Introduction
Cervical cancer (CC) is one of the most common
cancers among women worldwide and the second cause of cancer
mortality in developing countries (1). Human papillomavirus (HPV) is the
leading risk factor for CC development (2). However, different types of lesions
may be observed in the cervix prior to cancer establishment,
including grade 1, 2 and 3 cervical intraepithelial neoplasia, as
well as invasive carcinoma (3).
Apart from HPV infection, other risk factors have been reported to
be involved in the transformation process from normal to malignant
cells, including smoking, oral contraceptive use and steroid sex
hormones, among others (4–6). The tumor microenvironment (TME) is
crucial for the carcinogenic process, and hormones are a key factor
in this context. In addition, cells that belong to the innate
immune system are located in the TME, having the ability to kill
tumor cells (7). 17β-estradiol
(E2) and prolactin (PRL) have been reported to be present in the
TME (8–10); however, their role on immunological
mechanisms generated in the response to CC is poorly
understood.
Estrogens are sex hormones that belong to the
cholesterol-derived steroids group, whose three primary forms are
estrone (E1), E2 and estriol (E3), of which E2 has been reported to
exhibit an increased biological activity (11). The functions of E2 are mediated
through the estrogen receptors (ER)α and β, and the G
protein-coupled estrogen receptor 1 (GPER) (12,13).
Of note, ERα, ERβ and GPER have been shown to be overexpressed in
CC tissues compared with that in the premalignant lesion and normal
cervical epithelium (14,15). Studies using mice have demonstrated
that a temporary presence of E2 promotes CC, being ERα
signaling-dependent (16,17).
PRL is a lactogenic polypeptide hormone synthesized
primarily by the pituitary gland (18). Additionally, other studies have
demonstrated an extrapituitary PRL production by some tissues and
organs. PRL exerts its functions through its binding to the PRL
receptor (PRLR) (19,20). Previous studies have demonstrated
the expression of a 60 kDa PRL in CC tissues and CC-derived cells.
This PRL variant may regulate various processes, including
apoptosis, cytokine production and metabolism in THP-1 and
CC-derived cells (14,21,22).
PRLR is a member of the class I cytokine receptor
superfamily; it presents with various isoforms, one long, one
intermediate, and two short isoforms, with an average weight of
85–90, 65 and 40–50 kDa respectively (23). High PRL levels have been reported
in the serum of patients with CC (24). There is also evidence of the
increased expression of PRLR in premalignant lesions, CC tissues
and CC-derived cell lines (21).
The stimulation of CC-derived cells with PRL induces the expression
of anti-apoptotic gene through the signal transducer and activator
of transcription (STAT)-3 (25).
This evidence confirms the importance of PRL in CC pathogenesis and
some relevant events in the progression of the disease.
Natural killer (NK) cells are a major component of
the innate immunity against tumors and viral infections. They
constitute 5 to 15% of all lymphocytes and are phenotypically
defined by the expression of CD56 and the absence of CD3 (26). NK cells are equipped with a
repertoire of receptors that can both stimulate (activating
receptors) or prevent (inhibitory receptors) their reactivity
(27). The natural cytotoxicity
receptors (NCRs), including natural cytotoxicity triggering
receptor 3 (NKp30), natural cytotoxicity triggering receptor 2
(NKp44) and natural cytotoxicity triggering receptor 1 (NKp46),
have been reported to induce NK cell activation; however, their
corresponding ligands have not yet been well defined (28). Another activating receptor is
natural killer group 2D (NKG2D), a type 2 transmembrane protein,
whose ligands include MHC-I chain-related protein A and B (MICA and
MICB) and the UL16 binding proteins (ULBP) from 1 to 6 (29). In 2012, a previous study revealed
that NKG2D receptor expression in NK cells decreased when
interacting directly with CC cell lines (30). Another study revealed that the
expression of NKp30 and NKp46 receptors was decreased in squamous
intraepithelial lesions and CC; however, NKG2D was only decreased
in CC, and was negatively associated with NK cell cytotoxic
activity (31). Of note, tumors
evade the immune system through the liberation of MICA and MICB
from the cellular membrane to create a soluble form (32). This process has been reported to be
mediated by various metalloproteinases (33). The soluble form of MICA and MICB
has been found to be associated with the internalization and
degradation of NKG2D and the consequent decrease in the NK
cell-mediated cytotoxicity (34,35).
In cancers, such as CC, which is related to a viral infection, it
is crucial to understand whether the factors included in the TME,
including hormones, may modify the mechanisms that favor the
malignancy of the disease.
Both estrogen and PRL receptors have been identified
in human cell lines and murine NK cells. However, the expression of
GPER in these cells remains unclear (36,37).
There is evidence to indicate that estrogens have been linked to a
decrease in NK cell cytotoxicity using human and murine models,
while PRL exert opposite effects in human NK cell lines (NK-92 and
YT cell lines) (37). In addition,
the effects of these hormones may affect the regulation of proteins
that belong to cytotoxicity processes, including activating
receptors and their ligands (37–43).
Riera-Leal et al (14), observed the effects of E2 and PRL
on CC-cell line metabolism and concluded that the two hormones
increased cell metabolism, with PRL to a lesser extent than E2.
However, PRL appears to exert a more prominent effect over E2 when
simultaneously applied.
In the CC TME, E2 and PRL are present. Thus, the
present study aimed to investigate the effects of the E2 and PRL
stimuli, concurrently or separately applied on NKL cells and
CC-derived cell lines, as well as to evaluate the expression of
different molecules related to NK cell-mediated cytotoxicity,
including NCR, NKG2D and MICA/B.
Materials and methods
Cell culture and hormone stimuli
The HeLa, SiHa, C33A, MCF7 (all from ATCC) and HaCaT
(CLS Cell Lines Service GmbH) cell lines were cultured in DMEM
medium, supplemented with 10% fetal bovine serum (FBS), 1%
penicillin G (10,000 U/ml), and streptomycin (10,000 µg/ml) (Gibco;
Thermo Fisher Scientific, Inc.). Similarly, the NKL (kindly donated
by Dr Adriana Aguilar Lemarroy) and K562 (ATCC) cell lines were
grown in supplemented RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.). All cell lines were incubated at 37°C and 5%
CO2 until 80% of confluence was obtained. NKL, HeLa,
SiHa, C33A and HaCaT cell cultures were stimulated for 48 h with
PRL (200 ng/ml) isolated from HeLa cell supernatant, E2 (10 nM;
Sigma Aldrich; Merck KGaA), or both (E2 and PRL). HeLa, SiHa, C33A,
HaCaT and K562 cell lines were authenticated by Multiplexion GmbH,
using the multiplex human cell line authentication test.
Isolation and purification of the 60
kDa-weighted PRL
The isolation of PRL from the HeLa cell supernatant
was performed using magnetic beads (Protein G Microbeads
MultiMACS™; Miltenyi Biotec GmbH) following the
manufacturer's protocol. The 60 kDa PRL was purified employing the
50 kDa molecular cut-off filters (Amicon® Ultra 0.5 ml
centrifugal filters; cat. no. UFC505024; MilliporeSigma). The
procedure for the filtration was performed as follows: 14,000 × g
for 30 min (filtration phase); 1,000 × g for 2 min (recovery phase)
at 4°C. Once purified, the correct identification of the 60 kDa PRL
was determined using a 12% polyacrylamide gel for electrophoresis
at 95V for 90 min. Subsequently, silver nitrate (cat. no. 209139;
MilliporeSigma) staining was performed for 20 min at room
temperature to visualize the 60-kDa band belonging to PRL. Finally,
quantification of the purified protein was performed utilizing
Thermo Scientific NanoDrop 2000c Spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.).
NK cell cytotoxicity assay
The cytotoxicity of NK cells against the Green
Fluorescent Protein (GFP)-transfected K562 cell line (kindly
donated from Dr Adriana Aguilar Lemarroy) was evaluated in a
propidium iodide (PI) flow cytometry assay. K562 cells were seeded
with a constant number (150,000) with different effector (NK cells)
to target cell ratios [effector:target (E:T) 1:1, 5:1 and 10:1].
