Introduction
Advanced malignant solid tumors in children have the
characteristics of a short incubation period, extensive metastasis
and rapid growth, and are the second leading cause of
cancer-related mortality in children. Thus, advanced malignant
solid tumors are of serious threat to the health of children
worldwide (1–3). Peripheral blood stem cell
transplantation (PBSCT) is the most common transplantation
procedure performed in medicine; autologous PBSCs are the most
common source of stem cells used in the autologous transplantation
setting (4). PBSCs have previously
been shown to be capable of supporting rapid and complete
hematopoietic reconstitution when used for autologous
transplantation (5–7). Recently, autologous PBSCT (APBSCT) has
begun to replace autologous bone marrow transplantation due to its
rapid rate of engraftment and low transplant-related mortality
(8–11).
However, children with advanced malignant solid
tumors are usually of low weight, with a young age of onset
(12,13), thin venous access and a low total
blood volume, which makes it difficult to control the dosage of
anticoagulant drug and the side-effects of reinfusion or
post-reinfusion. These factors all increase the risk of the
recruitment, collection and reinfusion process of APBSC in children
with solid tumors (14,15). The present study reports the outcomes
of 38 children with advanced malignant solid tumors who received
high-dose chemotherapy (HDCT) and APBSCT treatment between
September 2005 and November 2011 in Beijing Tongren Hospital
(Capital Medical University, Beijing, China).
Patients and methods
Patients
The study was approved by the Ethics Committee of
Beijing Tongren Hospital, and was conducted in accordance with the
Declaration of Helsinki. Written informed consent was obtained from
all subjects or their parents. A total of 38 patients with advanced
malignant solid tumors were recruited between September 2005 and
November 2011. The 38 patients consisted of 27 males and 11
females, with a median age of 6.4 years (range, 1–14 years). The
median weight was 22.3 kg (range, 11–75 kg). The distribution of
tumor types was as follows: Neuroblastoma (NB) in 24 cases,
retinoblastoma in five cases, rhabdomysarcoma in four cases,
primitive neuroectodermal tumor in two cases, hepatoblastoma in one
case, Wilms' tumor in one case and malignant schwannoma in one
case. The pre-transplant conditions were ranked as complete
remission (CR), partial remission (PR) and progressive disease
(PD). The patient characteristics are summarized in Table I.
 | Table I.Pre-transplant conditions of 38
children patients who received autologous peripheral blood stem
cell transplantation. |
Table I.
Pre-transplant conditions of 38
children patients who received autologous peripheral blood stem
cell transplantation.
|
|
| Patient condition,
n | Gender, n |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Groups | Cases, n | CR | PR | DP | M | F | Age, years
(range) | Number of
chemotherapy courses prior to transplantation, n |
|---|
| NB | 24 | 11 | 6 | 7 | 17 |
|
6.22±3.09a
(1.5–14) |
3.76±2.45a |
| PNET | 2 | 2 |
|
| 1 | 17 |
6.00±3.00a
(3–9) |
7.00±1.00a |
| RB | 5 | 2 | 2 | 1 | 1 | 4 |
3.42±2.06a(1–6) |
9.8±4.3a |
| RMS | 4 |
| 4 |
| 3 | 1 |
8.75±4.57a (3–14) |
7.25±2.62a |
| HP | 1 |
|
| 1 | 1 |
| 11 | 65 |
| MSN | 1 |
|
| 1 |
| 1 | 10 | 45 |
| Wilms | 1 |
| 1 |
| 1 |
| 10 | 61 |
| Total | 38 | 14 | 14 | 10 | 23 | 15 |
6.3±3.4a |
|
Chemotherapy and PBSC collection
Pre-operatively, 6–61 cycles of induction
chemotherapy were administered to all children. Following initial
conventional chemotherapy, a physical examination and laboratory
tests were performed. Hematopoietic progenitor cells were mobilized
by chemotherapeutic treatment followed by administration of 5–10
µg/kg subcutaneous granulocyte colony-stimulating factor (G-CSF)
daily (Filgrastim; Amgen, Inc., Thousand Oaks, CA, USA) until the
level of peripheral white blood cells (WBCs) had decreased to
2×109/l (16). The
detailed mobilization programs and drugs used are listed in
Table II.
 | Table II.Mobilization program and pretreatment
treatment regimens for 38 children with advanced malignant solid
tumor. |
Table II.
