Introduction
Breast cancer is the most common malignant tumor in
women, and the leading cause of cancer mortality in females that
causes approximately half a million deaths each year worldwide
(1,2). Although recent substantial progress
has been achieved in treatments involving chemotherapy, surgery and
radiation therapy, breast cancer is still difficult to cure because
of the propensity of these tumors to form distant metastases
(3,4) and the distinct subtypes that exist
(5–7). Therefore, to cure breast cancer, we
should understand the relevant molecular mechanisms involved in
breast cancer metastasis. Evidence suggests that many
characteristics and markers, such as the progesterone receptor,
histological grade, HER2/ERBB2 status, the estrogen receptor, p53
mutational status and neogenin are able to classify heterogeneous
breast cancers (1,8). More recently, previous studies have
indicated that neogenin expression may be inversely correlated to
the tumorigenicity of human breast cancer (8); however, the specific function of
neogenin in the progression of breast cancer is unclear.
Neogenin, a homologue of the DCC (deleted in
colorectal cancer) receptor group, encodes a 1461 amino acid
identity. Neogenin is widely distributed in the CNS and is a
dependent receptor of the repulsive guidance molecule a (RGMa)
(9–11). Previous studies have suggested that
neogenin plays an important role in cell to cell recognition,
tissue growth regulation, cellular differentiation, cell migration,
cell apoptosis, angiogenesis, epithelial cell renewal and
histogenesis (12–16). It has been reported that neogenin is
expressed in many adult tissues, and abnormal expression of
neogenin has been found in a variety of human cancers, such as
pancreatic (17), colon cancer
(18), esophageal squamous cell
carcinoma (ESCC) (19), gliomas
(20) and breast cancer (8). Subsequent studies revealed that
altered expression of neogenin may lead to loss of pro-apoptotic
activity and may even cause tumorigenesis (21). There is some evidence to suggest
that downregulation of neogenin accelerates glioma progression
through promoter methylation and its overexpression in SHG-44
induced apoptosis (20). Moreover,
Lee and colleagues (8) reported
that neogenin expression is downregulated in human breast cancer
relative to the normal breast tissue.
A protein which can regulate cancer-relevant
cellular functions such as cellular proliferation and apoptosis may
be the potential source of molecular signaling pathways commonly
disrupted in cancer cells (22,23).
Evidence suggests that bone morphogenetic proteins (BMPs) regulate
many mammalian physiological and pathophysiological processes
(24). BMPs bind to kinase
receptors, thereby activating Smad transcription factors. Moreover,
it has been reported that neogenin is a receptor for BMPs (24). Thus, we speculated that neogenin
could modulate Smad signal transduction through binding with BMPs.
In the present study, we demonstrated that neogenin overexpression
can inhibit cell proliferation and migration; moreover, promoting
cell apoptosis. The present study provides the first direct
evidence in breast cancer cells that neogenin overexpression can
result in cell growth inhibition and apoptosis.
Materials and methods
Antibodies
A rabbit monoclonal phospho-specific antibody to
Smad1/5/8 was obtained from Cell Signaling Technology (Beverly, MA,
USA). Rabbit anti-Smad1 monoclonal antibody, rabbit anti-neogenin
monoclonal antibody, mouse anti-β-actin monoclonal antibody,
HRP-conjugated rabbit anti-mouse IgG and HRP-conjugated goat
anti-rabbit IgG were obtained from Abcam (Cambridge, MA, USA).
Cell culture and transfection
The human breast cancer cell lines MDA-MB-231, MCF-7
and T47D cells (all cell types from the American Type Culture
Collection, Manassas, VA, USA) were cultured in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum
(FBS; Gibco-BRL, Gaithersburg, MD, USA), 1% penicillin-streptomycin
and 1% glutamine. All the cells were grown and maintained at 37°C
under a humidified atmosphere of 5% CO2. MDA-MB-231
cells were transiently transfected in a 24-well plate with either
human neogenin cDNA (pcDNA3.1-neogenin) or the control vector
pcDNA3.1 using Lipofectamine 2000 reagent (Invitrogen, Carlsbad,
CA, USA) according to the manufacturer’s protocol. These cells were
assayed 24, 48, 72 and 96 h after transfection.
MTT proliferation assay
Cell proliferation in MDA-MB-231, MCF-7 and T47D
cells was detected using the MTT assay according to a method
previously described (4). Briefly,
transfected cells and control cells were plated in 96-well plates
at 5×103 cells/well and cultured in DMEM for 48 h. Next,
the culture medium was replaced with 100 µl of fresh DMEM,
then 20 µl MTT (5 mg/ml) was added to the cells for another
4 h at 37°C. Formazan crystals were dissolved in 200 µl of
dimethyl sulfoxide (DMSO) and the absorbance was measured at λ 595
nm with a spectrophotometer (Multiskan MK3; Thermo Fisher
Scientific, Waltham, MA, USA).
Transwell migration assays
MDA-MB-231 cell migration was detected according to
the method described in a previous study (16). Briefly, MDA-MB-231 cells were
transfected with neogenin, and then the cells (5×103
cells/well) were added to the upper Transwell (Corning Costar,
Corning, NY, USA) chambers with 0.5 mg/ml collagen type I (BD
Biosciences, Seoul, Korea) coated filters 24 h after transfection.
DMEM containing 10% fetal bovine serum, 1% penicillin-streptomycin
and 1% glutamine was added to the lower chamber and incubation was
continued for 24 h. Wide-field microscopy was used to quantify the
cells that migrated to the lower chamber. Cells were counted at
five randomly selected areas in each well.