The target cells were incubated alone to measure untreated control
cell death. Co-cultures between NKL and GFP-transfected K562 cells
(GFP-K562) (lymphoblasts derived from chronic myeloid leukemia),
characterized by its absence or decrease of MHC-I molecules, in
complex medium were performed for 4 h at 37°C and 5%
CO2. The cells were washed twice with 1% PBS and
incubated in the same buffer with PI (cat. no. P4170;
MilliporeSigma) for 20 min at room temperature in darkness. The
reading was performed using Attune® NxT acoustic focus
cytometer with the FACS Diva v3.1.2 software (BD Biosciences). The
cytotoxic activity was expressed as the % of specific lysis by
using the following formula:
Degranulation assay
CD107a was used as a marker of NKL degranulation
upon target recognition. A total of 30,000 NKL cells (effector)
were co-cultured with 30,000 K562 cells (target) cells at an E:T
ratio of 1:1 in 96-well plates for 4 h. At the start of the
incubation period, a 1:400 dilution of anti-CD107a-PE (cat. no.
555801; BD Biosciences) was added to each well. Monensin
(BioLegend, Inc.) was used as a protein transport blocker an added
for 1 h into the co-culture. To identify viable NKL cells from the
target cells, a 1:50 dilution of CD45 antibody (BioLegend, Inc.;
cat. no. 304027) and Zombie NIR dye (BioLegend, Inc.; cat. no.
423105) were used. The reading was performed using
Attune® NxT acoustic focus cytometer with the FACS Diva
Software v3.1.2 (BD Biosciences).
Western blot analysis
Total proteins were extracted from NKL and MCF7 cell
lines (obtained from ATCC) using RIPA lysis and extraction buffer
(cat. no. 89900; Thermo Fisher Scientific, Inc.), and the coomasie
plus (Bradford) assay (cat. no. 23238; Thermo Fisher Scientific,
Inc.) was used for protein quantification. A total of 50 µg protein
was mixed with loading buffer and then denatured at 95°C for 5 min.
Electrophoresis was performed on 10% polyacrylamide gels at 110 V
for 60 min, and subsequently, a PVDF-membrane electrical
transference (Bio-Rad Laboratories, Inc.) was performed for 90 min
at 240 V. The membranes were incubated overnight at 4°C with a
blocking solution of 1X PBS and 5% blotting-grade blocker (cat. no.
1706404; Bio-Rad Laboratories, Inc.). The dilution of the primary
antibodies used was 1:500 for ERα, ERβ and PRLR (cat. nos. sc-8002,
sc-373853, sc-20992, respectively; Santa Cruz Biotechnology, Inc.)
and GPER (cat. no. ab39742; Abcam) and 1:10,000 for β-actin (cat.
no. sc-47778; Santa Cruz Biotechnology, Inc.) in blocking solution
consisting of 1X PBS and 5% blotting-grade blocker, and incubated
overnight at 4°C. The membranes were washed five times for 7 min
with PBS and Tween-20 (cat. no. P1379; MilliporeSigma) and
incubated for 90 min with a dilution of 1:10,000 anti-mouse or
anti-rabbit secondary antibodies (cat. nos. sc-2005, sc-2357; Santa
Cruz Biotechnology, Inc.) at room temperature. Subsequently, the
membranes were washed 6 times for 10 min. Luminol and horseradish
peroxidase reagents (Immobilion; Merck KGaA) were used to perform
the chemiluminescence process. β-actin expression was used as an
internal control. The Microchemi 6.0 (DNR Bio-Imaging Systems Ltd.)
was used to visualize the membranes and GelQuant software V1.7.8
(BiochemLabSolutions) was utilized for densitometric
measurement.
Flow cytometry
For the NKL cell lines with and without hormonal
stimulation, the cell density was adjusted to 2×105
cells in total. The cells were washed with 1X PBS and centrifuged
at 1,800 × g for 10 min at 4°C. Subsequently, cells were incubated
with anti-NKG2D, anti-NKp30, anti-NKp44 and anti-NKp46 antibodies
at 1:100 dilution (cat. nos. 130-123-948, 130-121-995, 130-120-623
and 130-126-054, respectively; Miltenyi Biotec GmbH) at 4°C for 30
min in the dark. The cells were washed again and centrifuged at
1,800 × g for 5 min at 4°C. The cells were then fixed with 1 ml
0.05% PBS-formaldehyde solution. Following the same procedure,
CC-derived and HaCaT cell lines were labeled against anti-MICA/B
antibodies (cat. no. 130-100-889; Miltenyi Biotec GmbH) for
analysis using flow cytometry. The percentages and mean
fluorescence intensity (MFI) were determined with appropriate
protocols and controls to electronically compensate the overlapping
signals using the Attune® NxT Software v3.1.2 acoustic
focus cytometer (Invitrogen; Thermo Fisher Scientific, Inc.).
Soluble MICA quantification in cell
culture supernatants
Soluble MICA levels were analyzed using the Human
MICA ELISA kit (cat. no. RAB0358-1KT; MilliporeSigma) in the
supernatant of HeLa, SiHa, C33A and HaCaT cell lines stimulated
with E2 and PRL, according to the manufacturer´s instructions. The
results were obtained from two independent experiments using the
appropriate absorbance values (450 nm).
Statistical analysis
Data capture was performed using the statistical
program GraphPad 8.0.2 (GraphPad Software, Inc.). Statistical
analysis to compare the expression patterns of hormone receptors,
activating receptors, ligands and differences in the cytotoxicity
activity were carried out, using the ANOVA test followed by the
Tukey's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Antagonistic effects between E2 and
PRL on NK cell-mediated cytotoxicity
To evaluate the effect of hormones on NK
cell-mediated cytotoxicity, NKL cells were stimulated with E2 (10
nM) or PRL (200 ng/ml) either alone or in combination for 48 h and
subsequently co-cultured with GFP-K562 cells at various E:T ratios
(1:1, 5:1 and 10:1) for 4 h. The identification of dead target
cells was characterized as GFP+PI+ by flow
cytometric analysis (Fig. 1A).
 | Figure 1.Differential effects of hormones on
NK cytotoxic activity. NKL cells were stimulated with E2 (10 nM),
PRL (200 ng/ml), both (E2 + PRL) or untreated (control) for 48 h
and incubated in the presence of GFP-transfected K562 cells at
different E:T ratios (1:1, 5:1 and 10:1). The dead target cells are
characterized as GFP+PI+. (A) Representative
dot plot of three independent experiments. (B) Graphic of specific
lysis of target cells, data shown represent the mean from 3
independent experiments. (C) NKL cells were incubated with K562 at
1:1 E:T ratio. Zombie NIR dye, CD45 and CD107a were used to
identify viability, NKL population and degranulation respectively.
Statistical analysis was performed using ANOVA (*P<0.05). NK,
natural killer; E2, 17β-estradiol; PRL, prolactin; GFP, green
fluorescent protein; PI, propidium iodide; E:T, effector:target;
NIR, near infrared. |
Comparing the effect of hormones against untreated
control cells (without stimulation), it was demonstrated that
stimulation with E2 tends to decrease the lysis of GFP-K562 cells;
however, PRL stimulation tended to increase cytotoxicity against
GFP-K562 cells. Notably, at a 10:1 ratio, stimulation with PRL
exerted a positive effect on the cytotoxicity on NKL cells,
contrary to E2, which exerts an antagonistic effect as compared to
PRL (P<0.05). Notably, the combined effect of the two hormones
exerted similar effects as those observed with PRL alone, as
regards NKL cell-mediated cytotoxicity (P<0.05) (Fig. 1B). It was revealed that the
hormones located within the TME may have the ability to regulate
the cytotoxicity of NK cells with antagonistic outcomes.
