Introduction
Breast cancer is a heterogeneous disease with
various subtypes exhibiting distinct biological features, resulting
in differences in response patterns to various clinical strategies
and treatment modalities (1).
Currently, the research of breast cancer in regards to molecular
techniques and gene signatures has been a central issue for the
development of novel therapeutics (2). Angiogenesis, one of the established
approaches for targeting tumors, is a complex procedure containing
a succession of genetic alterations, of which VEGF is a key
mediator. In accordance with the results of various preclinical
studies, significant therapeutic effects of VEGF blockers have been
demonstrated in various types of cancers, even in some progressive
cancers (3). Our previous study on
12-deoxyphorbol 13-palmitate (DP) showed that it possesses an
anti-angiogenesis effect in vitro and in vivo through
the VEGFR-2-signaling pathway (4).
In the present study, we explored the ability of DP to inhibit the
expression of VEGF and hypoxia inducible factor-1α (HIF-1α) via the
PI3K/Akt/mTOR pathway.
VEGF is an endothelial cell-specific mitogen and
VEGF-mediated related signaling contributes to key aspects of tumor
angiogenesis and vascular permeability (5). The upstream gene of VEGF, HIF-1α, is
overexpressed in the early stages of mammary carcinogenesis, and is
correlated with diagnostic and prognostic indicators, making HIF-1α
a potential target for new approaches to inhibit angiogenesis
(6). HIF-1α, under hypoxic stress,
binds with HIF-1β, and controls the expression of multiple
downstream target genes by combining with hypoxia response elements
(HREs) of the target genes inside the nucleus to promote further
responses (7). The expression of
HIF-1α is controlled by multiple mechanisms, in which protein
ubiquitination is primarily involved. In addition, research has
found that HIF-1α accumulation depends on de novo protein
synthesis (9), and some pathways
participate in the regulation of HIF-1α, such as the PI3K/Akt/mTOR
pathway (10).
PI3K/Akt/mTOR, a crucial intracellular pathway, has
been well established to play a significant role in tumor cell
proliferation and growth (11).
Activation of PI3K transforms phosphatidylinositol 4,5-bisphosphate
(PIP2) to phosphatidylinositol 3,4,5-triphosphate (PIP3), and then
triggers Akt phosphorylation on Thr 308. Phosphorylated Akt
unleashes mTOR through inactivation of TSC1/TSC2 (12). mTOR, a protein kinase, is a
therapeutic target for cancer treatment which is ubiquitously
expressed in cells (13). As a
tumor-suppressor, the TSC-TSC2 complex emerges as a critical
negative regulator of signaling downstream of Akt and upstream of
mTOR (14). Accumulated evidence
from cancer and genetic biology indicates the benefit of targeting
the PI3K/Akt/mTOR signaling pathway to inhibit tumor angiogenesis.
Collectively, we hypothesized that DP possesses anticancer activity
in breast cancer by impacting tumor angiogenesis.
Materials and methods
Materials
DP was isolated from Euphorbia fischeriana
and its purity was >99% as characterized by gas chromatography.
The Valukine Human VEGF Immunoassay kit was purchased from R&D
Systems (Minneapolis, MN, USA). Wortmanin was purchased from Cayman
(Denver, CO, USA). Cell culture reagents were purchased from
HyClone (Logan, UT, USA). MTT, dimethylsulfoxide (DMSO) and
CoCl2 were purchased from Sigma-Aldrich (St. Louis, MO,
USA). RNAiso Plus, SYBR® Premix Ex Taq™ and
PrimeScript® RT reagent kit (Perfect Real Time) were
purchased from Takara (Dalian, Liaoning, China).
Cell culture
The human breast cancer cell line MCF-7 was
purchased from the American Type Culture Collection (ATCC)
(Rockville, MD, USA), and cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100
U/ml penicillin and 100 μg/ml streptomycin. The cells were
maintained at 37°C in a CO2 incubator with an atmosphere
composed of 5% CO2 and 95% air. To mimic a hypoxic
condition, the cells were treated with 150 μM
CoCl2 as the hypoxic control group.
Cell viability assay
The cells were divided into normoxic and hypoxic
control groups and treated with various concentrations (10–40
μM) of DP. Cell viability was determined by the MTT assay.
MCF-7 cells were seeded in 96-well plates at a density of
3×103 cells/well and incubated for 6, 12 and 24 h after
a 12-h adherence. Then, 20 μl of MTT solution (5 mg/ml) was
added into each well for another 4-h incubation at 37°C. After 4 h,
the medium was removed and the crystals were dissolved using 200
μl DMSO before constant shaking for 10 min. The absorbance
values were analyzed by an enzyme-labeling instrument at 570 nm.
Three replicate were conducted for the MTT assay.