Detection of apoptotic cells by flow
cytometry
At 48 h after transfection, apoptosis of MDA-MB-231
cells was detected by flow cytometry. Subsequently, the cells were
stained with Annexin V-FITC and propidium iodide (PI) for 20 min at
room temperature. The apoptotic cells were then analyzed by flow
cytometry (Beckman Coulter, Brea, CA, USA) according to the
instruction of the Annexin V-FITC Apoptosis detection kit (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China).
Total RNA extraction and quantitative
reverse transcription-PCR
Neogenin mRNA level was detected by the RT-PCR
method (25). Total RNA was
extracted using standard methods (26,27).
Approximately 2 µg of total RNA was reverse transcribed into
first strand cDNA using random primers for qRT-PCR analysis. The
primer pairs used for PCR are as follows: neogenin (24): forward, 5′-GGAAGGAGGGG AATGAGACC-3′
and reverse, 5′-AATCACGGGTAGGGT AGGTA-3′; β-actin forward,
5′-TCCCTGGAGAAGAGCTA CGA-3′ and reverse,
5′-AGGAAGGAAGGCTGGAAGAG-3′. All the primers were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). Quantitative RT-PCR was
done using the iQ SYBR Green Supermix (Bio-Rad Laboratories,
Hercules, CA, USA). Interpretation of the relative gene expression
was calculated using the 2−∆∆CT method (28). β-actin mRNA was used as an internal
control.
Western blot analysis
We performed western blot analysis as previously
described (29). The cells were
homogenized and lysed with RIPA lysis buffer (Beyotime, Nantong,
China). The protein concentration was measured using a BCA protein
assay kit (Beyotime). Equal amounts of protein lysate (40
µg/lane) were separated on 12% SDS-PAGE gels and
electrophoretically transferred to polyvinylidene fluoride (PVDF)
membranes. Then, the cells were incubated with primary antibodies
specific for neogenin, Smad1/5/8 and β-actin. The blots were rinsed
in TBST, and further incubated in HRP-conjugated rabbit anti-mouse
IgG or HRP-conjugated goat anti-rabbit IgG. Bound proteins were
visualized using enhanced chemiluminescence (ECL) reagent
(Boehringer Mannheim, Mannheim, Germany).
SiRNA transfection
Breast cancer MDA-MB-231 cells with the neogenin
protein were transfected with neogenin siRNA or the control siRNA
(siMock) using Lipofectamine 2000 (Invitrogen) following the
manufacturer’s instructions. The coding strand of human neogenin
siRNA (16) was 5′-AGAU
CUGGAGGUUUCACAUCUUUGG-3′. The siRNA oligonucleotides were obtained