To confirm the cytotoxicity assay results, a
degranulation assay in NKL cells was performed, and it was observed
that CD107a expression tended to decrease in NKL cells stimulated
with E2 compared to the untreated control cells. It was also
demonstrated that PRL stimulation was able to induce CD107a
expression compared to the unstimulated cells, which is consistent
with the antagonistic effects shown by the cytotoxicity assay. The
E2 + PRL stimulation did not cause a marked change in degranulation
marker expression in NKL cells (Fig.
1C).
Expression of hormonal receptors in
NKL cells
Once it was observed that hormones can regulate NKL
cell-mediated cytotoxicity, the expression of the estrogen
receptors (ERα, ERβ and GPER) and the PRL receptor were
characterized using western blotting with protein extracts from NKL
cells.
The expression of the four hormonal receptors is
depicted in Fig. 2. The expression
of ERα is denoted by the presence of a single 46-kDa band. The
bands indicating the expression of ERβ are 32, 45 and 56 kDa. Of
note, the 45-kDa band was thicker in the two cell lines. To the
best of our knowledge, this is the first study to demonstrate the
presence of GPER in NK cells. GPER was expressed as 42- and 100-kDa
bands. Notably, the highest GPER expression was the 100-kDa band,
which has been related to glycosylation of this receptor. Finally,
PRLR was observed in several bands of approximately 44, 50, 65 and
90 kDa in agreement with the various isoforms of the receptor. Of
note, the expression of PRLR was higher in the 50-kDa band, which
corresponds to a short isoform of this receptor. When compared with
that of the MCF7 cell line, which was used as a positive control,
the expression pattern of ERα and ERβ was similar to that observed
in the NKL cells. In the MCF7 cells, a higher expression level of
the normal form of GPER was observed compared with that in the NKL
cells. Finally, it was observed that MCF7 cells expressed the long
isoforms of PRLR in a greater proportion in comparison to the NKL
cells. The presence of all hormone receptors and their isoforms
contribute to a more detailed understanding of the hormonal effects
on NK cells.
 | Figure 2.Expression of estrogen and prolactin
receptors in NKL cells. The expression of hormonal receptors was
evaluated in protein extracts from NKL and MCF7 cell lines. Western
blot analysis revealed the expression of all four hormonal
receptors (ERα, ERβ, GPER and PRLR) and their different isoforms in
NKL (lane 1) and MCF7 cells (lane 2). ERα presented a unique 46-kDa
band, ERβ presented bands of approximately 32, 45 and 56 kDa, GPER
exhibited bands of 42 and 100 kDa and PRLR presented bands of ~44,
50, 65 and 90 kDa. β-actin was used as a loading control. NK,
natural killer; ER, estrogen receptor; GPER, G protein-coupled
estrogen receptor 1; PRLR, prolactin receptor. |
Expression of NCR and NKG2D in NKL
cells stimulated with E2 and PRL
To evaluate the effect of hormone stimuli on
activating receptors including NKp30, NKp44, NKp46 and NKG2D
expression on NKL cell surface, cells were stimulated with E2 and
PRL for 48 h and flow cytometric analysis was performed (Fig. 3). In the untreated control cells
(without stimulation), the percentage of NKG2D and NKp30 receptors
(100 and 96.2%, respectively) was higher than that for NKp46
(14.6%). As was expected, the percentage of positivity for the
NKp44 receptor was zero, as previously reported (44). The histograms demonstrated that the
percentages of positive NKL cells for the different receptors were
not markedly altered due to hormonal stimuli (Fig. 3A, C, E and G). However, PRL stimuli
induced a significant decrease in the expression of NKG2D compared
to the untreated control cells or E2 stimuli (P<0.05; Fig. 3B). The expression of NKp30, NKp44
and NKp46 receptors in NKL cells was not significantly altered by
E2 and PRL stimuli (Fig. 3D, F,
H). The downregulation of NKG2D due to PRL stimulation
demonstrated that it may be able to modulate signaling pathways
involved in NK cell cytotoxicity.
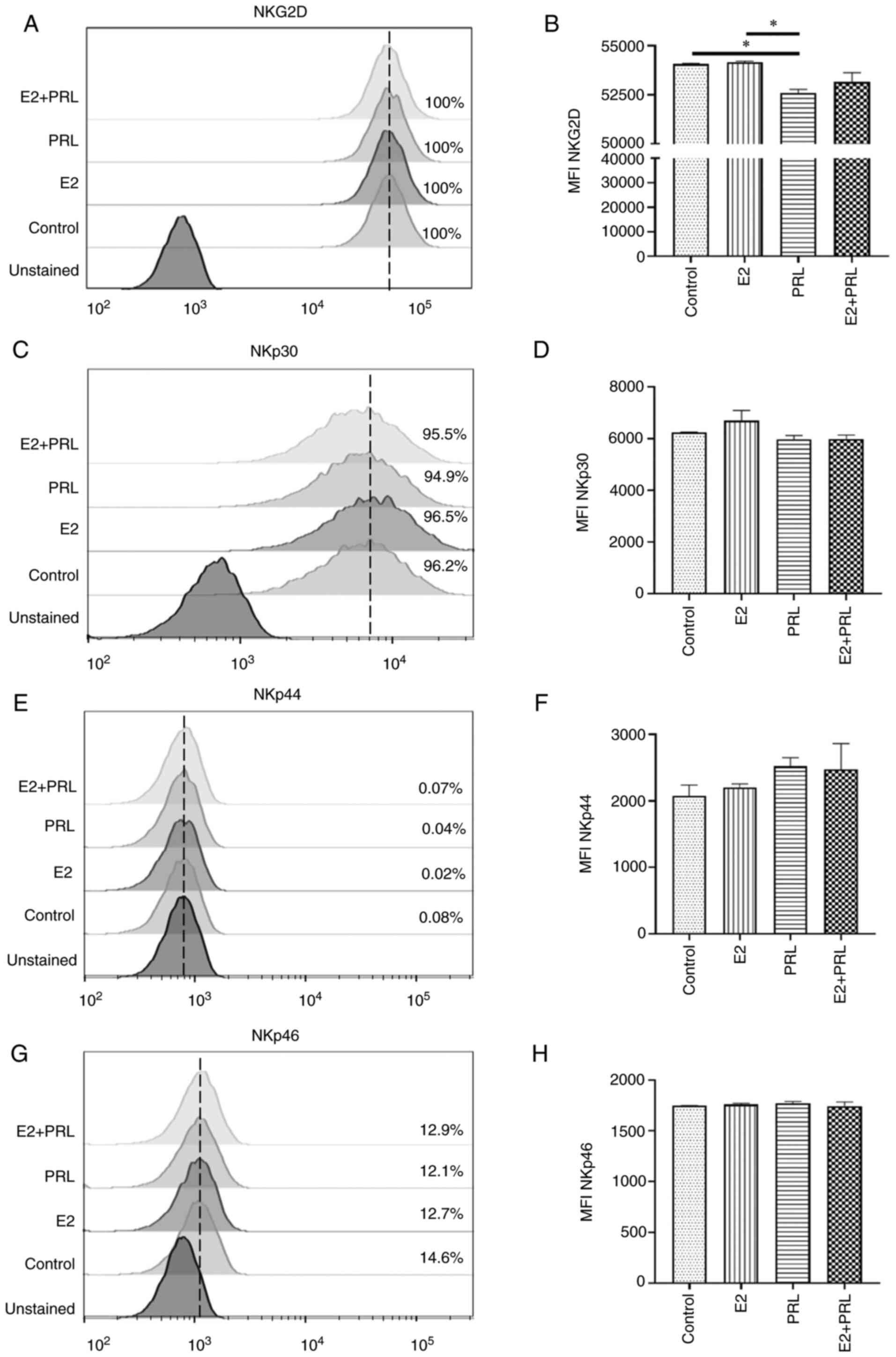 | Figure 3.NCR and NKG2D regulation by hormones
in NKL cells. The expression of (A) NKG2D, (C) NKp30, (E) NKp44,
and (G) NKp46 was analyzed using flow cytometry on NKL cells
stimulated with E2, PRL, both or untreated (control). The dotted
line (—) indicates the maximum peak located in the untreated
control cells. The MFI was expressed as the mean ± SD. (B, D, F and
H) Histograms of the MFI representative of each group. All
statistical analyses were performed using ANOVA (*P<0.05). NCR,
natural cytotoxicity receptors; NKG2D, natural killer group 2D;
NKp30, natural cytotoxicity triggering receptor 3; NKp44, natural
cytotoxicity triggering receptor 2; NKp46, natural cytotoxicity
triggering receptor 1; E2, 17β-estradiol; PRL, prolactin; MFI, mean
fluorescence intensity; SD, standard error. |
Modulation in the expression of NKG2D
ligands in CC-derived cells by hormonal stimuli
Since CC cells induce the expression of stress
ligands, including MICA and MICB (NKG2D receptor ligands in NK
cells), the present study then evaluated the effects of E2 and PRL
on MICA/B expression in CC-derived cell lines (HeLa, SiHa and C33A)
and a non-tumorigenic immortalized keratinocyte cell line (HaCaT).