Mobilization program and pretreatment
treatment regimens for 38 children with advanced malignant solid
tumor.
| Diagnosis | High-dose
therapy | Medication | Cases | Ratio, % |
|---|
| Neuroblastoma | CDV | Cy, 2.1
g/m2/day, days 1–2 | 17 | 70.8 |
|
|
| D, 25
mg/m2/day, days 1–3 |
|
|
|
|
| V, 0.67
mg/m2/day, days 1–3 |
|
|
|
| CiE | Ci, 50
mg/m2/day, days 1–4 | 3 | 12.5 |
|
|
| E, 200
mg/m2/day, days 1–3 |
|
|
|
| CHOP | Cy, 1
g/m2/day, days 1–3 | 3 | 12.5 |
|
|
| V, 1.5
mg/m2/day, day 1 |
|
|
|
|
| A, 25
mg/m2/day, days 1–2 |
|
|
|
|
| P, 100
mg/m2/day, days 1–5 |
|
|
|
| IT | I, 2
g/m2/day, days 1–5 | 1 | 4.2 |
|
|
| T, 100
mg/m2/day, days 1–4 |
|
|
| Retinoblastoma | CTV | Ca, 560
mg/m2/day, day 1 | 5 | 100.0 |
|
|
| T, 200
mg/m2/day, day 2 |
|
|
|
|
| V, 1.5
mg/m2/day, day 2 |
|
|
|
Rhabdomysarcoma | DEV | D, 450
µg/m2/day, days 2–6 | 1 | 25.0 |
|
|
| E, 100
mg/m2/day, days 2–4 |
|
|
|
|
| V, 1.5
mg/m2/day, days 1–8 |
|
|
|
| IEV | I, 1.5
mg/m2/day, days 2–6 | 3 | 75.0 |
|
|
| E, 100
mg/m2/day, days 2–6 |
|
|
|
|
| V, 1.5
mg/m2/day, days 1–8 |
|
|
| Primitive
neuroectodermal tumor | CDV | Cy, 2.1
g/m2/day, days 1–2 | 2 | 100.0 |
|
|
| D, 25
mg/m2/day, days 1–3 |
|
|
|
|
| V, 0.67
mg/m2/day, days 1–3 |
|
|
| Malignant
schwannoma | CDV | Cy, 2.1
g/m2/day, days 1–2 | 1 | 100.0 |
|
|
| D, 25
mg/m2/day, days 1–3 |
|
|
|
|
| V, 0.67
mg/m2/day, days 1–3 |
|
|
| Hepatoblastoma | ACP | A, 25
mg/m2/day, days 1–3 | 1 | 100.0 |
|
|
| Cy, 0.8–1
g/m2/day, day 1 |
|
|
|
|
| P, 20
mg/m2/day, days 1–5 |
|
|
| Wilms' tumor | DVA | D, 15
µg/m2/day, days 1–5 | 1 | 100.0 |
|
|
| V, 1.5
mg/m2/day, days 1–8 |
|
|
|
|
| A, 20
mg/m2/day, days 1–2 |
|
|
The collection of the APBSCs was performed when the
WBC count was >5×109/l, using a continuous flow blood
cell separator (CS-3000; Baxter, Deerfield, IL, USA). In this
series of patients, small volume collection chamber (SVCC)
separation and a groove clamp were used for patients weighing ≤20
kg. In all patients, the extracorporeal line was primed with 400 ml
of leukocyte-depleted red blood cells following regular priming
with normal saline. In Beijing Tongren Hospital, all patients who
weigh <30 kg automatically undergo this optional procedure.
Prior to collection, patients were required to take two pills of
calcium carbonate (600 mg), daily for two days. During the
collection procedure, acid-citrate-dextrose was used as an
anticoagulant in a ratio of 1:10 to 1:13 ml of whole blood, and
calcium gluconate (5 ml/1,500 ml of processed blood) was
administered continuously during apheresis to prevent hypocalcemia.
No other drugs were used and the patients were not sedated during
the procedure. A mononuclear cell (MNC) count of
>5×108/kg or a cluster of differentiation
(CD)34+ cell count of >3×106/kg was used
as the harvest criterion. At 6 h after the start of collection, the
number of platelets (PLTs) had to be ≥5.0×10−10/l,
otherwise 1–2 units of PLT was infused into these patients. The
harvested PBSCs were stored frozen at −196°C.
APBSCT
The pre-transplant pretreatment regimen consisted of
high-dose aclacinomycin, cyclophosphamide, cisplatin, carboplatin,
dactinomycin, daunorubicin, etoposide, ifosfamide, paclitaxel,
teniposide and vincristine. The detailed data is summarized in
Table II. In addition, oral
non-absorbed antibiotics (compound sulfamethoxazole tablets, 50
mg/kg, once) were administered to prevent infection, and
complementary medicine (0.6–1.2 g reduced glutathione, once and
5–10 ml polyene phosphatidylcholine, once) was administered
intravenously to protect the liver, the myocardium and the
gastrointestinal mucosa. Prior to reinfusion, antihistamines (5–10
mg loratadine, for 1–3 days) and intravenous hormone drugs (2 mg/kg
methylprednisolone, every 12 h, for 1–3 days) were administered to
all patients. On the 8th and 9th day of pretreatment, cryopreserved
PBSCs were rapidly thawed in a 39°C water bath and infused through
a central venous catheter without washing. During the whole
reinfusion process, the patient's vital signs were observed.