from Shanghai Sangon. Neogenin siRNA and siMock-transfected cells
were used for further experiments. Neogenin mRNA and protein levels
were determined by RT-PCR and western blotting 24 h after
transduction.
Statistics analysis
All data were obtained from at least three
independent experiments and are expressed as mean ± SD. Statistical
analysis was performed using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). Data were analyzed using analysis of variance
(ANOVA) and Student’s t-test. P<0.05 was considered to indicate
a statistically significant result.
Results
Increased neogenin levels in breast
cancer cell lines MDA-MB-231, MCF-7 and T47D cells transduced with
pcDNA3.1-neogenin
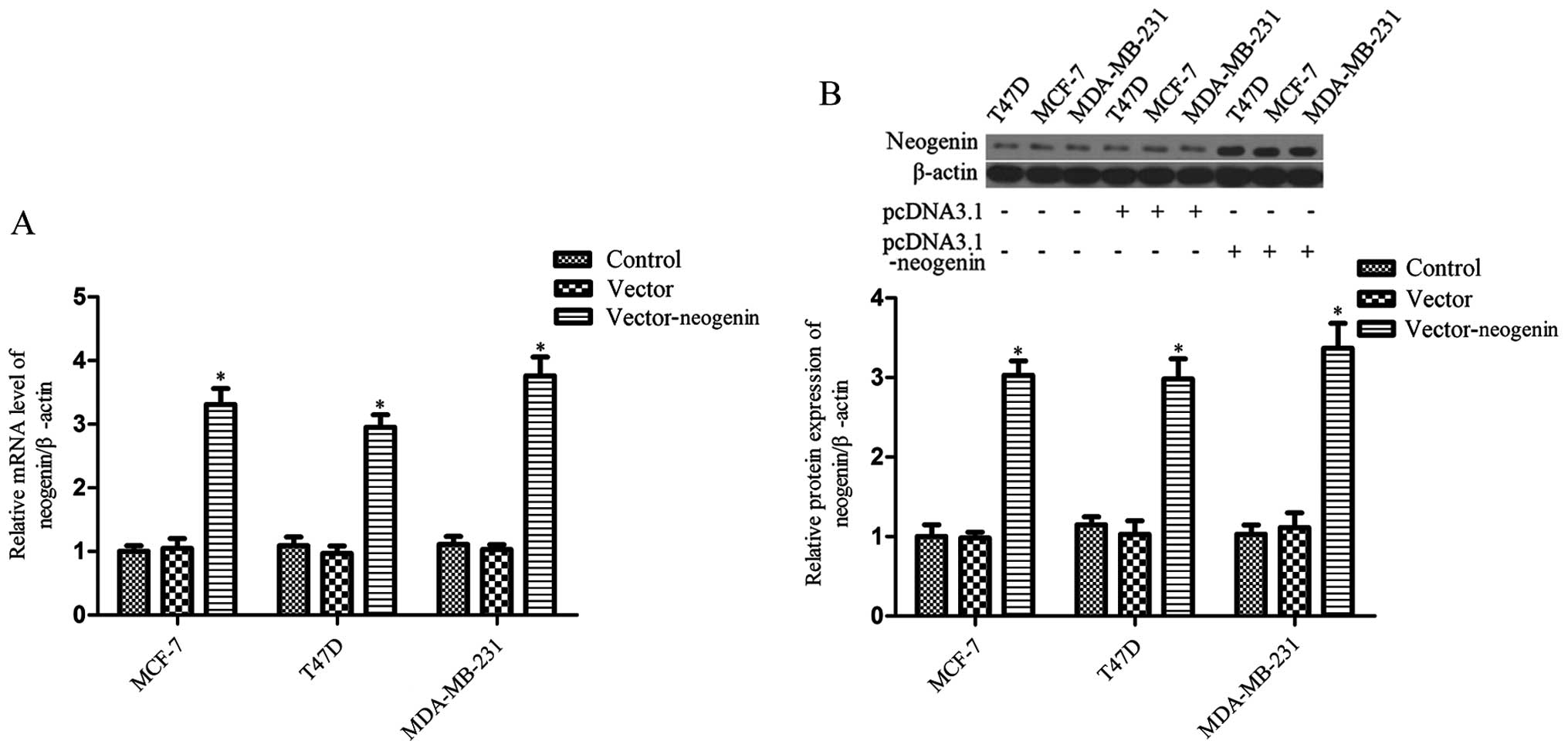
As a result of the RT-PCR and western blot analysis
of the three cell lines (MDA-MB-231, MCF-7 and T47D), the data show
that neogenin mRNA and protein expression was weak (Fig. 1). Then, in order to further
understand the role of neogenin in breast cancer, neogenin was
overexpressed in the MDA-MB-231, MCF-7 and T47D cell lines by
transfection. Cells were harvested after 48 h and neogenin
expression was analyzed by RT-PCR and western blot analysis. The
results show that neogenin mRNA and protein levels in the cells
that were transduced with pcDNA3.1-neogenin for 48 h were much
higher than in the control group (Fig.
1). The expression of neogenin was also upregulated in
MDA-MB-231, MCF-7 and T47D cells transduced with pcDNA3.1-neogenin
for 24, 72 and 96 h (data not shown).
Effect of the overexpression of neogenin
on breast cancer cell proliferation and migration
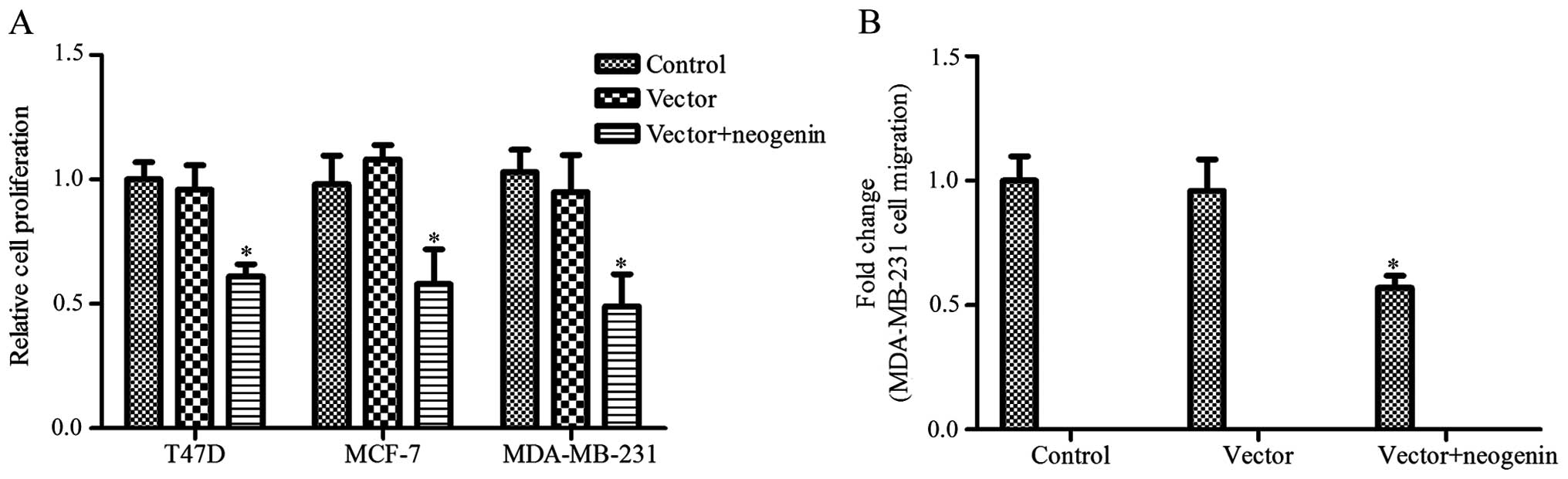
The neogenin overexpression vector was transfected
into MDA-MB-231, MCF-7 and T47D cells, and then cell proliferation
was measured by the MTT assay. As shown in Fig. 2A, the proliferation of MDA-MB-231,
MCF-7 and T47D cells was greatly decreased with neogenin
overexpression. Neogenin overexpression resulted in a 39, 42 and
51% decrease in the T47D, MCF-7 and MDA-MB-231 cell numbers,
respectively. These results indicate that neogenin overexpression
can inhibit the proliferation of all three breast cancer cell
lines. Thus, we selected the breast cancer cell line MDA-MB-231 for
further study. We also assessed the effects of neogenin
overexpression on MDA-MB-231 cell migration. The results show that
the migration of MDA-MB-231 cells was significantly decreased after
neogenin overexpression (Fig.
2B).