Enzyme-linked immunosorbent assay (ELISA)
for VEGF
For quantification of VEGF excreted by the MCF-7
cells, the cells were incubated overnight in 6-well dishes with
2×106 cells/well. Following DP treatments for 12 h, the
medium was collected and examined by a Valukine Human VEGF
Immunoassay kit following the manufacturer's instructions.
RT-qPCR
After DP treatments, total RNA was isolated from the
cells using total RNA extraction reagent RNAiso Plus according to
the product manual. Total RNA was reverse-transcribed to cDNA with
the PrimeScript® RT reagent kit following the
manufacturer's instructions. The amplification of cDNA was
performed in triplicate using SYBR® Premix Ex
Taq™ kit under conditions of 95°C for 30 sec, 95°C for 5 sec and
60°C for 31 sec. The VEGF, HIF-1α, TSC1 and TSC2 mRNA were analyzed
and the final data were presented using the 2−ΔΔCt
method. The primer sequences used for real-time PCR were: VEGF F,
5′-GTCCAACTTCTGGGCTGTTCT-3′ and R, 5′-CCCTCTCCTCTTCCTTCTCTTC-3′;
HIF-1α F, 5′-TAGCCGAGGAAGAACTATGAAC3′ and R, 5′-CACACT
GAGGTTGGTTACTGTTG3′; TSC1 F, 5′-GCACTCTTT CATCGCCTTTATG-3′ and R,
5′-ATCATTGGCTTGAC CACTTCTT-3′; TSC2 F, 5′-CAGACAATGGGAGACACAT
CAC-3′ and R, 5′-CtAAGTTCACCAGCACCAGAAG-3′.
Western blotting
MCF-7 cells were incubated overnight before
treatment with various concentrations of DP and wortmannin for 12
h. After washing with cold PBS twice, the cells were lysed with
RIPA lysis buffer (Beyotime, Shanghai, China), and total proteins
were quantitated with a BCA protein assay kit (CWBio, Beijing,
China). Equal protein (30 μg) of each sample was separated
by SDS-polyacrylamide gels (SDS-PAGE) and then blotted onto
polyvinylidene fluoride (PVDF) membranes. The membranes were
incubated with the primary mouse antibodies against GAPDH (CWBio),
HIF-1α (BD Biosciences, Minneapolis, MN, USA), TSC1 (Cell Signaling
Technology, Danvers, MA, USA) and rabbit antibodies against VEGF
(Abcam, Cambridge, UK), TSC2, p-Akt, Akt, p-PI3K, PI3K, mTOR and
p-mTOR at 4°C overnight followed by HRP-conjugated anti-rabbit IgG
or HRP-conjugated anti-mouse IgG antibody (both from Cell Signaling
Technology) incubated for 2 h at room temperature. Blots were then
visualized using the West Pico Chemiluminescent Substrate (Pierce,
Woburn, MA, USA). GAPDH was used as the internal reference.
Immunofluorescence
MCF-7 cells were plated in 6-well plates, then
treated with DP (0 and 20 μM) for 12 h. After incubation
overnight at 4°C with a 1:100 dilution of mouse anti-HIF-1α, the
cells were washed three times with PBS and then incubated with an
anti-mouse FITC-labeled secondary antibody for 1 h, and then cells
were stained with DAPI for 5 min after washing three times. The
immunofluorescence was observed with a confocal laser scanning
microscope (Olympus, Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± SD. Student's
t-tests were conducted to analyze the differences between
experimental groups and control groups using SPSS 13.0 (SPSS, Inc.,
Chicago, IL, USA). A P-value of ≤0.05 was considered to indicate a
statistically significant result.