As previously described in the literature, it was observed that, in
the untreated control cells, the HPV-18 and HPV-16 positive cell
lines (HeLa and SiHa, respectively) presented a higher percentage
of cells expressing MICA/B (99.7 and 99.8%, respectively) (45), contrary to HPV-negative cells (C33A
and HaCaT, 8.35 and 2.09%, respectively; Fig. 4A). Stimulation of HeLa cells with
PRL increased the expression of MICA/B compared to the untreated
control cells (P<0.05); however, the simultaneous stimulation
with E2 and PRL reversed this effect (P<0.05). By contrast, in
SiHa cells, the concurrent stimulation with E2 and PRL decreased
MICA/B expression compared to the untreated control cells and E2
stimulus (P<0.05; Fig. 4A).
Hormonal stimuli did not induce changes in MICA/B expression in the
C33A or HaCaT cells.
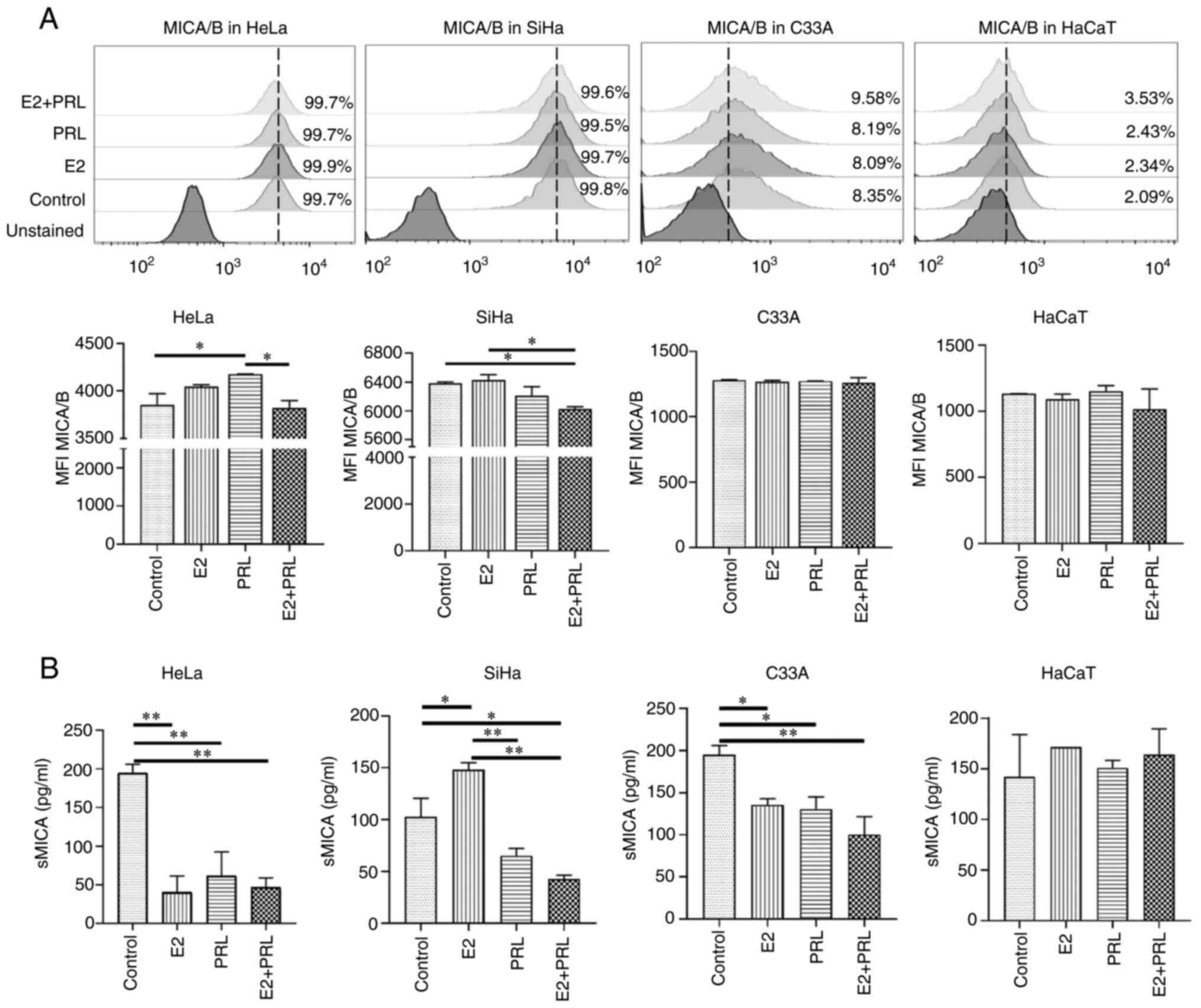 | Figure 4.Modulation of MICA/B by hormones in
different CC cell lines. (A) Cell surface MICA/B expression was
evaluated in HeLa, SiHa, C33A and HaCaT cells stimulated with E2,
PRL, both or untreated (control) using flow cytometry. The MFI is
expressed as the mean ± SD. (B) The expression of the soluble form
of MICA was measured by ELISA assays from HeLa, SiHa, C33A and
HaCaT supernatants after 48 h stimuli with E2 (10 nM) and PRL (200
ng/ml). The data shown represent the mean ± SD of absorbance values
(450 nm) from two independent experiments. All statistical analyses
were performed using ANOVA (*P<0.05 and
**P<0.01). MHC class I polypeptide-related sequence A/B;
CC, cervical cancer; E2, 17β-estradiol; PRL, prolactin; MFI, Mean
Fluorescence Intensity; sMICA, soluble MICA; SD, standard
error. |
Among the escape mechanisms of tumor cells towards
immunological recognition is the release or secretion of soluble
forms of activating ligands. In line with this, it has been
discovered that MIC molecules can be released into the
extracellular matrix and thereby promote an immune escape strategy
for tumor cells (32). For this
reason, in the present study, the levels of soluble MICA (sMICA) in
CC-derived and HaCaT cell line supernatants, stimulated for 48 h
with E2, PRL or both was evaluated (Fig. 4B). In comparison to the untreated
control cells, stimulation with E2 or PRL alone, and E2 and PRL in
combination, decreased the liberation of MICA into the supernatant
of all CC-derived cell lines (P<0.05), apart from the SiHa
cells, where E2 stimulation resulted in increased sMICA levels
(P<0.05). This effect was abrogated by stimulation with PRL
alone, and with E2 and PRL in combination (P<0.01) in SiHa
cells. Notably, the hormone stimuli had no effect on MICA secretion
in the HaCaT cell supernatant. Both the membrane and soluble form
of the MICA ligand are regulated by E2 and PRL in CC-derived cell
lines.