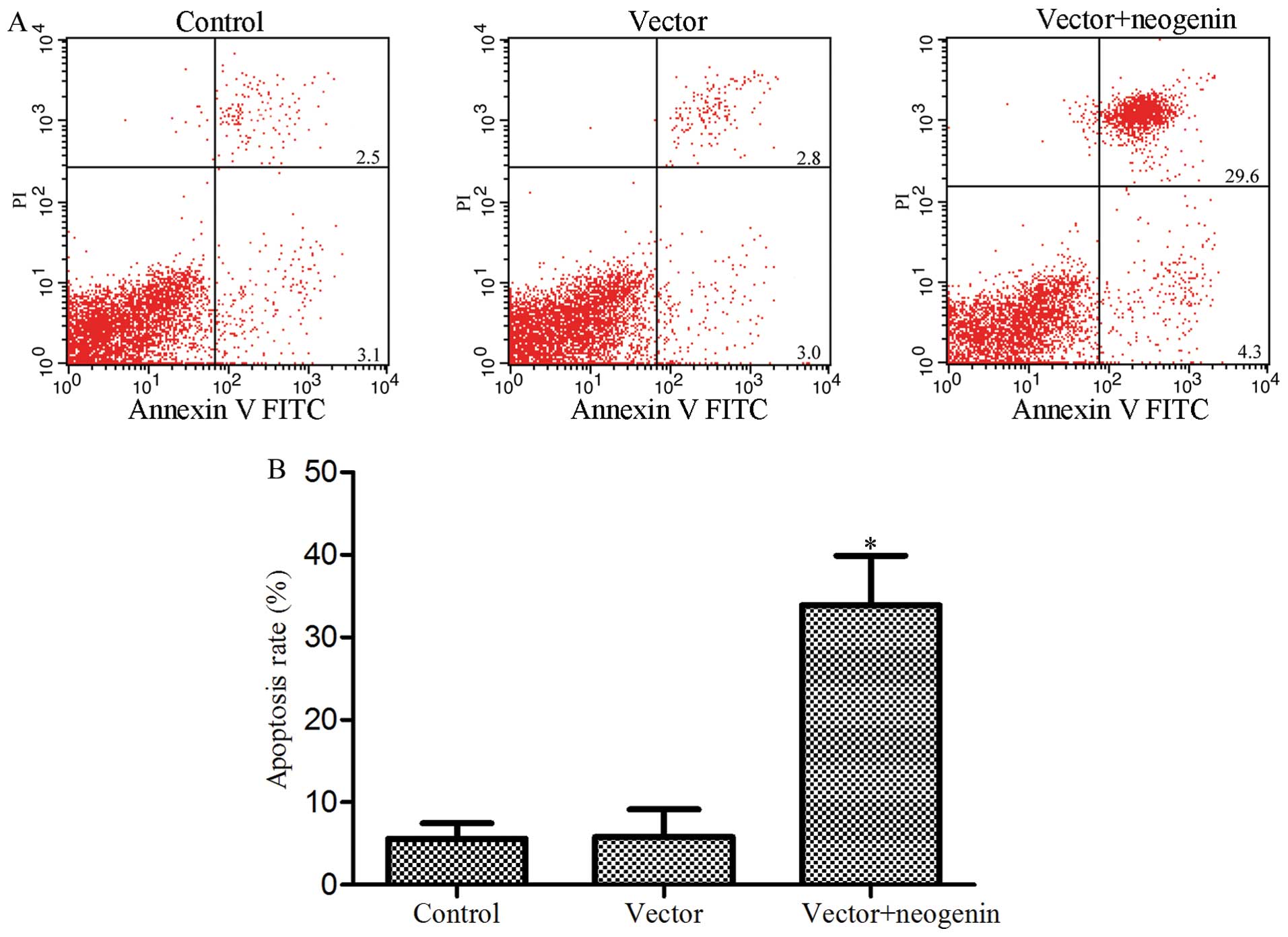
Induction of apoptosis after neogenin
overexpression in the breast cancer cell line MDA-MB-231
Reports have shown that neogenin overexpression can
induce apoptosis in the human glioma cell line SHG-44 (20). Moreover, the present study indicates
that neogenin overexpression inhibits breast cancer cell
proliferation. Thus, we tested whether the change in the MDA-MB-231
cell numbers in our studies was also mediated by neogenin-induced
apoptosis. Apoptosis was measured by flow cytometric analysis; the
results are shown in Fig. 3. Flow
cytometry showed that 33.9% of the cells that were transfected with
neogenin underwent apoptosis compared to 5.6% in the control group
which were not transfected (P<0.05) and 5.8% in the vector group
which were transfected with the empty vector (P<0.05). Similar
to those reported in literature (8), these results further suggest that
neogenin may be a breast cancer suppressor by inducing apoptosis in
breast cancer cells.