Results
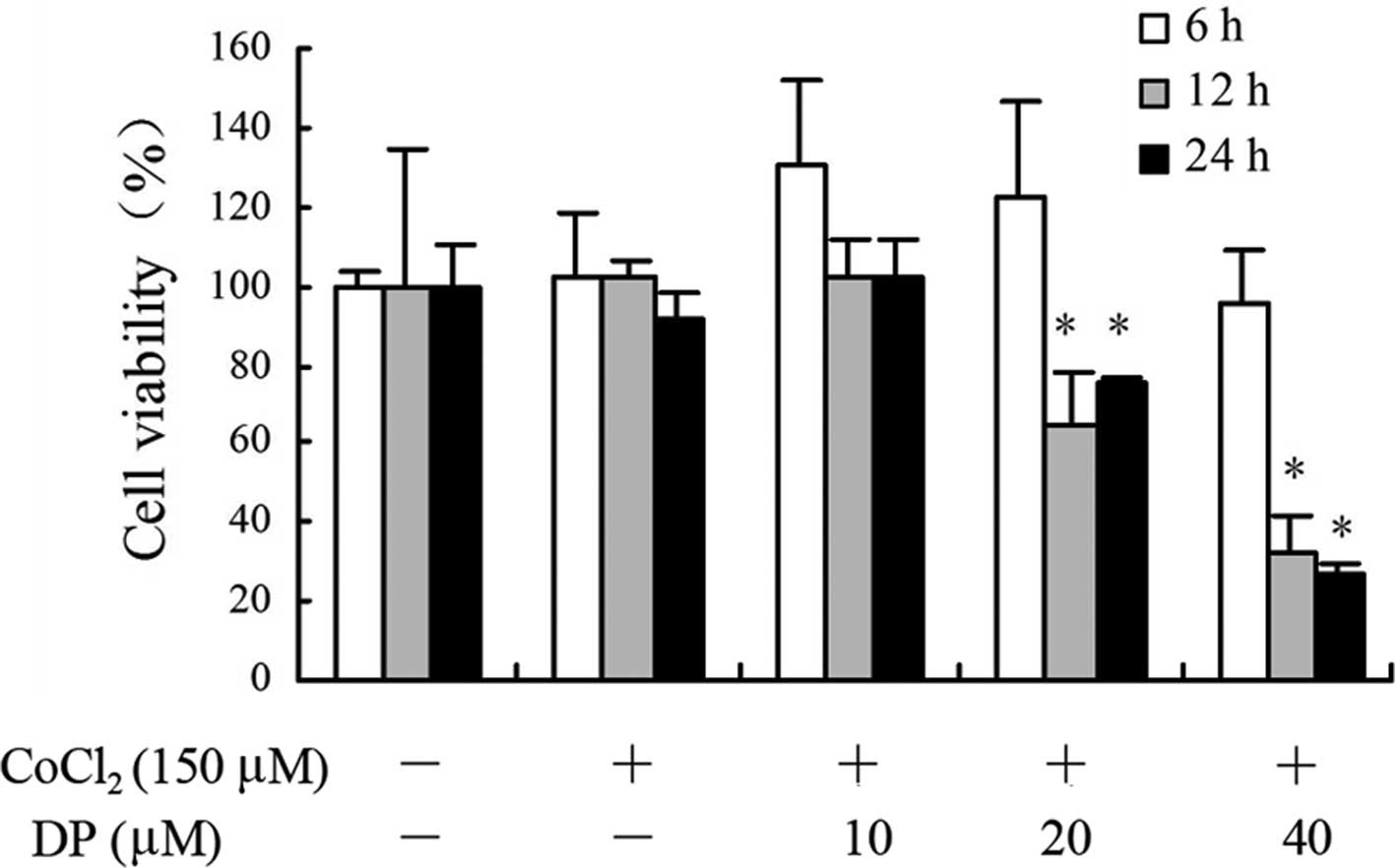
DP inhibits the proliferation of MCF-7
cells
In this experiment, we tested the effect of DP on
the proliferation of MCF-7 cells using MTT assay. Results of the
MTT assays showed that following treatment with 150 μM
CoCl2, the cell viability did not exhibit significant
differences compared with the normoxic control (Fig. 1). DP decreased the viability of
MCF-7 cells in a time- and dose-dependent manner. DP reduced the
viability slightly at 6 h without any statistical differences
following treatments at 10, 20 and 40 μM. Treatment at 10
μM showed no inhibition when compared with the hypoxic
control at 6, 12 and 24 h. Treatments at 20 and 40 μM
inhibited the cell viability which ranged between 26 and 75% at 12
and 24 h.