After day 1 of transplantation, recombinant human
G-CSF (rhG-CSF) was administered intravenously at a dose of 5–10
µg/kg on each day (+1 day). Engraftment was confirmed by recovery
of peripheral WBC counts to >2×109/l, a granulocyte
count of >0.5×109/l, an absolute neutrophil count of
>0.5×109/l, a hemoglobin concentration of >80 g/l
and a PLT count of >20×109/l, without dependence on
PLT transfusion for three consecutive days.
Medical evaluation and follow-up
The observed indicators during APBSC collection
included heart rate, breathing rate, blood oxygen level, blood
pressure value and other adverse reactions, such as paleness,
sweating, nausea, vomiting, numbness and chills.
The adverse reactions during reinfusion were as
follows: Abnormal heart rate, breathing, blood oxygen, blood
pressure, and heart, liver and renal function indicators, and other
adverse reactions, such as hemoglobinuria, headache, nausea,
vomiting and diarrhea.
Acute toxicity following reinfusion (on days 0, 7,
14, 21 and 28 post-transplantation) was defined according to the
criteria proposed by Bearman et al (17). Lung function was observed after 30
days, and the viscera could be assessed after 60 days. A review was
performed after every three months.
The prognosis of the children in this study was
categorized according to integrated efficacy standards (18) as follows: i) CR, the tumor disappeared
completely following the treatment and no evidence of tumor residue
was observed in the imaging examination; ii) PR, the tumor shrank
by >50% and no new lesions were observed; iii) PD, the tumor
volume increased by >25% and new tumor lesions appeared during
the treatment; and iv) mortality.
During follow-up, the general situation, physical
examination, onset site imaging, and analysis of the blood, bone
marrow and associated tumor markers, were performed monthly for
three months post-transplantation and then every third month of the
first year, followed by every six months thereafter until five
years post-transplantation.
Statistical analysis
SPSS software version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for data analysis. All data were expressed as the
mean ± standard deviation, median (range) or n (%), as appropriate.
Fisher's exact test or the χ2 test were used to examine
the association between qualitative variables. Survival and
event-free curves were determined by the Kaplan-Meier method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
APBSC collection data
The mean number of collected MNCs and the
CD34+ cell count from the 38 patients was
5.6±2.2×108/kg and 3.8±2.6×106/kg,
respectively. Of these 38 patients, the number of stem cells
collected from 31 cases (81.6%) accorded with the transplantation
standards (Table III).
 | Table III.Association between age and cycles
chemotherapy, and the amount of stem cells obtained. |
Table III.
Association between age and cycles
chemotherapy, and the amount of stem cells obtained.
| Variable | Cases, n | Collections, n
(mean ±SD) | MNC
(1×108/kg) | CD34+
(1×106/kg) |
|---|
| Age, years |
|
|
|
|
| ≤5 | 15 | 1.7±0.7 | 6.3±2.2 | 6.2±4.1 |
|
>5 | 23 | 2.6±0.9 | 5.7±2.2 | 3.0±2.4 |
|
P-value |
| 0.004 | 0.417 | 0.004 |
| Cycles of
chemotherapy |
|
|
|
|
|
≤10 | 23 |
| 6.0±2.0 | 5.2±3.9 |
|
>10 | 15 |
| 5.8±2.5 | 2.9±2.2 |
|
P-value |
|
| 0.792 | 0.042 |
The amount of CD34+ stem cells and the
collection time were statistically different (P<0.05) between
the two groups of children aged >5 and ≤5 years, showing that
children <5 years of age can contribute to the collection and
acquisition of stem cells. No significant difference with respect
to the amount of MNCs was observed between the two groups.
The amount of CD34+ cells in the patients
receiving <10 chemotherapy cycles was significantly higher in
comparison to those receiving >10 cycles (P<0.05; Table III), suggesting that a greater
number of chemotherapy cycles is detrimental to the acquisition of
CD34+ cells.
Toxicity and adverse reactions of stem
cell collection and reinfusion
All 38 patients developed class 2 or 3
gastrointestinal reactions and class 3 hair loss following
chemotherapy. No hypotension, paleness, sweating, nausea, vomiting,
numbness or chills were observed in the process of stem cell
collection. Of the 38 cases, 32 patients displayed an increased
heart rate and sinus tachycardia. The average amount of PLT loss
was 48% (range, 30–55%). Irradiated PLTs were administered into
eight patients due to excessive stem cell collection.