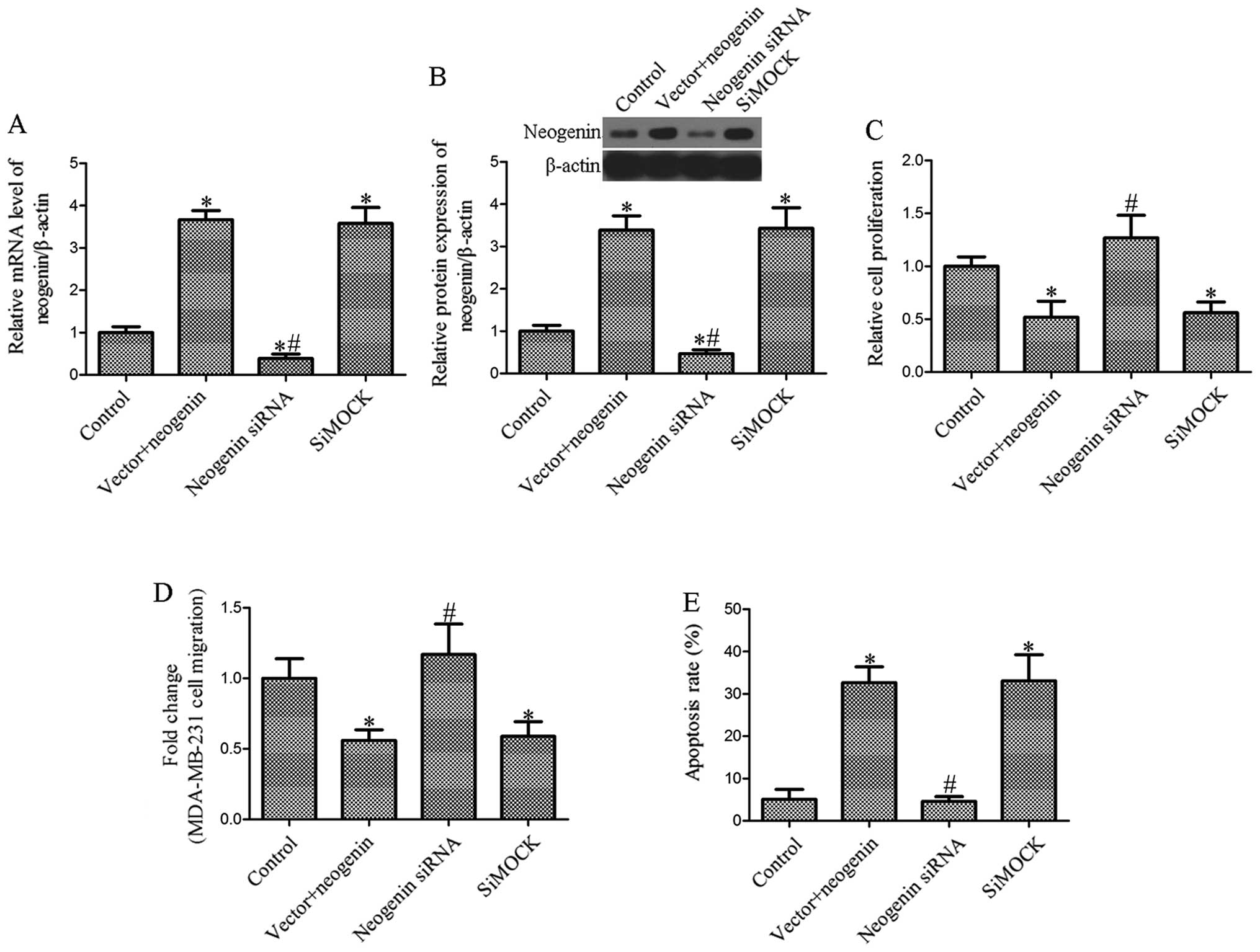
Effect of the ablation of neogenin on
breast cancer cell proliferation, migration and apoptosis
To determine whether siRNAs inhibit the expression
of neogenin, we first investigated the effects of siRNA on neogenin
mRNA and protein expression in MDA-MB-231 cells transfected with
neogenin. The level of neogenin was measured by RT-PCR and western
blot analysis after transfection of siRNAs into the breast cancer
cells. The results showed that the level of neogenin in breast
cancer MDA-MB-231 cells transfected with neogenin siRNA was
significantly decreased (P<0.05; Fig. 4A and B). Then, we determined the
effect of neogenin silencing on cell proliferation, migration and
apoptosis. As shown in Fig. 4C,
MDA-MB-231 cell growth was significantly increased in the neogenin
siRNA-transfected group compared with the siMock-transfected group.
Furthermore, we found that cell migration following the ablation of
neogenin considerably increased the migration of MDA-MB-231 cells
(Fig. 4D). Moreover, MDA-MB-231
cell apoptosis was also markedly decreased in the siRNA-transfected
group (Fig. 4E). Our results showed
that the effect of neogenin on proliferation, migration and
apoptosis is associated with the overexpression of neogenin.
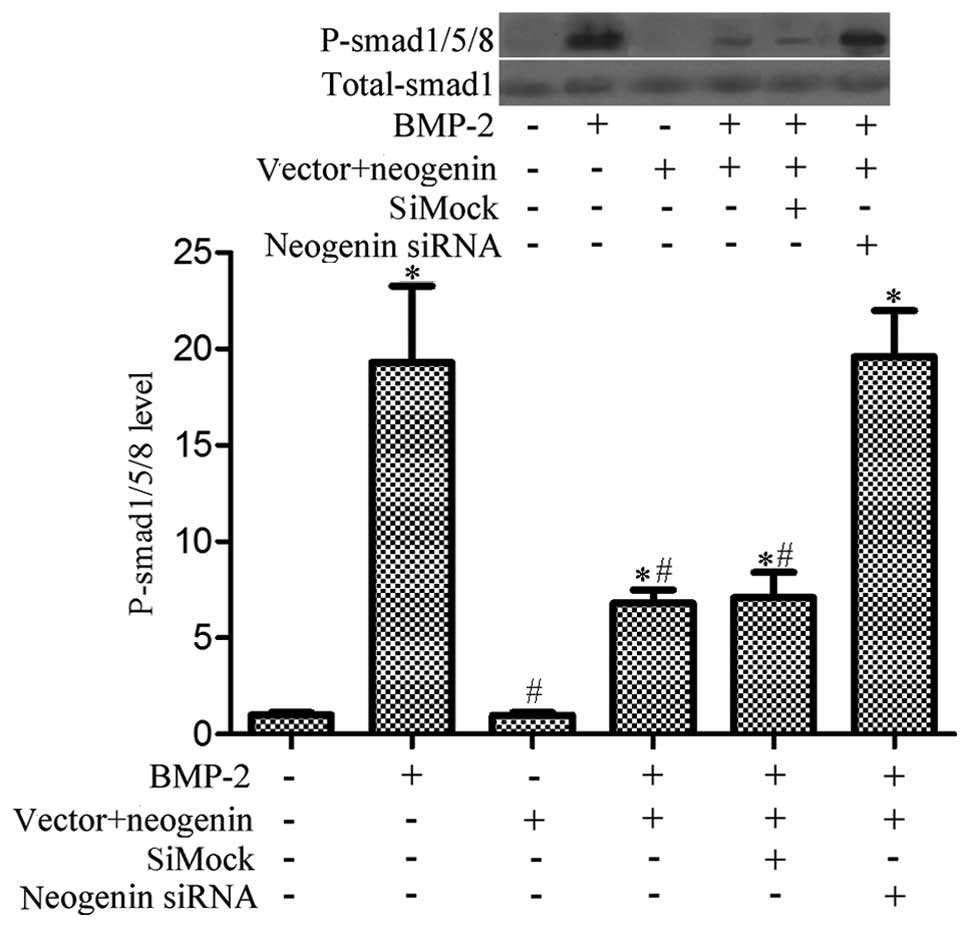
Overexpression of neogenin suppresses
BMP-2-induced phosphorylation of Smad1/5/8 in breast cancer
cells
Reports have suggested that BMP2 may act as a tumor
suppressor by promoting apoptosis in many cell types, such as
mature colonic epithelial and human colorectal cancer cells
(30,31). Moreover, some evidence indicates
that BMP-2 can induce the phosphorylation of Smad1/5/8, which is
prevented by neogenin (24).