Discussion
CC represents one of the main health issues in
women. Of note, 604,000 new cases worldwide were estimated in 2020.
The main risk factor associated with CC is HPV infection, which is
present in >99% of patients with CC (1). However, it has been revealed that HPV
infection alone is not sufficient for CC to manifest (16). In this sense, the hormonal role
constitutes an important factor for the carcinogenesis of this type
of tumor. Hormones, including 17β-estradiol and PRL are related to
the genesis, persistence and development of CC (14,16,21,46),
since in addition to being present in the TME of this cancer type,
they can contribute to anti-apoptotic, proliferative, invasive,
survival effects and metabolic adaptation of CC cells (10,15,21,22,25,47).
In addition, they can regulate the expression of HPV oncogenes
(48). The functionality of these
hormones within the TME may also depend on a bilateral regulation
between the two hormones, since there are studies demonstrating the
possible regulation of PRLR by E2, as well as the regulation of
estrogen receptors exerted by PRL effects (49–51).
In the TME there are also cells of the innate immune
system, including NK cells, which have the potential to kill cells
transformed and infected by HPV (52). As regards CC, studies have revealed
that there is a poor infiltration of NK cells, and therefore this
may be associated with a decrease in their cytotoxic activity
against tumor cells (53,54). In both in vitro models and
patients, it has been observed that CC cells are capable of
regulating NK cell cytotoxicity given that tumor-infiltrating NK
cells decrease the expression of perforins, activating receptors
and IFN-γ, and on the other hand increasing the expression of
inhibition receptors (54,55). Likewise, the expression of
activating receptors, such as NKG2D, and the expression of cell
stress ligands with CC have been related (30,31,45,56).
The present study demonstrated that E2 decreased the cytotoxicity
of NKL cells, as well as CD107a expression, which is consistent
with the findings in the studies by Hao et al (57,58)
underlining that various concentrations of E2 may have a negative
effect on proliferative capacity, IFN-γ expression and the
cytotoxic effects of the NK cells extracted from mouse spleens
against the YAC-1 target cells. A possible explanation for this
phenomenon is the indirect decrease in granzyme B levels, due to
the effect of E2, where it has been demonstrated that estrogen
induces the expression of inhibitory proteinase 9, a potent
inhibitor of granzyme B (38,58).
Subsequently, when confirming the effect of E2 on
the cytotoxicity of NKL cells, the present study analyzed the
possible isoforms of the estrogen receptors that these cells
express, with the aim of determining the pathway through which E2
may exert such an effect. As regards ERα, it has been revealed that
it presents with three main isoforms, known as ERα66, ERα46 and
ERα36, named for their characteristic molecular weights (59). NKL cells express the 46 kDa isoform
of ERα, which has also been previously detected in lymphocytes with
CD3+ CD8+ and CD3−CD56+
phenotypes obtained from peripheral blood (60). This isoform is characterized by the
lack of the first 173 amino acids of the amino terminal AF-1 domain
and has been associated with an inhibitory role on tumor cell
growth, as in breast cancer cells ERα46 may inhibit the estrogenic
effects of ERα66, inducing in turn cell proliferation and cell
cycle progression. It has been suggested that these effects occur
due to a functional competition between both isoforms (59,61,62).
In relation to ERβ, in other human cancer models it has been
demonstrated that this receptor presents with various isoforms,
known as ERβ1, β2, β3, β4 and β5 whose molecular weights range from
50 to 59 kDa (63). NKL cells
strongly express ERβ, represented as a 45-kDa band and a weaker
expression of a 56-kDa band. Similarly, in peripheral blood
lymphocytes the expression of ERβ with weights lower than 56 kDa
has been detected (60).
Furthermore, in breast cancer cells the presence of ERβ isoforms
with molecular weights of around 44 kDa has also been observed.
This may be attributed to the fact that exons 5 and 6 of the ERβ
mRNA are eliminated, thereby generating a protein of lower
molecular weight (60,64). To date, the possible role of these
ERβ isoforms with molecular weights <50 kDa is unknown;
therefore, further studies are warranted to achieve a better
understanding at the functional level of these variants.
Another receptor through which E2 has been reported
to exert its effects is the G protein-coupled estrogen receptor,
GPER, which has been reported to be associated with non-genomic
pathways through kinase-dependent signaling for rapid gene
regulation (65). Recent findings
have revealed that GPER is overexpressed in biopsies of patients
with CC and its agonistic activation increases mitochondrial
permeability, as well as apoptosis, as well decreases the
proliferation of CC cells (15).
NKL cells express a weak band of 42 kDa and a strong band of around
100 kDa. Currently, no evidence has been reported concerning the
presence of GPER in these cells; however, the high weight of GPER
has been related to glycosylated forms and/or dimerization of this
receptor (66,67). It has been demonstrated that GPER
glycosylation may occur mainly in an asparagine residue known as
Asn44 and this post-translational modification has been
associated with its location in the plasma membrane, where GPER can
regulate the rapid non-genomic response of estrogens (68,69).
By contrast, in the present study it was observed
that stimulation with PRL induced an increase in NKL cell-mediated
cytotoxicity and CD107a expression. This is in line with previous
studies by Sun et al (37,70),
where NK cells extracted from mice treated with PRL and also from
cell lines including NK-92 were used. This increase in NK
cell-mediated cytotoxicity may be attributed to the fact that PRL,
in conjunction with IL-2 and IL-15, may increase the expression of
IFN-γ, perforins and Fas-L (37).
Considering that PRL has been reported to exert its effects through
its receptor, it was decided to visualize the possible isoforms by
which PRL could exert this effect on cytotoxicity. PRLR is
expressed in a number of isoforms, including a long (between 80 and
90 kDa), an intermediate (65 kDa) and 2 short isoforms (between 40
and 55 kDa) (23). The variant
with the highest expression in NKL cells was the short isoform
corresponding to the 50-kDa band, characterized by the lack of the
Box 2 region, which is crucial for the interaction with proteins
containing an SH2 domain, including STAT proteins, leading to a
negative regulation on the effects triggered by the long isoform of
the PRLR (18,23). Further more detailed studies are
required to determine whether the different PRLR isoforms may have
a functional effect on NK cells. Notably, the concurrent
stimulation of E2 and PRL also increased the cytotoxicity of NKL
cells, as observed with PRL alone. It was observed that PRL may
overcome the effects of E2 and as previously mentioned, this may be
attributed to both hormones having a bilateral regulation (49–51).
This is in line with Riera-Leal et al (14), who observed in a context of CC cell
metabolism, that PRL may have a greater impact over the estrogenic
effects induced by E2.
Subsequently, the present study aimed to evaluate
whether the differences in the cytotoxicity of NKL cells by
hormones may be attributed to changes in the expression of
activation receptors, including NCR and NKG2D. The data obtained
did not indicate that these effects were related to the NCR, since
it was observed that the hormones did not modify the expression of
NKp30, NKp44 and NKp46. By contrast, it was observed that PRL may
decrease the expression of NKG2D in NKL cells. This is in line with
a previous study by Ma et al (71) in 2010, where it was demonstrated
that the expression of NKG2D may decrease in T lymphocytes from
patients with prolactinoma.
When the change in the expression of NKG2D by these
hormones was observed, it was decided to evaluate MICA and MICB
ligands of this receptor, which are expressed in the membrane, as
well as in soluble forms. MICA and MICB are known as stress
proteins and these ligands have been reported to be elevated in CC
patient biopsies and to be also overexpressed in CC-derived cell
lines, including SiHa, HeLa, CALO and INBL (45,54,71).
The results of the present study are consistent with the findings
from the study by del Toro-Arreola et al (45), with MICA/B being expressed mainly
in HaCaT, C33A, HeLa and SiHa cells. Furthermore, sMICA was
detected in cell supernatants at relatively similar levels. Of
note, it was demonstrated that PRL may increase MICA/B expression
on the HeLa cell surface, while decreasing sMICA release in the
supernatant of all CC-derived cells. In the context of the
interaction that exists in the CC microenvironment, the increase in
cytotoxicity which was observed under the effect of PRL may be
explained by the increase in MICA/B in the membrane, which can bind
to NKG2D; this is also supported by the decrease in sMICA. To the
best of our knowledge, there no studies available to date that
relate the effect of PRL with the release of MIC molecules.