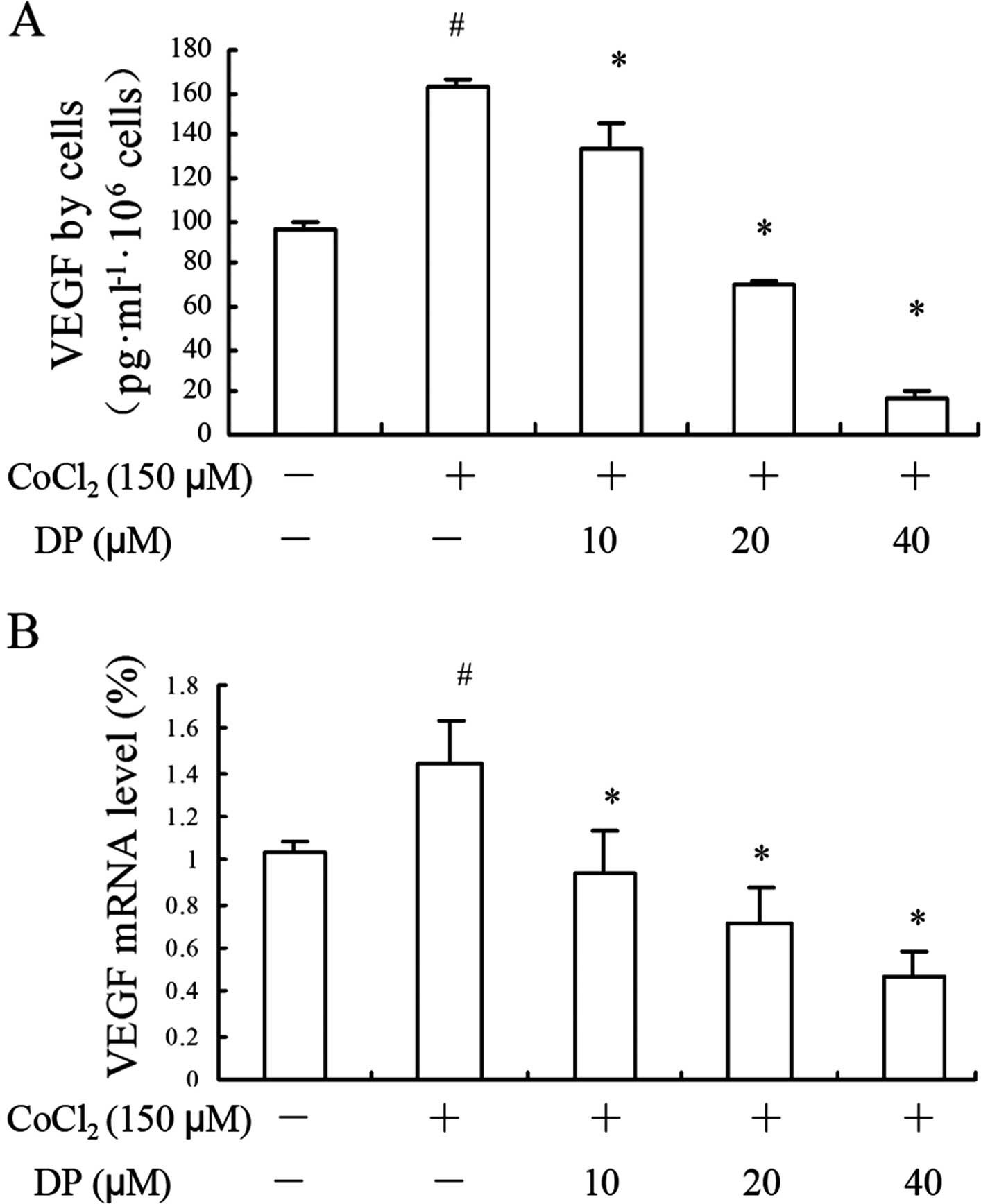
DP reduces the transcription and
excretion of VEGF
VEGF is activated not only in normal human tissues
yet is also associated with oncogenesis of breast cancer (15). As an important pro-angiogenic
factor, VEGF excreted from tumor cells promotes the development of
nearby vessels. In the present study, we investigated the impact of
DP on VEGF protein and mRNA levels by ELISA and RT-qPCR assays,
respectively. In the ELISA assay, the VEGF accumulation is
presented as normalized data based on the cell number. A notable
increase in VEGF level was detected when cells were exposed to a
hypoxia condition, and DP downregulated VEGF at both the protein
(Fig. 2A) and mRNA (Fig. 2B) levels. The results indicated that
DP reversed the expression and transcription of VEGF induced by
hypoxia, which confirms it to be a potential inhibitor for reducing
neovascularity.