Furthermore, an important role for the Smad1/5/8 signaling pathway
in migration was described in the bone marrow stromal cells
(32). Therefore, we investigated
if neogenin-induced breast cancer cell migration and growth
inhibition is related to the BMP-2-induced Smad1/5/8 signaling
pathway. We treated MDA-MB-231 cells with rhBMP-2 (0.1 mg/ml) for
30 min, and then analyzed the phosphorylation state of the receptor
proteins Smad1/5/8 using an antibody that specifically recognizes
phosphorylated Smad1/5/8. The results showed that phosphorylation
of Smad1/5/8 was significantly decreased in neogenin overexpressing
cells (Fig. 5). These data indicate
that the Smad1/5/8 pathway is inhibited in neogenin-transfected
cells.
Discussion
The main findings of the present study are as
follows: i) neogenin is weakly expressed in breast cancer cells,
and neogenin overexpression can inhibit breast cancer cell growth
and migration; ii) neogenin overexpression can promote breast
cancer MDA-MB-231 cell apoptosis; iii) neogenin silencing has no
apparent effect on MDA-MB-231 cell growth, migration or apoptosis;
and iv) neogenin overexpression is able to inhibit BMP-2-induced
Smad1/5/8 phosphorylation. The results of the present study
indicate that neogenin inhibits the progression of breast cancer
in vitro, which can be explained by the growth and migration
inhibition and pro-apoptosis effects of neogenin in breast cancer
cells.
Breast cancer, a serious threat to the health of
females, is a malignant tumor associated with the fastest growing
female mortality rate, far surpassing lung cancer (33,34).
The incidents of breast cancer are increasing at an annual rate of
3% in China (34). Treatment for
breast cancer is far from satisfactory, and some evidence suggests
that breast cancer is a genetic disease (35). The balance of oncogenes and tumor
suppressor genes plays an important role in the regulation of
cellular physiological processes and an abnormal balance may affect
cell proliferation, differentiation, apoptosis and drug resistance
(36,37). Thus, it is possible that identifying
novel targets may prevent or enhance the treatment of breast cancer
(38). Evidence has suggested that
neogenin is abnormally expressed in various cancers, including
bladder cancer (8).
Although Meyerhardt and co-workers (39) suggested that neogenin is expressed
in breast cancer cell lines and indicated that neogenin expression
is unchanged in cancer, including bladder cancer, some studies have
shown that neogenin expression is lower, in prostate (40), colon (41) and breast cancer (8). Our results are consistent with the
existing data (8) which suggest
that the expression of neogenin in breast cancer cells is inversely
associated with the tumorigenicity of breast cancer. Considering
the results of RT-PCR and western blot analysis on the three cell
lines (T47D, MCF-7 and MDA-MB-231), the data show that neogenin was
weakly expressed in these cells (Fig.
1). In order to study the effects of neogenin on the
progression of breast cancer, we transfected the recombinant
expression vector pcDNA3.1-neogenin into the breast cancer cell
lines T47D, MCF-7 and MDA-MB-231. The RT-PCR and western blot
analysis results show that the expression of neogenin in the three
cell lines was significantly upregulated (Fig. 1). Then, we demonstrated that a high
level of neogenin was correlated with a decrease in cell
proliferation and migration and an increase in cell apoptosis
(Figs. 2 and 3). Neogenin siRNA was used to silence the
expression of neogenin in neogenin-transfected cells. The results
suggest that neogenin siRNA increased the cell number and migration
and decreased apoptosis (Fig. 4).
The data from the present study indicate that neogenin was able to
inhibit the progression of breast cancer.
The functions of BMPs in cancer are situational and
complex (42); our observations
show that neogenin expression inhibits BMP-2-induced
phosphorylation of Smad1/5/8 in breast cancer cells. Treatment of
these cells with rh-BMP-2 led to an increase in the level of
phosphorylation of Smad1/5/8; however, neogenin overexpression
induced a marked decrease in the phosphorylation of Smad1/5/8.