However, it has been revealed that metalloprotease 9, which has the
ability to cleave MICA, decreases its expression due to the effects
of PRL, possibly explaining the aforementioned result (72,73).
By contrast, in the present study, E2 decreased sMICA in HeLa and
C33A cells, whereas an opposite effect was observed in SiHa cells,
possibly indicating that the effects of E2 vary depending on the
cell type. Although an effect of E2 on MICA/B surface expression,
when stimulating the cells with both hormones was not detected, it
was observed that sMICA expression decreased in all CC-derived cell
lines. This may indicate that the joint effect of the hormones may
be related to the increase in the cytotoxicity of NKL cells and
also supporting the regulatory effect of one hormone on the
other.
In conclusion, the results of the present study
suggested that E2 and PRL, which are overexpressed in the CC
microenvironment, may antagonistically regulate the cytotoxicity of
NK cells. Furthermore, NKL cells express different variants of the
hormone receptors by which their effects may be exerted. By
contrast, hormones regulate the expression of molecules, including
the NKG2D receptor, MICA/B ligands and their soluble forms, which
may be involved in the cytotoxicity of NK cells. This knowledge
revealed an overview that may help in understanding further the
mechanism by which these hormones may contribute to the development
of CC. It would be of interest to evaluate the possible molecules
involved in pathways triggered by E2 and PRL on NK cell-mediated
cytotoxicity in future studies, using next-generation RNA
sequencing, ultimately aiming to identify novel therapeutic targets
involved in CC.
Acknowledgements
Not applicable.
Funding
This study was supported by the Sectorial Research Fund for
Education, SEP-CONACYT (grant no. A1-S-51207), and the Jalisco
Scientific Development Fund (FODECIJAL) to Attend State Problems
2019 (Project #8168). The study was supported by a CONACYT
fellowship (#885574).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AGP and CDHS performed the experiments. MGC assisted
in the flow cytometry data analysis and in the interpretation of
the results. ARdA, JCVP, IGRL, AAL and MGC contributed to the
statistical analysis and the critical review of the manuscript.
JSZN participated in the ELISA. AGP and MGC confirm the
authenticity of all the raw data. ALPS designed the study and wrote
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wójcik L, Samulak D, Makowska M,
Romanowicz H, Kojs Z, Smolarz B and Michalska MM: The role of human
papillomavirus in cervical cancer. Int J Cancer Clin Res.
6:1252019.
|
|
3
|
Van hede D, Langers I, Delvenne P and
Jacobs N: Origin and immunoescape of uterine cervical cancer.
Presse Med. 43:e413–e421. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Xu H, Zhang L and Qiao Y:
Cervical cancer: Epidemiology, risk factors and screening. Chin J
Cancer Res. 32:720–728. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Momenimovahed Z and Salehiniya H:
Incidence, mortality and risk factors of cervical cancer in the
world. Biomed Res Ther. 4:1795–1811. 2017. View Article : Google Scholar
|
|
6
|
Chung SH, Franceschi S and Lambert PF:
Estrogen and ERalpha: Culprits in cervical cancer? Trends
Endocrinol Metab. 21:504–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barros MR Jr, de Melo CML, Barros MLCMGR,
de Cássia Pereira de Lima R, de Freitas AC and Venuti A: Activities
of stromal and immune cells in HPV-related cancers. J Exp Clin
Cancer Res. 37:1372018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding L, Liu C, Zhou Q, Feng M and Wang J:
Association of estradiol and HPV/HPV16 infection with the
occurrence of cervical squamous cell carcinoma. Oncol Lett.
17:3548–3554. 2019.
|
|
9
|
Adurthi S, Kumar MM, Vinodkumar HS,
Mukherjee G, Krishnamurthy H, Acharya KK, Bafna UD, Uma DK,
Abhishekh B, Krishna S, et al: Oestrogen Receptor-α binds the FOXP3
promoter and modulates regulatory T-cell function in human cervical
cancer. Sci Rep. 7:172892017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lopez-Pulido EI, Muñoz-Valle JF, Del
Toro-Arreola S, Jave-Suárez LF, Bueno-Topete MR, Estrada-Chávez C
and Pereira-Suárez AL: High expression of prolactin receptor is
associated with cell survival in cervical cancer cells. Cancer Cell
Int. 13:1032013. View Article : Google Scholar
|
|
11
|
Gruber CJ, Tschugguel W, Schneeberger C
and Huber JC: Production and actions of estrogens. N Engl J Med.
346:340–352. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shanle EK and Xu W: Endocrine disrupting
chemicals targeting estrogen receptor signaling: Identification and
mechanisms of action. Chem Res Toxicol. 24:6–19. 2011. View Article : Google Scholar
|
|
13
|
Mizukami Y: In vivo functions of
GPR30/GPER-1, a membrane receptor for estrogen: From discovery to
functions in vivo. Endocr J. 57:101–107. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Riera-Leal A, Ramírez De Arellano A,
Ramírez-López IG, Lopez-Pulido EI, Dávila Rodríguez JR,
Macías-Barragan JG, Ortiz-Lazareno PC, Jave-Suárez LF,
Artaza-Irigaray C, Del Toro Arreola S, et al: Effects of 60 kDa
prolactin and estradiol on metabolism and cell survival in cervical
cancer: Co-expression of their hormonal receptors during cancer
progression. Oncol Rep. 40:3781–3793. 2018.PubMed/NCBI
|
|
15
|
Hernandez-Silva CD, Riera-Leal A,
Ortiz-Lazareno PC, Jave-Suárez LF, Ramírez De Arellano A,
Lopez-Pulido EI, Macías-Barragan JG, Montoya-Buelna M,
Dávila-Rodríguez JR, Chabay P, et al: GPER overexpression in
cervical cancer versus premalignant lesions: Its activation induces
different forms of cell death. Anticancer Agents Med Chem.
19:783–791. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brake T and Lambert PF: Estrogen
contributes to the onset, persistence, and malignant progression of
cervical cancer in a human papillomavirus-transgenic mouse model.
Proc Natl Acad Sci USA. 102:2490–2495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chung SH, Wiedmeyer K, Shai A, Korach KS
and Lambert PF: Requirement for estrogen receptor alpha in a mouse
model for human papillomavirus-associated cervical cancer. Cancer
Res. 68:9928–9934. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bernard V, Young J, Chanson P and Binart
N: New insights in prolactin: Pathological implications. Nat Rev
Endocrinol. 11:265–275. 2015. View Article : Google Scholar
|
|
19
|
Marano RJ and Ben-Jonathan N: Minireview:
Extrapituitary prolactin: An update on the distribution,
regulation, and functions. Mol Endocrinol. 28:622–633. 2014.
View Article : Google Scholar
|
|
20
|
Brooks CL: Molecular mechanisms of
prolactin and its receptor. Endocr Rev. 33:504–525. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ascencio-Cedillo R, López-Pulido EI,
Muñoz-Valle JF, Villegas-Sepúlveda N, Del Toro-Arreola S,
Estrada-Chávez C, Daneri-Navarro A, Franco-Topete R, Pérez-Montiel
D, García-Carrancá A and Pereira-Suárez AL: Prolactin and prolactin
receptor expression in cervical intraepithelial neoplasia and
cancer. Pathol Oncol Res. 21:241–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramírez De Arellano A, Riera Leal A,
Lopez-Pulido EI, González-Lucano LR, Macías Barragan J, Del Toro
Arreola S, García-Chagollan M, Palafox-Sánchez CA, Muñoz-Valle JF
and Pereira-Suárez AL: A 60 kDa prolactin variant secreted by
cervical cancer cells modulates apoptosis and cytokine production.