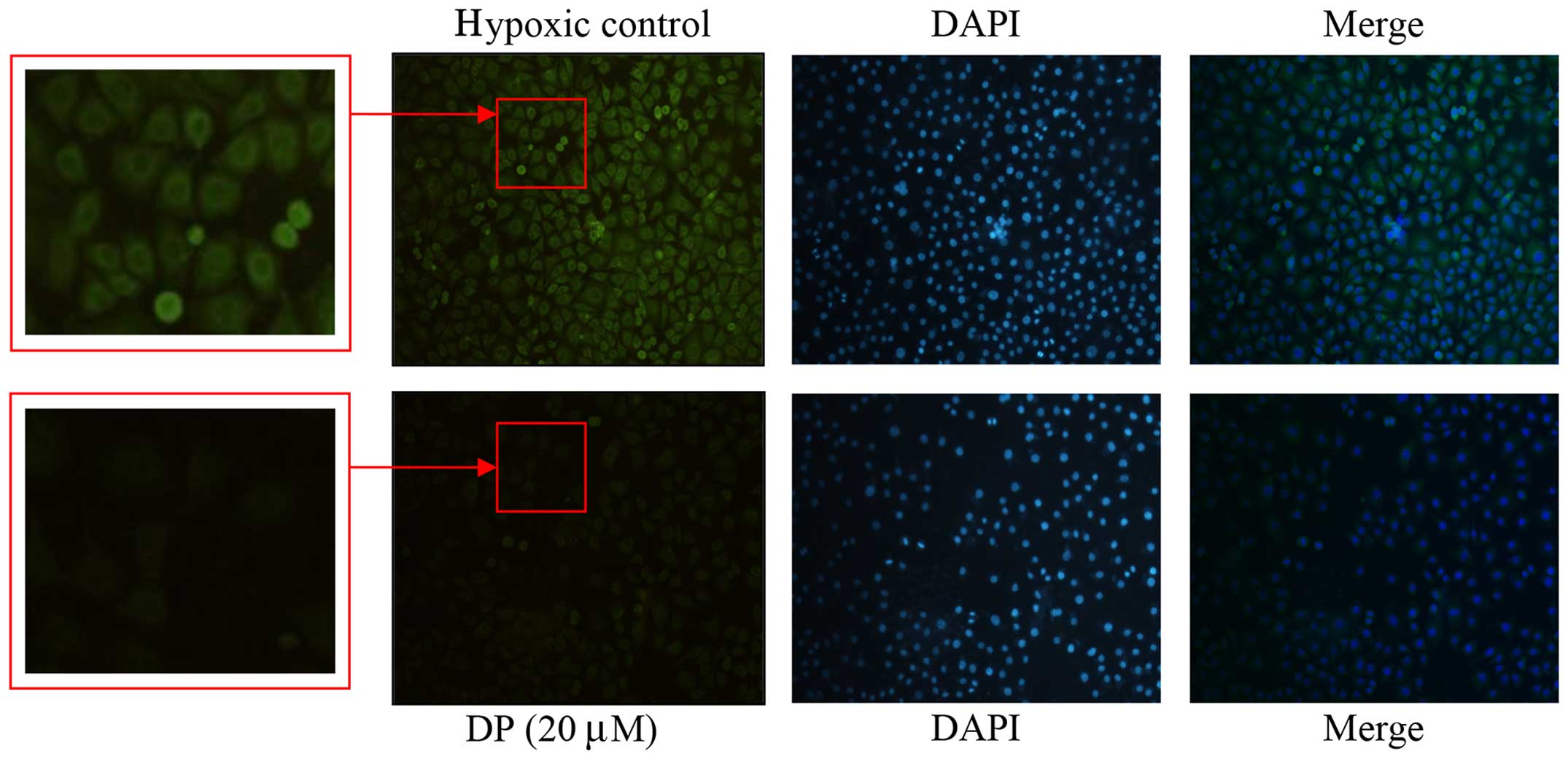
DP re-distributes the location of
HIF-1α
An increase and stabilization of nuclear
translocation of HIF-1α is a key factor to VEGF gene expression and
angiogenesis (16). To explore the
effect of DP on the location of HIF-1α, immunofluorescence was
used. As shown in Fig. 3, in the
presence of DP, the HIF-1α fluorescent intensity became weaker.
Simultaneously, the localization of HIF-1α was re-distributed in
the cells. The expression of HIF-1α was obviously concentrated in
and around the nucleus in the hypoxic control, yet this
accumulation decreased after the addition of DP (20 μM),
which confirmed that DP interrupts nuclear HIF-1α
translocation.