All 38 patients developed hemoglobinuria on the day
of reinfusion, and recovered within 12 h following hydration and
urine alkalization. The detailed data is presented in Table IV. Oxygen saturation was within the
normal range during reinfusion. Prior to intervention, the number
of patients who developed grade 0–1, grade 2 and grade 3–4 adverse
reactions was 5, 14 and 19, respectively, whereas 36, 2 and 0
patients, respectively, developed these adverse reactions following
intervention (P<0.001). All adverse reactions were restored to
normal within 24 h of symptomatic treatment.
 | Table IV.Toxicity and adverse reactions during
the process of autologous peripheral blood stem cell
reinfusion. |
Table IV.
Toxicity and adverse reactions during
the process of autologous peripheral blood stem cell
reinfusion.
| Toxic reaction | Cases, n | Occurrence rate,
% |
|---|
| Hemoglobinuria | 38 | 100 |
| Hypertension | 18 | 47.4 |
| Nausea,
vomiting | 16 | 42.1 |
| Abdominal pain | 9 | 23.7 |
| Arrhythmia | 8 | 21.1 |
| Headache | 8 | 21.1 |
| Hypoxemia | 7 | 18.4 |
| Fever | 5 | 13.2 |
| Diarrhea | 4 | 10.5 |
Toxicity and adverse reactions during
the process of bone marrow suppression following reinfusion
Three and 14 days after pretreatment in the 38
cases, there were 19 cases of grade I, 11 cases of grade II, five
cases of grade III and three cases of grade IV adverse reaction.
One patient with NB succumbed on the day 4 following HDCT during
the process of bone marrow suppression, due to multiple organ
failure and primary cardiac damage with low immune tolerance.
According to Bearman's criteria, the toxicity and adverse reactions
in the organs of the remaining 37 cases were summarized and are
presented in Table V.
 | Table V.Distribution of organ toxicity in 37
surviving patients. |
Table V.
Distribution of organ toxicity in 37
surviving patients.
|
| Stage, n |
|---|
|
|
|
|---|
| Organ | 0 | I | II | III | IV |
|---|
| Heart | 31 | 2 | 2 | 1 | 1 |
| Bladder | 36 | 1 | 0 | 0 | 0 |
| Kidney | 37 | 0 | 0 | 0 | 0 |
| Liver | 21 | 13 | 3 | 0 | 0 |
| Central nervous
system | 37 | 0 | 0 | 0 | 0 |
| Oral | 12 | 13 | 7 | 4 | 1 |
|
Gastrointestinal | 12 | 15 | 7 | 2 | 1 |
| Infection | 1 | 9 | 10 | 15 | 2 |
| Lung | 37 | 0 | 0 | 0 | 0 |
All 37 cases developed different degrees of oral
ulceration, vomiting, diarrhea and plasma imbalance, which were
gradually restored to normal on the 22nd day after mucosal damage.
There were four cases of sepsis, 19 of respiratory infections,
eight of gastrointestinal infections, 3 of urinary tract infections
and four of an unknown fever. All the diseases or associated
clinical symptoms were controlled using broad-spectrum antibiotics.
Of these 37 cases, one patient developed hypovolemic shock due to
gastrointestinal bleeding caused by intestinal mucosal damage
following chemotherapy, and recovered by symptomatic treatment
within 22 days. Five cases presented with myocardial damage,
arrhythmia and cardiac dysfunction due to HDCT (manifested as
elevated creatine kinase-MB), but recovered within 60 days
following cardiac treatment. Another 17 cases exhibited elevated
levels of liver transaminases and three cases developed jaundice;
these patients recovered following triple protection and
symptomatic treatment for one week. One case whose mucosal was
damaged by melphalan developed hemorrhagic cystitis, but recovered
within one month following hydration and urine alkalization.
Efficacy and prognosis of APBSCs
The average time for bone marrow reconstitution in
the 37 cases was 12.3±3.1 days after reinfusion. The quantity of
reinfused CD34+ cells was negatively correlated with the
reconstitution time of the bone marrow (rs=-0.634; P=0.001),
showing that a high level of CD34+ cell collection can
contribute to bone marrow reconstitution.