Oncol Rep. 39:1253–1260. 2018.
|
|
23
|
Abramicheva PA and Smirnova OV: Prolactin
receptor isoforms as the basis of tissue-specific action of
prolactin in the norm and pathology. Biochemistry (Mosc).
84:329–345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsu CT, Yu MH, Lee CY, Jong HL and Yeh MY:
Ectopic production of prolactin in uterine cervical carcinoma.
Gynecol Oncol. 44:166–171. 1992. View Article : Google Scholar
|
|
25
|
Ramírez de Arellano A, Lopez-Pulido EI,
Martínez-Neri PA, Estrada Chávez C, González Lucano R,
Fafutis-Morris M, Aguilar-Lemarroy A, Muñoz-Valle JF and
Pereira-Suárez AL: STAT3 activation is required for the
antiapoptotic effects of prolactin in cervical cancer cells. Cancer
Cell Int. 15:832015. View Article : Google Scholar
|
|
26
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar
|
|
27
|
Vivier E, Raulet DH, Moretta A, Caligiuri
MA, Zitvogel L, Lanier LL, Yokoyama WM and Ugolini S: Innate or
Adaptive Immunity? The example of natural killer cells. Science.
331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brandt CS, Baratin M, Yi EC, Kennedy J,
Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et
al: The B7 family member B7-H6 is a tumor cell ligand for the
activating natural killer cell receptor NKp30 in humans. J Exp Med.
206:1495–1503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Srivastava RM, Savithri B and Khar A:
Activating and inhibitory receptors and their role in natural
killer cell function. Indian J Biochem Biophys. 40:291–299.
2003.PubMed/NCBI
|
|
30
|
Jimenez-Perez MI, Jave-Suarez LF,
Ortiz-Lazareno PC, Bravo-Cuellar A, Gonzalez-Ramella O,
Aguilar-Lemarroy A, Hernandez-Flores G, Pereira-Suarez AL,
Daneri-Navarro A and del Toro-Arreola S: Cervical cancer cell lines
expressing NKG2D-ligands are able to down-modulate the NKG2D
receptor on NKL cells with functional implications. BMC Immunol.
13:72012. View Article : Google Scholar
|
|
31
|
Garcia-Iglesias T, Del Toro-Arreola A,
Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE,
Ramirez-Dueñas MG, Balderas-Peña LM, Bravo-Cuellar A,
Ortiz-Lazareno PC and Daneri-Navarro A: Low NKp30, NKp46 and NKG2D
expression and reduced cytotoxic activity on NK cells in cervical
cancer and precursor lesions. BMC Cancer. 9:1862009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y,
Wang Y, Xiong F, Guo C, Li Y, et al: Natural killer group 2D
receptor and its ligands in cancer immune escape. Mol Cancer.
18:292019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
López-Soto A, Huergo-Zapico L,
Acebes-Huerta A, Villa-Alvarez M and Gonzalez S: NKG2D signaling in
cancer immunosurveillance: NKG2D signaling. Int J Cancer.
136:1741–1750. 2015. View Article : Google Scholar
|
|
34
|
Baragaño Raneros A, Suarez-Álvarez B and
López-Larrea C: Secretory pathways generating immunosuppressive
NKG2D ligands: New targets for therapeutic intervention.
Oncoimmunology. 3:e284972014. View Article : Google Scholar
|
|
35
|
Chitadze G, Bhat J, Lettau M, Janssen O
and Kabelitz D: Generation of soluble NKG2D ligands: Proteolytic
cleavage, exosome secretion and functional implications. Scand J
Immunol. 78:120–129. 2013. View Article : Google Scholar
|
|
36
|
Curran EM, Berghaus LJ, Vernetti NJ,
Saporita AJ, Lubahn DB and Estes DM: Natural killer cells express
estrogen receptor-alpha and estrogen receptor-beta and can respond
to estrogen via a non-estrogen receptor-alpha-mediated pathway.
Cell Immunol. 214:12–20. 2001. View Article : Google Scholar
|
|
37
|
Sun R, Li AL, Wei HM and Tian ZG:
Expression of prolactin receptor and response to prolactin
stimulation of human NK cell lines. Cell Res. 14:67–73. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang X, Ellison SJ, Alarid ET and Shapiro
DJ: Interplay between the levels of estrogen and estrogen receptor
controls the level of the granzyme inhibitor, proteinase inhibitor
9 and susceptibility to immune surveillance by natural killer
cells. Oncogene. 26:4106–4114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang X, Orr BA, Kranz DM and Shapiro DJ:
Estrogen induction of the granzyme B inhibitor, proteinase
inhibitor 9, protects cells against apoptosis mediated by cytotoxic
T lymphocytes and natural killer cells. Endocrinology.
147:1419–1426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mavoungou E, Bouyou-Akotet MK and Kremsner
PG: Effects of prolactin and cortisol on natural killer (NK) cell
surface expression and function of human natural cytotoxicity
receptors (NKp46, NKp44 and NKp30). Clin Exp Immunol. 139:287–296.
2005. View Article : Google Scholar
|
|
41
|
Basu S, Pioli PA, Conejo-Garcia J, Wira CR
and Sentman CL: Estradiol regulates MICA expression in human
endometrial cells. Clin Immunol. 129:325–332. 2008. View Article : Google Scholar
|
|
42
|
Ren J, Nie Y, Lv M, Shen S, Tang R, Xu Y,
Hou Y, Zhao S and Wang T: Estrogen upregulates MICA/B expression in
human non-small cell lung cancer through the regulation of ADAM17.
Cell Mol Immunol. 12:768–776. 2015. View Article : Google Scholar
|
|
43
|
Wolfson B, Padget MR, Schlom J and Hodge
JW: Exploiting off-target effects of estrogen deprivation to
sensitize estrogen receptor negative breast cancer to immune
killing. J Immunother Cancer. 9:e0022582021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gunesch JT, Angelo LS, Mahapatra S,
Deering RP, Kowalko JE, Sleiman P, Tobias JW, Monaco-Shawver L,
Orange JS and Mace EM: Genome-wide analyses and functional
profiling of human NK cell lines. Mol Immunol. 115:64–75. 2019.
View Article : Google Scholar
|
|
45
|
del Toro-Arreola S, Arreygue-Garcia N,
Aguilar-Lemarroy A, Cid-Arregui A, Jimenez-Perez M, Haramati J,
Barros-Nuñez P, Gonzalez-Ramella O, Del Toro-Arreola A,
Ortiz-Lazareno P, et al: MHC class I-related chain A and B ligands
are differentially expressed in human cervical cancer cell lines.
Cancer Cell Int. 11:152011. View Article : Google Scholar
|
|
46
|
Huang Y, Li J, Xiang L, Han D, Shen X and
Wu X: 17β-Oestradiol activates proteolysis and increases invasion
through phosphatidylinositol 3-kinase pathway in human cervical
cancer cells. Eur J Obstet Gynecol Reprod Biol. 165:307–312. 2012.
View Article : Google Scholar
|
|
47
|
Riera Leal A, Ortiz-Lazareno PC,
Jave-Suárez LF, Ramírez De Arellano A, Aguilar-Lemarroy A,
Ortiz-García YM, Barrón-Gallardo CA, Solís-Martínez R, Luquin De
Anda S, Muñoz-Valle JF and Pereira-Suárez AL: 17β-estradiol-induced
mitochondrial dysfunction and Warburg effect in cervical cancer
cells allow cell survival under metabolic stress. Int J Oncol.
56:33–46. 2020.
|
|
48
|
Ramírez-López IG, Ramírez de Arellano A,
Jave-Suárez LF, Hernández-Silva CD, García-Chagollan M,
Hernández-Bello J, Lopez-Pulido EI, Macias-Barragan J,
Montoya-Buelna M, Muñoz-Valle JF and Pereira-Suárez AL: Interaction
between 17β-estradiol, prolactin and human papillomavirus induce
E6/E7 transcript and modulate the expression and localization of
hormonal receptors. Cancer Cell Int. 19:2272019. View Article : Google Scholar
|
|
49
|
Leondires MP, Hu ZZ, Dong J, Tsai-Morris
CH and Dufau ML: Estradiol stimulates expression of two human
prolactin receptor isoforms with alternative exons-1 in T47D breast
cancer cells. J Steroid Biochem Mol Biol. 82:263–268. 2002.
