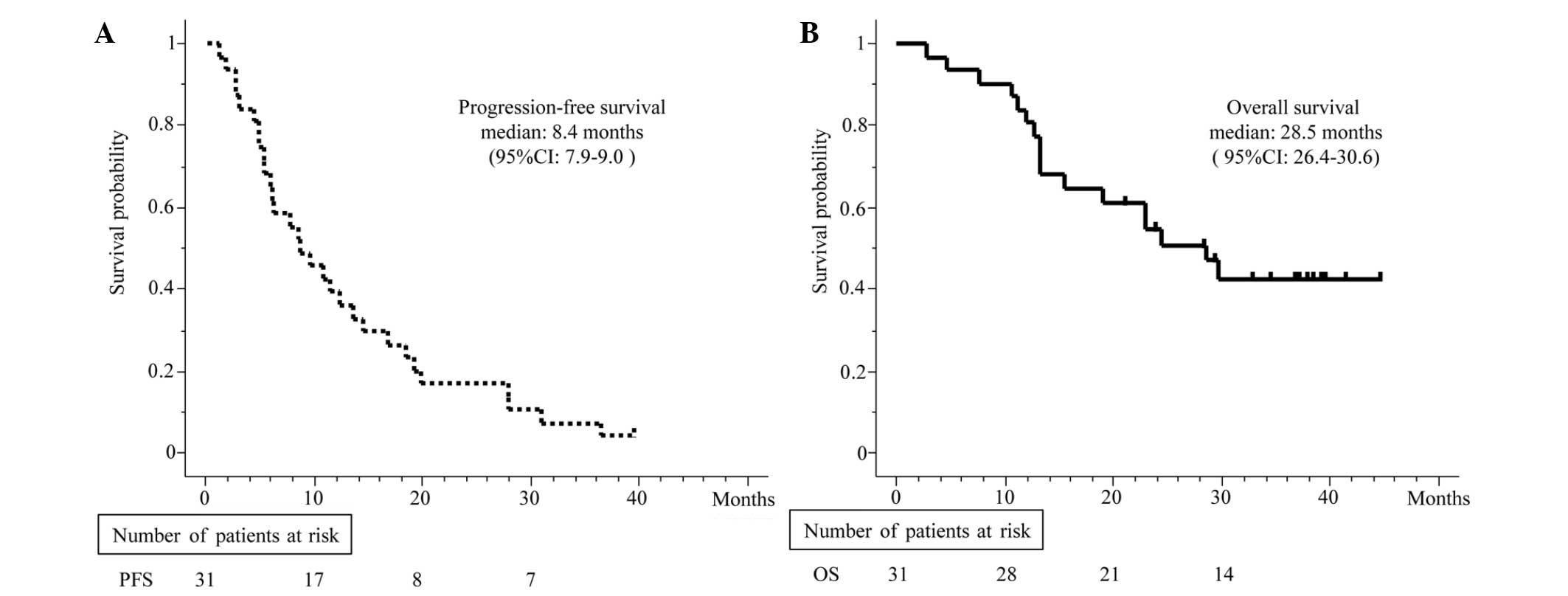
CI, 41–74%; Fig. 1). The median PFS
and OS time were 8.4 months (95% CI, 7.9–9.0; Fig. 2A) and 28.5 months (95% CI, 26.4–30.6
months; Fig. 2B), respectively.
Safety analysis
Observed hematological toxicities of grade III or
higher included neutropenia (9 cases; 29.0%), decreased hemoglobin
levels (1 case; 3.2%) and thrombocytopenia (1 case; 3.2%). No
adverse events classified as non-hematological toxicities or
febrile neutropenia were observed, however, 4 cases of grade III
hypertension (12.9%) and 1 case of colitis (3.2%; (Table II)) did occur. Grade II hemoptysis
was observed in 1 case (3.2%) during the first course of treatment.