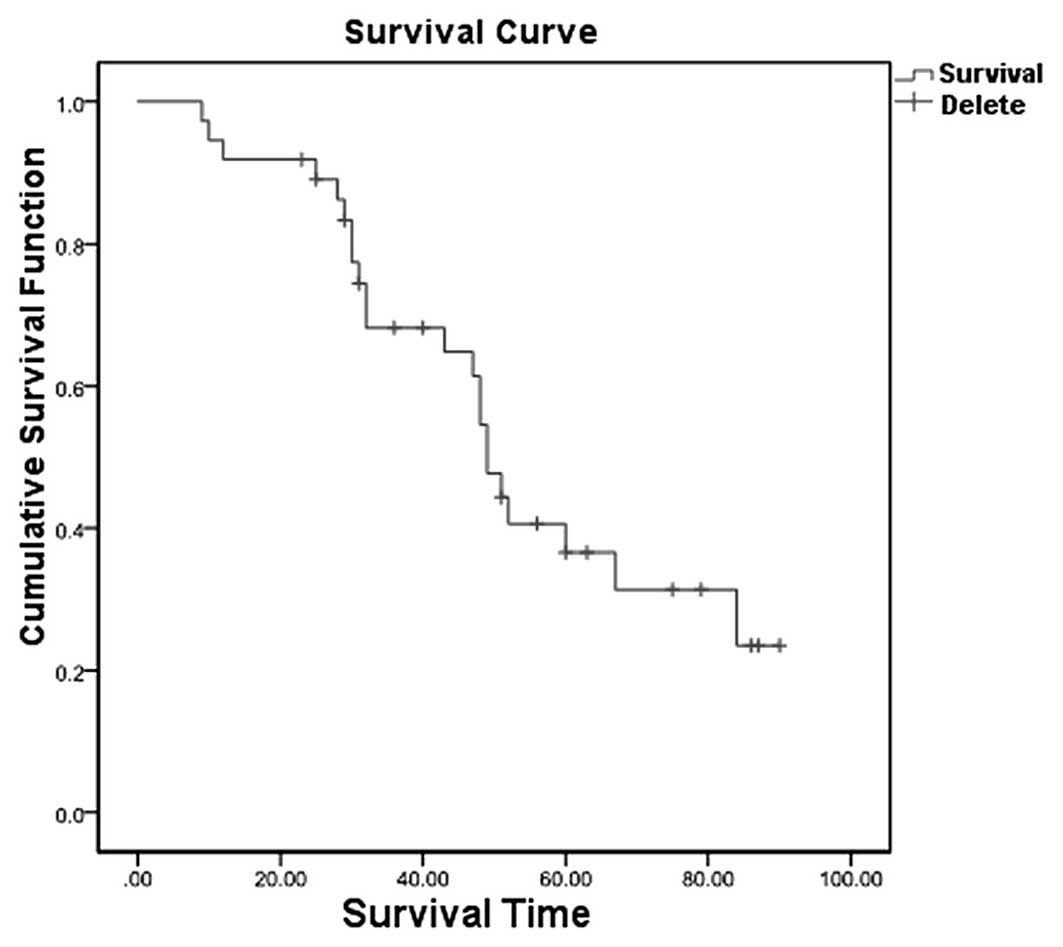
Subsequent to APBSCT treatment, the cumulative
survival rates of the 37 patients at one, three and five years were
91.9, 68.2 and 36.6%, respectively (95% confidence interval,
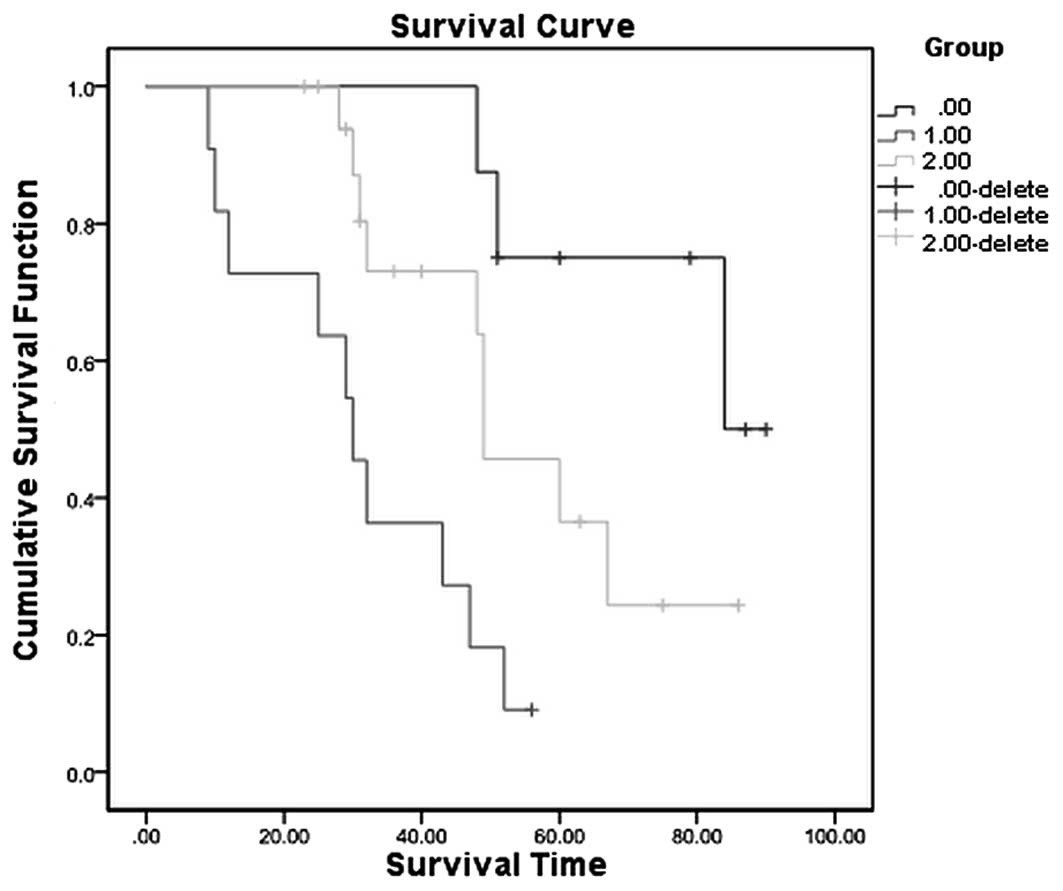
44.89–53.11) (Fig. 1). Next, the 37
patients were subdivided into three groups, as follows: The CR
group (13 cases), the PR group (14 cases) and the PD group (10
cases). The survival rates at 5 years post-treatment were 75.0% in
the CR group, 36.5% in the PR group and 9.1% in the PD group
(Fig. 2).