View Article : Google Scholar
|
|
50
|
Adamson AD, Friedrichsen S, Semprini S,
Harper CV, Mullins JJ, White MR and Davis JR: Human prolactin gene
promoter regulation by estrogen: Convergence with tumor necrosis
factor-alpha signaling. Endocrinology. 149:687–694. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
González L, Zambrano A, Lazaro-Trueba I,
Lopéz E, González JJA, Martín-Pérez J and Aranda A: Activation of
the unliganded estrogen receptor by prolactin in breast cancer
cells. Oncogene. 28:1298–1308. 2009. View Article : Google Scholar
|
|
52
|
Sasagawa T, Takagi H and Makinoda S:
Immune responses against human papillomavirus (HPV) infection and
evasion of host defense in cervical cancer. J Infect Chemother.
18:807–815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Garzetti G, Ciavattini A, Muzzioli M,
Goteri G, Mannello B, Romanini C and Fabris N: Natural killer cell
activity in patients with invasive cervical carcinoma: Importance
of a longitudinal evaluation in follow-up. Gynecol Obstet Invest.
40:133–138. 1995. View Article : Google Scholar
|
|
54
|
Textor S, Dürst M, Jansen L, Accardi R,
Tommasino M, Trunk MJ, Porgador A, Watzl C, Gissmann L and Cerwenka
A: Activating NK cell receptor ligands are differentially expressed
during progression to cervical cancer. Int J Cancer. 123:2343–2353.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chang WC, Li CH, Chu LH, Huang PS, Sheu BC
and Huang SC: Regulatory T cells suppress natural killer cell
immunity in patients with human cervical carcinoma. Int J Gynecol
Cancer. 26:156–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Arreygue-Garcia NA, Daneri-Navarro A, del
Toro-Arreola A, Cid-Arregui A, Gonzalez-Ramella O, Jave-Suarez LF,
Aguilar-Lemarroy A, Troyo-Sanroman R, Bravo-Cuellar A, Delgado-Rizo
V, et al: Augmented serum level of major histocompatibility complex
class I-related chain A (MICA) protein and reduced NKG2D expression
on NK and T cells in patients with cervical cancer and precursor
lesions. BMC Cancer. 8:162008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hao S, Zhao J, Zhou J, Zhao S, Hu Y and
Hou Y: Modulation of 17beta-estradiol on the number and
cytotoxicity of NK cells in vivo related to MCM and activating
receptors. Int Immunopharmacol. 7:1765–1775. 2007. View Article : Google Scholar
|
|
58
|
Hao S, Li P, Zhao J, Hu Y and Hou Y:
17beta-estradiol suppresses cytotoxicity and proliferative capacity
of murine splenic NK1.1+ cells. Cell Mol Immunol. 5:357–364. 2008.
View Article : Google Scholar
|
|
59
|
Arnal JF, Lenfant F, Metivier R, Flouriot
G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P,
Katzenellenbogen B and Katzenellenbogen J: Membrane and nuclear
estrogen receptor alpha actions: from tissue specificity to medical
implications. Physiol Rev. 97:1045–1087. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pierdominici M, Maselli A, Colasanti T,
Giammarioli AM, Delunardo F, Vacirca D, Sanchez M, Giovannetti A,
Malorni W and Ortona E: Estrogen receptor profiles in human
peripheral blood lymphocytes. Immunol Lett. 132:79–85. 2010.
View Article : Google Scholar
|
|
61
|
Penot G, Le Péron C, Mérot Y,
Grimaud-Fanouillère E, Ferrière F, Boujrad N, Kah O, Saligaut C,
Ducouret B, Métivier R and Flouriot G: The human estrogen
receptor-alpha isoform hERalpha46 antagonizes the proliferative
influence of hERalpha66 in MCF7 breast cancer cells. Endocrinology.
146:5474–5484. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Miller MM, McMullen PD, Andersen ME and
Clewell RA: Multiple receptors shape the estrogen response pathway
and are critical considerations for the future of in vitro-based
risk assessment efforts. Crit Rev Toxicol. 47:570–586. 2017.
View Article : Google Scholar
|
|
63
|
Leung YK, Mak P, Hassan S and Ho SM:
Estrogen receptor (ER)-beta isoforms: A key to understanding
ER-beta signaling. Proc Natl Acad Sci USA. 103:13162–13167. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Saunders PT, Millar MR, Williams K,
Macpherson S, Bayne C, O'Sullivan C, Anderson TJ, Groome NP and
Miller WR: Expression of oestrogen receptor beta (ERbeta1) protein
in human breast cancer biopsies. Br J Cancer. 86:250–256. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gaudet HM, Cheng SB, Christensen EM and
Filardo EJ: The G-protein coupled estrogen receptor, GPER: The
inside and inside-out story. Mol Cell Endocrinol. 418:207–219.
2015. View Article : Google Scholar
|
|
66
|
Sandén C, Broselid S, Cornmark L,
Andersson K, Daszkiewicz-Nilsson J, Mårtensson UE, Olde B and
Leeb-Lundberg LM: G protein-coupled estrogen receptor 1/G
protein-coupled receptor 30 localizes in the plasma membrane and
traffics intracellularly on cytokeratin intermediate filaments. Mol
Pharmacol. 79:400–410. 2011. View Article : Google Scholar
|
|
67
|
Jala VR, Radde BN, Haribabu B and Klinge
CM: Enhanced expression of G-protein coupled estrogen receptor
(GPER/GPR30) in lung cancer. BMC Cancer. 12:6242012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Pupo M, Bodmer A, Berto M, Maggiolini M,
Dietrich PY and Picard D: A genetic polymorphism repurposes the
G-protein coupled and membrane-associated estrogen receptor GPER to
a transcription factor-like molecule promoting paracrine signaling
between stroma and breast carcinoma cells. Oncotarget.
8:46728–46744. 2017. View Article : Google Scholar
|
|
69
|
Gonzalez de Valdivia E, Sandén C, Kahn R,
Olde B and Leeb-Lundberg LMF: Human G protein-coupled receptor 30
is N-glycosylated and N-terminal domain asparagine 44 is required
for receptor structure and activity. Biosci Rep.
39:BSR201824362019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sun R, Wei H, Zhang J, Li A, Zhang W and
Tian ZG: Recombinant human prolactin improves antitumor effects of
murine natural killer cells in vitro and in vivo.
Neuroimmunomodulation. 10:169–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma L, Li G, Su Y, He Q, Zhang C and Zhang
J: The soluble major histocompatibility complex class I-related
chain A protein reduced NKG2D expression on natural killer and T
cells from patients with prolactinoma and non-secreting pituitary
adenoma. J Clin Neurosci. 17:241–247. 2010. View Article : Google Scholar
|
|
72
|
Zaga-Clavellina V, Parra-Covarrubias A,
Ramirez-Peredo J, Vega-Sanchez R and Vadillo-Ortega F: The
potential role of prolactin as a modulator of the secretion of
proinflammatory mediators in chorioamniotic membranes in term human
gestation. Am J Obstet Gynecol. 211:48.e1–e6. 2014. View Article : Google Scholar
|
|
73
|
Shiraishi K, Mimura K, Kua LF, Koh V,
Siang LK, Nakajima S, Fujii H, Shabbir A, Yong WP, So J, et al:
Inhibition of MMP activity can restore NKG2D ligand expression in
gastric cancer, leading to improved NK cell susceptibility. J
Gastroenterol. 51:1101–1111. 2016. View Article : Google Scholar
|



