This patient subsequently developed grade III anorexia and nausea
during the first course of treatment; therefore, BEV was
discontinued and the therapy was changed from CDDP to CBDCA from
the second course of treatment onward in this patient. No
treatment-associated mortality was observed.
 | Table II.Toxicities and grades of patients with
advanced non-squamous non small-cell lung cancer. |
Table II.
Toxicities and grades of patients with
advanced non-squamous non small-cell lung cancer.
| Patients, %
(n=31) |
|---|
|
|---|
| Toxicity | G1 | G2 | G3 | G4 | G3/4 |
|---|
| Neutropenia | 0 | 6 | 29 | 0 | 29 |
| Anemia | 19 | 23 | 3 | 0 | 3 |
| Thrombocytopenia | 3 | 6 | 0 | 3 | 3 |
| Febrile
neutropenia | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 3 | 42 | 13 | 0 | 10 |
| Bleeding |
|
|
|
|
|
|
Hemopytsis | 0 | 3 | 0 | 0 | 3 |
|
Epistaxis | 5 | 0 | 0 | 0 | 0 |
| Pulmonary
thromboembolism | 0 | 0 | 0 | 3 | 0 |
| Congestive heart
disease | 0 | 0 | 0 | 0 | 0 |
| Proteinuria | 10 | 0 | 0 | 0 | 0 |
| Fatigue | 13 | 10 | 3 | 0 | 3 |
| Anorexia | 58 | 16 | 6 | 0 | 6 |
| Vomiting | 6 | 6 | 0 | 0 | 0 |
| Diarrhea | 3 | 0 | 0 | 0 | 0 |
| Constipation | 19 | 3 | 0 | 0 | 0 |
| Gastric ulcer | 0 | 0 | 3 | 0 | 3 |
| Colitis | 0 | 0 | 3 | 0 | 3 |
| Pneumonitis | 0 | 0 | 0 | 0 | 0 |
| Elevated AST | 16 | 6 | 0 | 0 | 0 |
| Elevated ALT | 13 | 6 | 0 | 0 | 0 |
| Elevated
creatinine | 32 | 3 | 0 | 0 | 0 |
Discussion
Two phase III studies (E4599 and AVAiL) demonstrated
extension in the OS and PFS times (primary endpoints) following
treatment with BEV in combination with standard platinum doublet
chemotherapy (13,19). Based on these findings, this
therapeutic regime has become a standard for the treatment of
patients with advanced NSCLC, excluding cases of squamous cell
carcinoma. However, in all phase III studies conducted thus far,
the effects of adding BEV to platinum doublet chemotherapy have
been evaluated only for platinum doublet CP or CDDP plus GEM
(doublets that are commonly used at the start of such studies). To
the best of our knowledge, no studies have been designed to
evaluate the effect of adding BEV (dose, 15 mg/kg) to platinum
doublet CDDP plus PEM therapy. Thus, the present retrospective
study allowed clarification of the tolerability of combined CDDP,
PEM and BEV (dose, 15 mg/kg) therapy in patients with non-squamous
NSCLC.
In the current study, CPB therapy at a BEV dose of
15 mg/kg resulted in a median PFS time of 10.5 months and a median
OS time of 27.2 months. Therefore, the outcome of this treatment
strategy dose not appear to be inferior to the outcomes of
alternative platinum doublet plus BEV (15 mg/kg) regimes reported
in previous phase II studies (24,25). In
addition, the outcome of the present study is comparable to the
outcome of the JO19907 study, which yielded a median PFS time of
6.1 months (20), and the subgroup
analysis of Asian subjects in the AVAiL study, which yielded median
PFS times of 8.5 (15 mg/kg) and 8.2 months (7.5 mg/kg) (26). Furthermore, the response rate to CPB
therapy in the present study was 71.0% (95% CI, 41–74%), a value
not inferior to the response rate of 61% recorded in the CP plus
BEV (15 mg/kg) therapeutic group of a previously conducted domestic
randomized phase II trial (20).