Moreover, the extent of the Smad1/5/8 phosphorylation in the siMock
group was less than that in the neogenin siRNA group.
In summary, neogenin may play an important role in
the progression of breast cancer. Upregulation of neogenin reduced
breast cancer cell proliferation, inhibited migration and induced
apoptosis. Collectively, neogenin can be considered a tumor
suppressor in breast cancer. We demonstrated that neogenin
expression may be inversely correlated to breast cancer. However,
the specific mechanism of action of neogenin in breast cancer cells
remains to be determined. Future studies on the role of neogenin in
breast cancer will address these issues and enhance our knowledge
of breast cancer.
References
|
1
|
Blanco MA and Kang Y: Signaling pathways
in breast cancer metastasis - novel insights from functional
genomics. Breast Cancer Res. 13:2062011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang M, Chen J, Su F, Yu B, Su F, Lin L,
Liu Y, Huang JD and Song E: Microvesicles secreted by macrophages
shuttle invasion-potentiating microRNAs into breast cancer cells.
Mol Cancer. 10:1172011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li L, Luo J, Wang B, Wang D, Xie X, Yuan
L, Guo J, Xi S, Gao J, Lin X, et al: MicroRNA-124 targets
flotillin-1 to regulate proliferation and migration in breast
cancer. Mol Cancer. 12:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burstein HJ, Griggs JJ, Prestrud AA and
Temin S: American Society of Clinical Oncology clinical practice
guideline update on adjuvant endocrine therapy for women with
hormone receptor-positive breast cancer. J Oncol Pract. 6:243–246.
2010. View Article : Google Scholar :
|
|
6
|
Dawood S, Merajver SD, Viens P, Vermeulen
PB, Swain SM, Buchholz TA, Dirix LY, Levine PH, Lucci A,
Krishnamurthy S, et al: International expert panel on inflammatory
breast cancer: Consensus statement for standardized diagnosis and
treatment. Ann Oncol. 22:515–523. 2011. View Article : Google Scholar :
|
|
7
|
Ashok M, Griffin P and Halpern M: Impact
of clinical and nonclinical factors on the choice of HER2 test for
breast cancer. Cancer Invest. 28:735–742. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JE, Kim HJ, Bae JY, Kim SW, Park JS,
Shin HJ, Han W, Kim SW, Kang KS and Noh DY: Neogenin expression may
be inversely correlated to the tumorigenicity of human breast
cancer. BMC Cancer. 5:1542005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fitzgerald DP, Bradford D and Cooper HM:
Neogenin is expressed on neurogenic and gliogenic progenitors in
the embryonic and adult central nervous system. Gene Expr Patterns.
7:784–792. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamashita T, Mueller BK and Hata K:
Neogenin and repulsive guidance molecule signaling in the central
nervous system. Curr Opin Neurobiol. 17:29–34. 2007. View Article : Google Scholar
|
|
11
|
Matsunaga E and Chedotal A: Repulsive
guidance molecule/neogenin: A novel ligand-receptor system playing
multiple roles in neural development. Dev Growth Differ.
46:481–486. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lejmi E, Leconte L, Pédron-Mazoyer S,
Ropert S, Raoul W, Lavalette S, Bouras I, Feron JG, Maitre-Boube M,
Assayag F, et al: Netrin-4 inhibits angiogenesis via binding to
neogenin and recruitment of Unc5B. Proc Natl Acad Sci USA.
105:12491–12496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wilson NH and Key B: Neogenin: One
receptor, many functions. Int J Biochem Cell Biol. 39:874–878.
2007. View Article : Google Scholar
|
|
14
|
Cole SJ, Bradford D and Cooper HM:
Neogenin: A multifunctional receptor regulating diverse
developmental processes. Int J Biochem Cell Biol. 39:1569–1575.
2007. View Article : Google Scholar
|
|
15
|
Wilson NH and Key B: Neogenin interacts
with RGMa and netrin-1 to guide axons within the embryonic
vertebrate forebrain. Dev Biol. 296:485–498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SJ, Wang YG, Lee HW, Kang HG, La SH,
Choi IJ, Irimura T, Ro JY, Bresalier RS and Chun KH: Up-regulation
of neogenin-1 increases cell proliferation and motility in gastric
cancer. Oncotarget. 5:3386–3398. 2014.PubMed/NCBI
|
|
17
|
Link BC, Reichelt U, Schreiber M, Kaifi
JT, Wachowiak R, Bogoevski D, Bubenheim M, Cataldegirmen G, Gawad
KA, Issa R, et al: Prognostic implications of netrin-1 expression
and its receptors in patients with adenocarcinoma of the pancreas.
Ann Surg Oncol. 14:2591–2599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song S, Mazurek N, Liu C, Sun Y, Ding QQ,
Liu K, Hung MC and Bresalier RS: Galectin-3 mediates nuclear
beta-catenin accumulation and Wnt signaling in human colon cancer
cells by regulation of glycogen synthase kinase-3beta activity.
Cancer Res. 69:1343–1349. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu YC, Lam KY, Law S, Wong J and
Srivastava G: Identification of differentially expressed genes in
esophageal squamous cell carcinoma (ESCC) by cDNA expression array:
Overexpression of Fra-1, Neogenin, Id-1, and CDC25B genes in ESCC.
Clin Cancer Res. 7:2213–2221. 2001.PubMed/NCBI
|
|
20
|
Wu X, Li Y, Wan X, Kayira TM, Cao R, Ju X,
Zhu X and Zhao G: Down-regulation of neogenin accelerated glioma
progression through promoter methylation and its overexpression in
SHG-44 induced apoptosis. PLoS One. 7:e380742012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujita Y, Taniguchi J, Uchikawa M, Endo M,
Hata K, Kubo T, Mueller BK and Yamashita T: Neogenin regulates
neuronal survival through DAP kinase. Cell Death Differ.