Discussion
With the widespread use of chemotherapy, surgery,
radiation and biological immunotherapy, the survival rates of
children with advanced malignant solid tumors has improved
significantly (19–21). However, due to the short incubation
period, rapid growth and distant metastasis, regular treatment of
advanced solid tumors only improves short-term survival, and rarely
improves long-term survival (22–24).
APBSCT was introduced into the clinic in 1985 and confers certain
advantages, including rapid engraftment, rapid and complete
hematopoietic reconstitution, a low risk of recurrence, fewer
transplant-related complications, high safety and a low cost.
Currently, APBSCT is one of the most effective methods for
prolonging the lifespans of patients with malignant solid tumors,
particularly advanced solid tumors (25,26).
In the present study, the median survival time of
the children was 49.0 months. The survival rate at one, three and
five years post-treatment was 91.9, 68.2 and 36.6%, respectively.
These findings were consistent with the results of a previous study
by Kushner et al (26).
However, for the 10 cases with PD, the five year survival rate was
only 9.1%. Although APBSCT cannot alter the prognosis, it can
prolong the survival time and reduce the tumor burden of PD in an
individual patient. The present findings also indicated that
children who were <5 years of age and underwent fewer
chemotherapy cycles contribute to the collection and acquisition of
stem cells. In addition, the long-term prognosis of CR and PR
patients was also excellent, suggesting that powerful induction
therapy in the early stages can contribute to CR.
For children with malignant solid tumors, the method
for controlling the risk during the mobilization, collection and
reinfusion processes, and a reduction in the possibility of failing
to receive APBSCT is important for increasing the success rate of
the treatment and prolonging survival (27–29). In
this study, the ABPSCTs of 38 patients were successfully mobilized,
collected and reinfused, and engraftment and hematopoietic
reconstitution was achieved following pre-transplant conditioning
in all patients, with the exception of one, who succumbed to
multiple organ failure. Additionally, it was demonstrated that the
processed blood volume was positively associated with the
CD34+ cell count in PBSC collection and the age of the
children, which was in accordance with a previous study by Sevilla
et al (30). Sufficient MNC
and CD34+ cell counts were obtained from 31 of the 38
cases for PBSCT. During the process of PBSC collection, the
children exhibited a low blood volume, resulting in vasovagal
reactions, such as hypotension, tachycardia, paleness and severely
hypovolemic shock. Therefore, continuous electrocardiogram and
blood pressure monitoring in the process of PBSC collection
contributes to the early detection of vasovagal reactions. Eight
cases in the present study sustained a 48% rate of PLT loss
following PBSC collection, and the corresponding measure was a
timely intravenous infusion of PLTs.
According to the standards set in the study by
Balduzzi et al (31), 31 cases
(81.6%) in the present study reached the required threshold value
of stem cell transplantation. The remaining seven cases were all
from patients with PD, which accounted for 70% (7/10) of the total
patients with PD, suggesting that the quantity of obtained stem
cells was closely associated with the condition of the patient. An
explanation for these findings is that patients with PD generally
have a poorer physical condition, tolerance and hematopoietic bone
marrow function following radiotherapy, chemotherapy and surgery,
which leads to a decrease in the stem cell counts in comparison to
patients in the remission stage.