In the present study, no previously unknown
toxicities associated with BEV and no treatment-associated
mortalities were noted. In a previous study (JO19907) (20), the CP plus BEV therapeutic group
developed grade III or higher neutropenia, decreased hemoglobin
levels and thrombocytopenia at incidences of 91, 12 and <5%,
respectively. In addition, the incidence of febrile neutropenia was
8%. In contrast to the results of the JO19907 study, febrile
neutropenia was not observed in the present study, and the
incidence of hematological toxicities was typically lower in
patients treated with combined CDDP plus PEM therapy.
The adverse events associated with BEV treatment
were comparable between the present and aforementioned studies. In
a subgroup analysis of data regarding Asian subjects obtained in
the AVAiL study (27), the incidence
of grade III or higher hypertension, bleeding and proteinuria in
the 15 mg/kg BEV group was 9.1, 3.0 and 6.1%, respectively.
Therefore, the indicators of BEV-specific toxicities observed in
the present study were not markedly different from those identified
in previous studies. Furthermore, in consideration of the inclusion
and exclusion criteria adopted in previous clinical analyses of
BEV, the present study excluded patients exhibiting: i) A history
of hemoptysis, ii) void formation and iii) tumor invasion of large
blood vessels. As a result, no subjects developed grade III or
higher hemoptysis or pulmonary bleeding in the present study.
The most frequent reason for the non-applicability
of CPB therapy was the presence of brain metastases. BEV is not
contraindicated for patients with brain metastases in Western
countries. By contrast, BEV was contraindicated for patients with
brain metastasis following its approval under the National Health
Insurance program in Japan (during the current study period).
However, the relative contraindication of BEV for patients with
brain metastasis was lifted in Japan in June 2012. Therefore, CPB
therapy will be applied in more cases as it begins to be
administered to patients with brain metastasis. Furthermore, a
recent report demonstrated the effectiveness of CDDP plus PEM
therapy in patients with brain metastasis (28). Thus, a prospective evaluation of CPB
therapy in patients exhibiting brain metastasis with a poor
prognosis is required.
In conclusion, the present single center
retrospective study clarified the feasibility, including the
efficacy and safety, of CDDP plus PEM combined with 15 mg/kg BEV as
a primary therapeutic strategy for patients with advanced
non-squamous NSCLC.
References
|
1
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taylor EC, Kuhnt D, Shih C, et al: A
dideazatetrahydrofolate analogue lacking a chiral center at C-6,
N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic
acid, is an inhibitor of thymidylate synthase. J Med Chem.
35:4450–4454. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schultz RM, Patel VF, Worzalla JF and Shih
C: Role of thymidylate synthase in the antitumor activity of the
multitargeted antifolate, LY231514. Anticancer Res. 19:437–443.