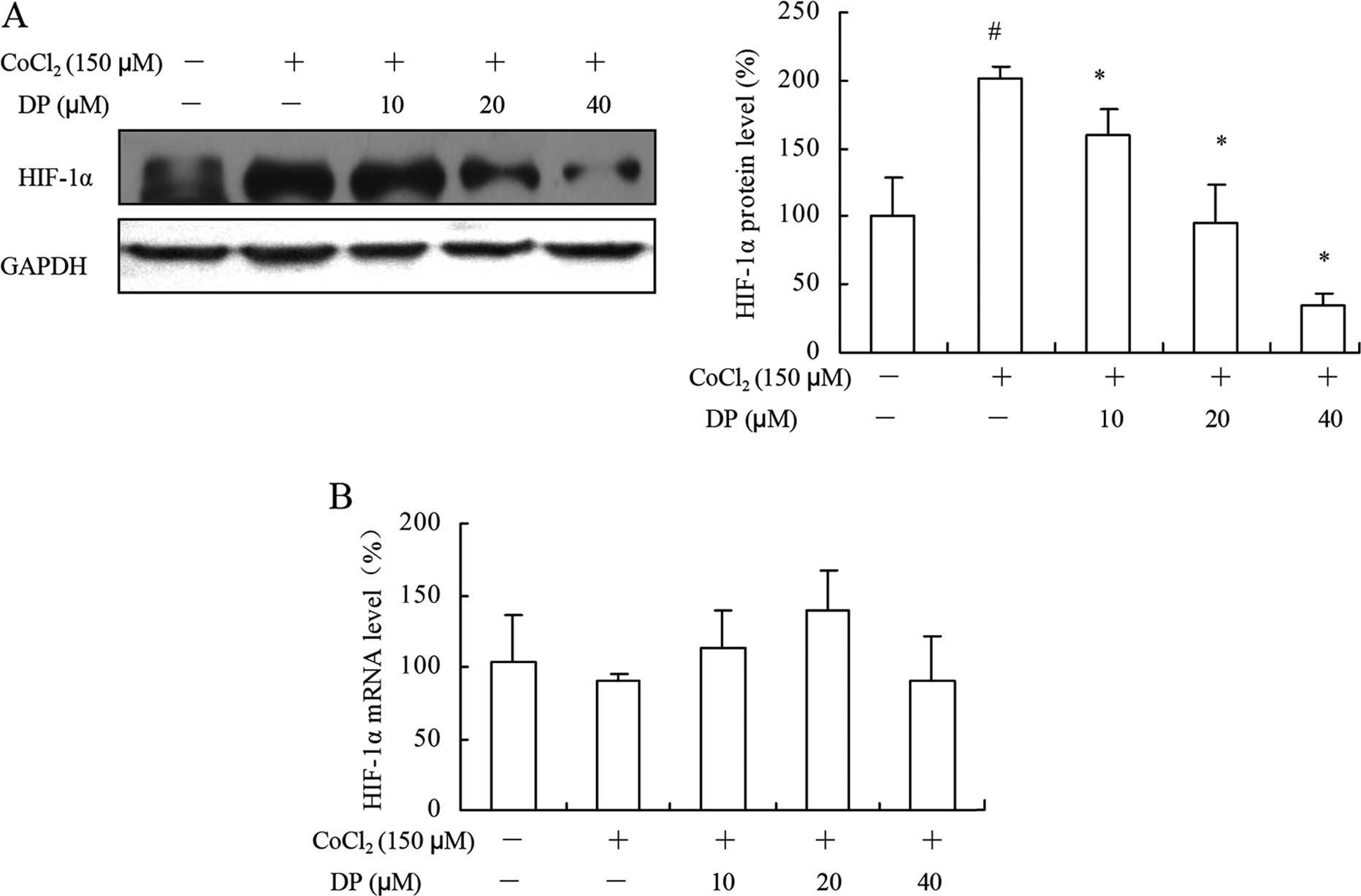
DP inhibits the expression of HIF-1α
without affecting its transcriptional activity
Hypoxia is involved in the process of angiogenesis,
and a growing number of studies have focused on regulating the HIF
family to alter the expression of VEGF (17). To further confirm the results above,
western blotting and RT-qPCR were performed to examine the total
HIF-1α expression in the cells. As shown in Fig. 4A, MCF-7 cells were treated with
different concentrations of DP under a hypoxia condition for 12 h.
The protein level of HIF-1α under a hypoxia condition was markedly
increased when compared with the level under a normoxic condition,
and this increase was abolished by DP in a concentration-dependent
manner. However, the protein level was downregulated while the mRNA
level of HIF-1α remained unchanged (Fig. 4B), suggesting that DP may
downregulate the expression of HIF-1α without affecting it at the
transcriptional level in the MCF-7 cells.