Previous studies have shown that the occurrence of
hemoglobinuria during the process of reinfusion is associated with
erythrocyte lysis and the use of dimethyl sulfoxide in
cryopreserved and thawed peripheral blood stem cell autografts
(32,33). In the present study, the incidence of
acute renal dysfunction was reduced through a series of methods,
including the use of hormones, hydration, alkalization and diuretic
measures to ensure restoration to normal within 12 hours. In
addition, it was found that 37 out of the 38 children developed
severe infections, which may have been due to neutropenia and
pretreatment in the bone marrow suppression and function recovery
stages during the process of transplantation, thus conferring an
increased risk of associated infection (34,35). In
the present study, the joint use of broad-spectrum antibiotic drugs
played an important role in preventing and controlling infection
from pediatric APBSCT.
In summary, the present study has demonstrated the
safety and efficacy of HDCT followed by APBSCT for the treatment of
children with advanced malignant solid tumors and suggests that
HDCT with APBSCT may be used as a post-remission therapy. A
prospective study will be necessary to analyze whether HDCT with
auto-PBSCT will affect outcomes in the treatment of children with
advanced malignant solid tumors.
Acknowledgements
This study was supported by a branch project of the
Capital Characteristic Clinical Project from the Beijing Science
and Technology Commission in China (grant no.
Z121107001012057).
References
|
1
|
Shi X, Tian L, Zhu XD, Wang HM and Qin H:
Effect of Chinese drugs combining with chemotherapy on quality of
life in 146 children with solid tumor. Chin J Integr Med. 17:31–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Müller HL, Oh Y, Lehrnbecher T, Blum WF
and Rosenfeld RG: Insulin-like growth factor-binding protein-2
concentrations in cerebrospinal fluid and serum of children with
malignant solid tumors or acute leukemia. J Clin Endocrinol Metab.
79:428–434. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Duan W, Jin X, Xiu Y, et al: Expression of
the novel all-trans retinoic acid-related resistance gene HA117 in
pediatric solid tumors. J Pediatr Hematol Oncol. 36:45–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Körbling M and Freireich EJ: Twenty-five
years of peripheral blood stem cell transplantation. Blood.
117:6411–6416. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheridan WP, Morstyn G, Wolf M, et al:
Granulocyte colony-stimulating factor and neutrophil recovery after
high-dose chemotherapy and autologous bone marrow transplantation.
Lancet. 2:891–895. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nademanee A, Sniecinski I, Schmidt GM, et
al: High-dose therapy followed by autologous peripheral-blood
stem-cell transplantation for patients with Hodgkin's disease and
non-Hodgkin's lymphoma using unprimed and granulocyte
colony-stimulating factor-mobilized peripheral-blood stem cells. J
Clin Oncol. 12:2176–2186. 1994.PubMed/NCBI
|
|
7
|
Bensinger WI, Clift R, Martin P, et al:
Allogeneic peripheral blood stem cell transplantation in patients
with advanced hematologic malignancies: a retrospective comparison
with marrow transplantation. Blood. 88:2794–2800. 1996.PubMed/NCBI
|
|
8
|
Körbling M, Fliedner TM, Holle R, et al:
Autologous blood stem cell (ABSCT) versus purged bone marrow
transplantation (pABMT) in standard risk AML: influence of source
and cell composition of the autograft on hemopoietic reconstitution
and disease-free survival. Bone Marrow Transplant. 7:343–349.
1991.PubMed/NCBI
|
|
9
|
Mehta J, Powles R, Singhal S and Treleaven
J: Peripheral blood stem cell transplantation may result in
increased relapse of acute myeloid leukaemia due to reinfusion of a
higher number of malignant cells. Bone Marrow Transplant.
15:652–653. 1995.PubMed/NCBI
|
|
10
|
Reiffers J, Korbling M, Labopin M, Henon P
and Gorin NC: Autologous blood stem cell transplantation versus
autologous bone marrow transplantation for acute myeloid leukemia
in first complete remission. Int J Cell Cloning. 10 (Suppl
S1):111–113. 1992. View Article : Google Scholar
|
|
11
|
Demirer T, Petersen FB, Bensinger WI, et
al: Autologous transplantation with peripheral blood stem cells
collected after granulocyte colony-stimulating factor in patients
with acute myelogenous leukemia. Bone Marrow Transplant. 18:29–34.
1996.PubMed/NCBI
|
|
12
|
Gignac GA and Wexler LH: Effects of
therapy for solid tumorsFertility Preservation in Male Cancer
Patients. Mulhall JP: Cambridge University Press; Cambridge: pp.
119–128. 2013, View Article : Google Scholar
|
|
13
|
Taurin S and Greish K: Enhanced vascular
permeability in solid tumors: a promise for anticancer
nanomedicineTight Junctions in Cancer Metastasis. Martin TA and
Jiang WG: Springer; Dordrecht: pp. 81–118. 2013
|
|
14
|
Cesaro S, Nesi F, Tridello G, et al: A
randomized, non-inferiority study comparing efficacy and safety of
a single dose of pegfilgrastim versus daily filgrastim in pediatric
patients after autologous peripheral blood stem cell transplant.