1999.PubMed/NCBI
|
|
5
|
Shih C, Habeck LL, Mendelsohn LG, Chen VJ
and Schultz RM: Multiple folate enzyme inhibition: mechanism of a
novel pyrrolopyrimidine-based antifolate LY231514 (MTA). Adv Enzyme
Regul. 38:135–152. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, et al: Phase III study comparing cisplatin plus
gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive
patients with advanced-stage non-small-cell lung cancer. J Clin
Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grønberg BH, Bremnes RM, Fløtten O, et al:
Phase III study by the Norwegian lung cancer study group:
pemetrexed plus carboplatin compared with gemcitabine plus
carboplatin as first-line chemotherapy in advanced non-small-cell
lung cancer. J Clin Oncol. 27:3217–3224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanna N, Shepherd FA, Fossella FV, et al:
Randomized phase III trial of pemetrexed versus docetaxel in
patients with non-small-cell lung cancer previously treated with
chemotherapy. J Clin Oncol. 22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciuleanu T, Brodowicz T, Zielinski C, et
al: Maintenance pemetrexed plus best supportive care versus placebo
plus best supportive care for non-small-cell lung cancer: a
randomised, double-blind, phase 3 study. Lancet. 374:1432–1440.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
therapeutic implications. Semin Oncol. 29:(Suppl 16). 10–14. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seto T, Higashiyama M, Funai H, et al:
Prognostic value of expression of vascular endothelial growth
factor and its flt-1 and KDR receptors in stage I non-small-cell
lung cancer. Lung Cancer. 53:91–96. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gray R, Bhattacharya S, Bowden C, Miller K
and Comis RL: Independent review of E2100: a phase III trial of
bevacizumab plus paclitaxel versus paclitaxel in women with
metastatic breast cancer. J Clin Oncol. 27:4966–4972. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Escudier B, Pluzanska A, Koralewski P, et
al: AVOREN Trial Investigators: Bevacizumab plus interferon alfa-2a
for treatment of metastatic renal cell carcinoma: a randomised,
double-blind phase III trial. Lancet. 370:2103–2111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman HS, Prados MD, Wen PY, et al:
Bevacizumab alone and in combination with irinotecan in recurrent
glioblastoma. J Clin Oncol. 27:4733–4740. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnson DH, Fehrenbacher L, Novotny WF, et
al: Randomized phase II trial comparing bevacizumab plus
carboplatin and paclitaxel with carboplatin and paclitaxel alone in
previously untreated locally advanced or metastatic non-small-cell
lung cancer. J Clin Oncol. 22:2184–2191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reck M, von Pawel J, Zatloukal P, et al:
Phase III trial of cisplatin plus gemcitabine with either placebo
or bevacizumab as first-line therapy for nonsquamous non-small-cell
lung cancer: AVAil. J Clin Oncol. 27:1227–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niho S, Kunitoh H, Nokihara H, et al:
JO19907 Study Group: Randomized phase II study of first-line
carboplatin-paclitaxel with or without bevacizumab in Japanese
patients with advanced non-squamous non-small-cell lung cancer.
Lung Cancer. 76:362–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barlesi F, Gervais R, Lena H, et al:
Pemetrexed and cisplatin as first-line chemotherapy for advanced
non-small-cell lung cancer (NSCLC) with asymptomatic inoperable
brain metastases: a multicenter phase II trial (GFPC 07–01). Ann
Oncol. 22:2466–2470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Common Terminology Criteria for Adverse
Events (CTCAE). v.4.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmJanuary.
2014
|
|
23
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
William WN Jr, Kies MS, Fossella FV, et
al: Phase 2 study of carboplatin, docetaxel and bevacizumab as
frontline treatment for advanced nonsmall-cell lung cancer. Cancer.
116:2401–2408. 2010.PubMed/NCBI
|
|
25
|
Clément-Duchêne C, Krupitskaya Y, Ganjoo
K, et al: A phase II first-line study of gemcitabine, carboplatin
and bevacizumab in advanced stage nonsquamous non-small cell lung
cancer. J Thorac Oncol. 5:1821–1825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Paz-Ares L, de Marinis F, Dediu M, et al:
Maintenance therapy with pemetrexed plus best supportive care
versus placebo plus best supportive care after induction therapy
with pemetrexed plus cisplatin for advanced non-squamous
non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3,
randomised controlled trial. Lancet Oncol. 13:247–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mok TS, Hsia TC, Tsai CM, et al: Efficacy
of bevacizumab with cisplatin and gemcitabine in Asian patients
with advanced or recurrent non-squamous non-small cell lung cancer
who have not received prior chemotherapy: a substudy of the Avastin
in Lung trial. Asia Pac J Clin Oncol. 7:(Suppl 2). 4–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Besse B, Lasserre SF, Compton P, Huang J,
Augustus S and Rohr UP: Bevacizumab safety in patients with central
nervous system metastases. Clin Cancer Res. 16:269–278. 2010.
View Article : Google Scholar : PubMed/NCBI
|