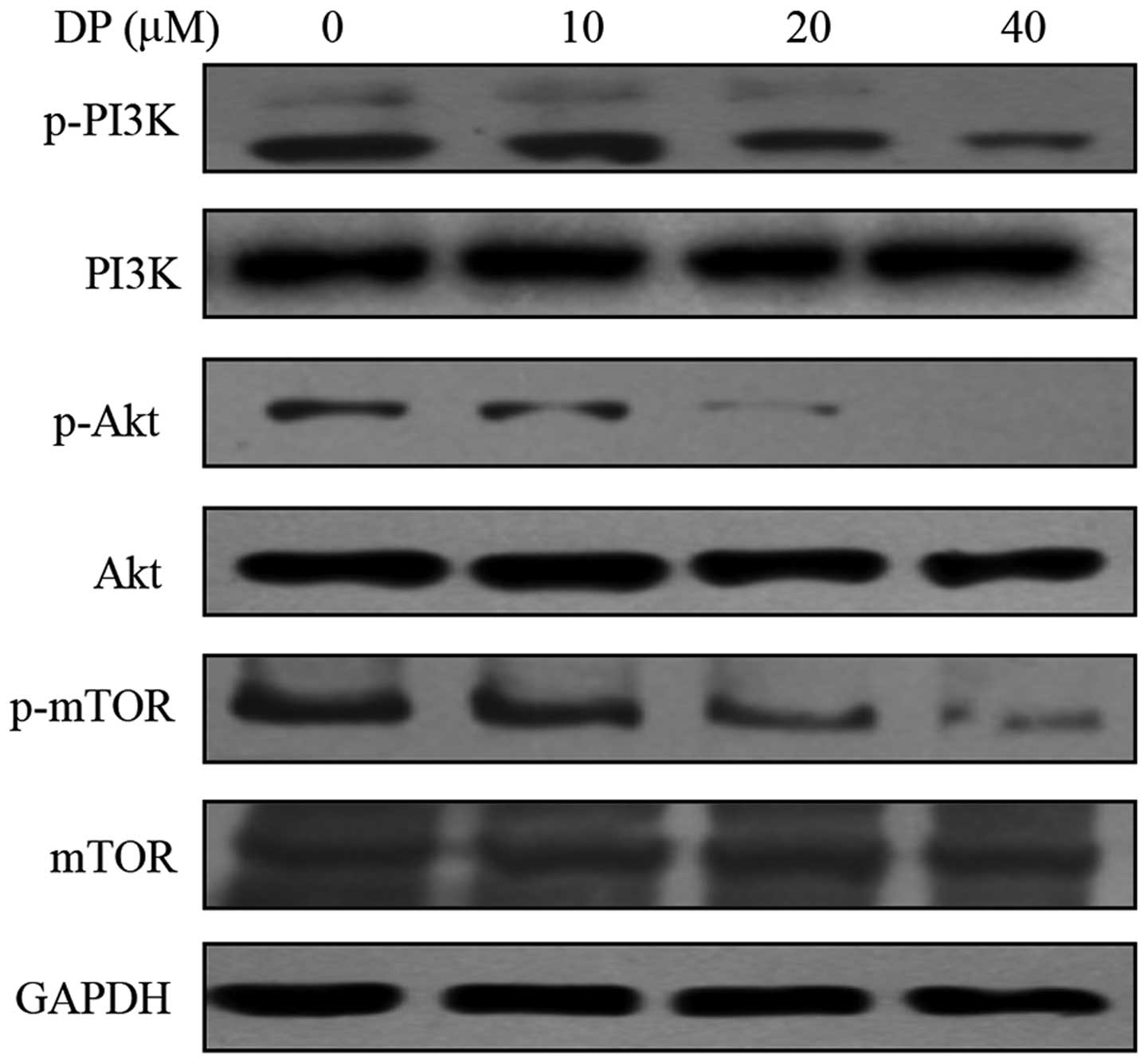
DP inhibits the PI3K/Akt/HIF-1α signaling
pathway in MCF-7 cells
The PI3K/Akt/HIF-1α pathway is important in the
majority of human types of cancers. Research has found that the
PI3K/Akt pathway is involved in tumor angiogenesis by an
HIF-1-dependent mechanism (18). To
evaluate the effect of DP on the PI3K/Akt/HIF-1α pathway, we
detected the phosphorylation of PI3K, Akt and mTOR. The MCF-7 cells
were treated with DP at various concentrations under a hypoxic
condition for 12 h. Treatment of DP resulted in a decrease in the
expression of p-PI3K, which was correlated with its effectors p-Akt
and p-mTOR, whereas DP did not affect the total protein levels of
these proteins (Fig. 5).