PloS One. 8:e532522013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eto T, Takase K, Miyamoto T, et al:
Autologous peripheral blood stem cell transplantation with
granulocyte colony-stimulating factor combined conditioning regimen
as a postremission therapy for acute myelogenous leukemia in first
complete remission. Int J Hematol. 98:186–196. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pulsipher MA, Chitphakdithai P, Logan BR,
et al: Acute toxicities of unrelated bone marrow versus peripheral
blood stem cell donation: results of a prospective trial from the
National Marrow Donor Program. Blood. 121:197–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bearman SI, Appelbaum FR, Buckner CD, et
al: Regimen-related toxicity in patients undergoing bone marrow
transplantation. J Clin Oncol. 6:1562–1568. 1988.PubMed/NCBI
|
|
18
|
Corrias MV, Haupt R, Carlini B, et al:
Peripheral blood stem cell tumor cell contamination and survival of
neuroblastoma patients. Clin Cancer Res. 12:5680–5685. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Widemann BC, Salzer WL, Arceci RJ, et al:
Phase I trial and pharmacokinetic study of the farnesyltransferase
inhibitor tipifarnib in children with refractory solid tumors or
neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol.
24:507–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Crist WM and Kun LE: Common solid tumors
of childhood. N Engl J Med. 324:461–471. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hartmann O, Benhamou E, Beaujean F, et al:
High-dose busulfan and cyclophosphamide with autologous bone marrow
transplantation support in advanced malignancies in children: a
phase II study. J Clin Oncol. 4:1804–1810. 1986.PubMed/NCBI
|
|
23
|
Green DM, Kun LE, Matthay KK, et al:
Relevance of historical therapeutic approaches to the contemporary
treatment of pediatric solid tumors. Pediatr Blood Cancer.
60:1083–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Macy ME, Duncan T, Whitlock J, et al: A
multi-center phase Ib study of oxaliplatin (NSC#266046) in
combination with fluorouracil and leucovorin in pediatric patients
with advanced solid tumors. Pediatr Blood Cancer. 60:230–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon SY, Won SC, Han JW, Shin YJ and Lyu
CJ: Feasibility of sequential high-dose chemotherapy in advanced
pediatric solid tumors. Pediatr Hematol Oncol. 27:1–12. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kushner BH, Kramer K, Meyers PA, Wollner N
and Cheung NK: Pilot study of topotecan and high-dose
cyclophosphamide for resistant pediatric solid tumors. Med Pediatr
Oncol. 35:468–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hoffman JA, Shah AJ, Ross LA and Kapoor N:
Adenoviral infections and a prospective trial of cidofovir in
pediatric hematopoietic stem cell transplantation. Biol Blood
Marrow Transplant. 7:388–394. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ljungman P, Deliliers GL, Platzbecker U,
et al: Cidofovir for cytomegalovirus infection and disease in
allogeneic stem cell transplant recipients. The Infectious Diseases
Working Party of the European Group for Blood and Marrow
Transplantation. Blood. 97:388–392. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klein HG, Spahn DR and Carson JL: Red
blood cell transfusion in clinical practice. Lancet. 370:415–426.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sevilla J, González-Vicent M, Madero L,
García-Sánchez F and Angel Diaz M: Large volume leukapheresis in
small children: safety profile and variables affecting peripheral
blood progenitor cell collection. Bone Marrow Transplant.
31:263–267. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balduzzi A, Gooley T, Anasetti C, et al:
Unrelated donor marrow transplantation in children. Blood.
86:3247–3256. 1995.PubMed/NCBI
|
|
32
|
Okamoto Y, Takaue Y, Saito S, et al:
Toxicities associated with cryopreserved and thawed peripheral
blood stem cell autografts in children with active cancer.
Transfusion. 33:578–581. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Attarian H, Feng Z, Buckner CD, MacLeod B
and Rowley SD: Long-term cryopreservation of bone marrow for
autologous transplantation. Bone Marrow Transplant. 17:425–430.
1996.PubMed/NCBI
|
|
34
|
Wingard JR, Merz WG, Rinaldi MG, Johnson
TR, Karp JE and Saral R: Increase in Candida krusei
infection among patients with bone marrow transplantation and
neutropenia treated prophylactically with fluconazole. N Engl J
Med. 325:1274–1277. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Howell A, Gumpel JM and Watts RW:
Depression of bone marrow colony formation in gold-induced
neutropenia. Br Med J. 1:432–434. 1975. View Article : Google Scholar : PubMed/NCBI
|