15:1593–1608. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bolos V, Blanco M, Medina V, Aparicio G,
Diaz-Prado S and Grande E: Notch signalling in cancer stem cells.
Clin Transl Oncol. 11:11–19. 200PubMed/NCBI
|
|
23
|
Zardawi SJ, O’Toole SA, Sutherland RL and
Musgrove EA: Dysregulation of Hedgehog, Wnt and Notch signalling
pathways in breast cancer. Histol Histopathol. 24:385–398.
2009.PubMed/NCBI
|
|
24
|
Hagihara M, Endo M, Hata K, Higuchi C,
Takaoka K, Yoshikawa H and Yamashita T: Neogenin, a receptor for
bone morphogenetic proteins. J Biol Chem. 286:5157–5165. 2011.
View Article : Google Scholar :
|
|
25
|
Yang ZQ, Liu G, Bollig-Fischer A, Haddad
R, Tarca AL and Ethier SP: Methylation-associated silencing of
SFRP1 with an 8p11–12 amplification inhibits canonical and
non-canonical WNT pathways in breast cancers. Int J Cancer.
125:1613–1621. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang ZQ, Streicher KL, Ray ME, Abrams J
and Ethier SP: Multiple interacting oncogenes on the 8p11–p12
amplicon in human breast cancer. Cancer Res. 66:11632–11643. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang ZQ, Imoto I, Fukuda Y, Pimkhaokham A,
Shimada Y, Imamura M, Sugano S, Nakamura Y and Inazawa J:
Identification of a novel gene, GASC1, within an amplicon at
9p23–24 frequently detected in esophageal cancer cell lines. Cancer
Res. 60:4735–4739. 2000.PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Feng X, Wu Z, Wu Y, Hankey W, Prior TW, Li
L, Ganju RK, Shen R and Zou X: Cdc25A regulates matrix
metalloprotease 1 through Foxo1 and mediates metastasis of breast
cancer cells. Mol Cell Biol. 31:3457–3471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Chen X, Qiao M, Zhang BQ, Wang N,
Zhang Z, Liao Z, Zeng L, Deng Y, Deng F, et al: Bone morphogenetic
protein 2 inhibits the proliferation and growth of human colorectal
cancer cells. Oncol Rep. 32:1013–1020. 2014.PubMed/NCBI
|
|
31
|
Hardwick JC, van den Brink GR, Bleuming
SA, Ballester I, van den Brande JM, Keller JJ, Offerhaus GJ, van
Deventer SJ and Peppelenbosch MP: Bone morphogenetic protein 2 is
expressed by, and acts upon, mature epithelial cells in the colon.
Gastroenterology. 126:111–121. 2004. View Article : Google Scholar
|
|
32
|
Hu Y, Du Y, Jiang H and Jiang GS: Cerium
promotes bone marrow stromal cells migration and osteogenic
differentiation via Smad1/5/8 signaling pathway. Int J Clin Exp
Pathol. 7:5369–5378. 2014.PubMed/NCBI
|
|
33
|
Engebraaten O, Vollan HK and Borresen-Dale
AL: Triplenegative breast cancer and the need for new therapeutic
targets. Am J Pathol. 183:1064–1074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li N, Zheng RS, Zhang SW, Zou XN, Zeng HM,
Dai Z and Chen WQ: Analysis and prediction of breast cancer
incidence trend in China. Zhonghua Yu Fang Yi Xue Za Zhi.
46:703–707. 2012.in Chinese. PubMed/NCBI
|
|
35
|
Yang S and Han H: Effect of
cycloxygenase-2 silencing on the malignant biological behavior of
MCF-7 breast cancer cells. Oncol Lett. 8:1628–1634. 2014.PubMed/NCBI
|
|
36
|
Veeck J, Noetzel E, Bektas N, Jost E,
Hartmann A, Knüchel R and Dahl E: Promoter hypermethylation of the
SFRP2 gene is a high-frequent alteration and tumor-specific
epigenetic marker in human breast cancer. Mol Cancer. 7:832008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X and Munster PN: New protein kinase
inhibitors in breast cancer: afatinib and neratinib. Expert Opin
Pharmacother. 15:1277–1288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
De Los Santos JF, Cantor A, Amos KD,
Forero A, Golshan M, Horton JK, Hudis CA, Hylton NM, McGuire K,
Meric-Bernstam F, et al: Magnetic resonance imaging as a predictor
of pathologic response in patients treated with neoadjuvant
systemic treatment for operable breast cancer. Translational Breast
Cancer Research Consortium trial 017. Cancer. 119:1776–1783. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Meyerhardt JA, Look AT, Bigner SH and
Fearon ER: Identification and characterization of neogenin, a
DCC-related gene. Oncogene. 14:1129–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Latil A, Chêne L, Cochant-Priollet B,
Mangin P, Fournier G, Berthon P and Cussenot O: Quantification of
expression of netrins, slits and their receptors in human prostate
tumors. Int J Cancer. 103:306–315. 2003. View Article : Google Scholar
|
|
41
|
Li VS, Yuen ST, Chan TL, Yan HH, Law WL,
Yeung BH, Chan AS, Tsui WY, So S, Chen X, et al: Frequent
inactivation of axon guidance molecule RGMA in human colon cancer
through genetic and epigenetic mechanisms. Gastroenterology.
137:176–187. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ye L, Lewis-Russell JM, Kyanaston HG and
Jiang WG: Bone morphogenetic proteins and their receptor signaling
in prostate cancer. Histol Histopathol. 22:1129–1147.
2007.PubMed/NCBI
|