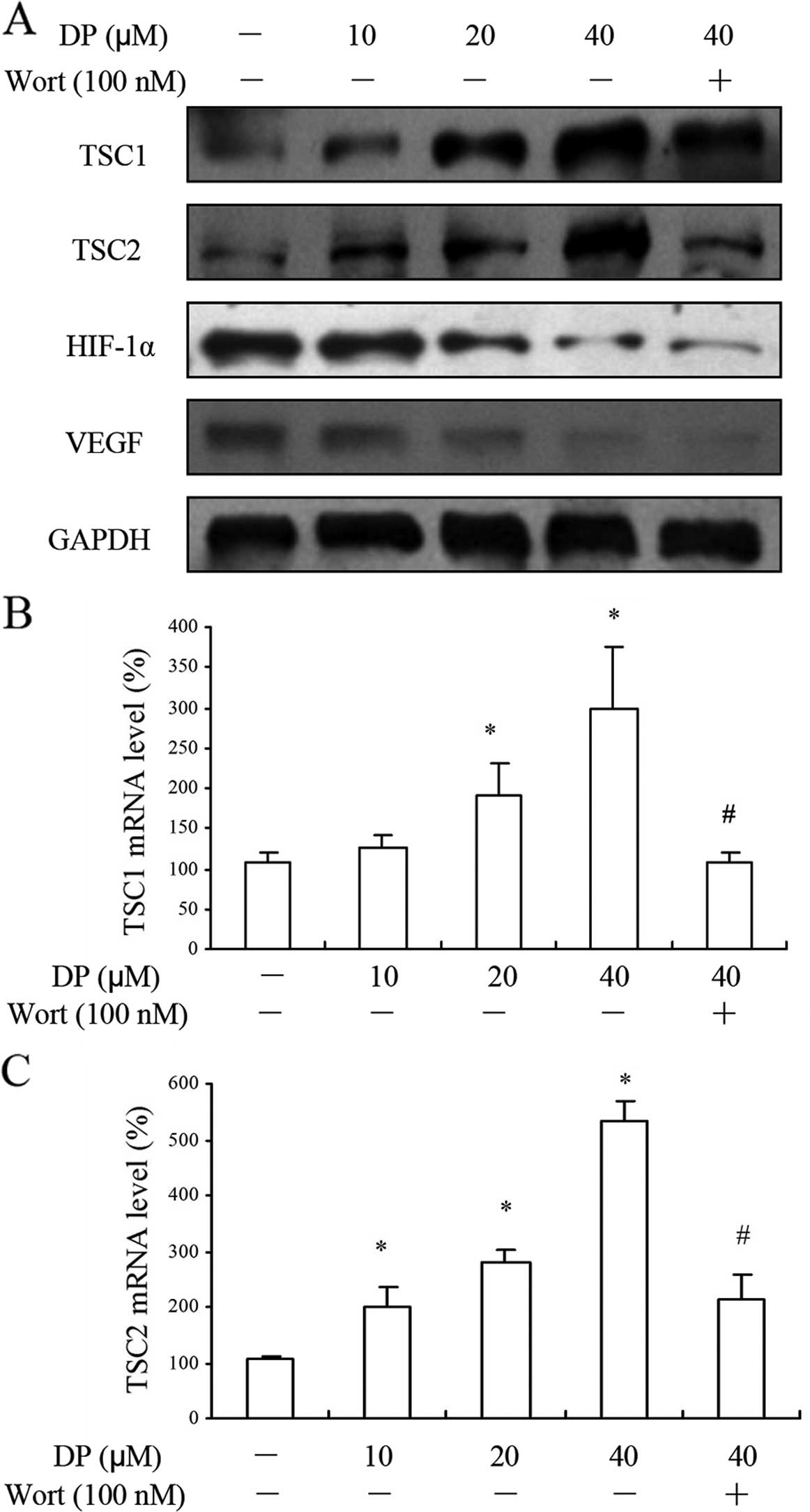
DP regulates HIF-1α and VEGF in a PI3K
and TSC1/TSC2-dependent manner
TSC1 and TSC2 are tumor-suppressor genes, and
important negative regulators of mTOR, with responsiveness to the
growth factors related to PI3K and Akt (19). DP enhanced the protein levels of
TSC1 and TSC2 in the MCF-7 cells in a concentration-dependent
manner, and this enhancement by DP required the activity of PI3K,
as the addition of the PI3K inhibitor wortmannin (100 nM)
significantly reversed this effect. Next, we found that
co-treatment of wortmannin with DP (40 μM) downregulated the
expression of HIF-1α and VEGF slightly by western blotting
(Fig. 6A). Furthermore, the RT-qPCR
results exhibited the same tendency of TSC1 and TSC2 mRNA with the
protein levels (Fig. 6B and C).
These results collectively suggest that DP inhibited VEGF and
HIF-1α through a PI3K-related signaling pathway.
Discussion
Breast cancer is the leading cause of cancer-related
death in females. It is a heterogeneous disease and is commonly
associated with epigenetic and multiple genetic abnormalities
(20,21). Breast cancer is
angiogenesis-dependent, which explains its vulnerability to various
angiogenesis-related factors such as vascular endothelial growth
factor (VEGF) in preclinical and clinical research (22). Reich et al found that the
blockade of VEGF by small interfering RNA (siRNA) inhibited
neovascularization in a mouse model (23). Considering the central importance of
VEGF emphasized by clinical trials (24), bevacizumab, a humanized murine
monoclonal antibody with strong affinity for VEGF, is the most
popular anti-angiogenic agent used in cancer therapy (25). Therefore, in the present study, we
found that 12-deoxyphorbol 13-palmitate (DP) suppressed the mRNA
level and excretion of VEGF in MCF-7 cells. Thus, we speculated
that DP influences the growth of blood vessel in breast tumors.
Hypoxia-inducible factor-1α (HIF-1α) is a strong
stimulator of neovascularization and excretion of VEGF at the
transcription level (26–29). We identified that DP has the
potential to inhibit VEGF expression in MCF-7 cells. Thus, we next
detected the effect of DP on HIF-1α. HIF-1α is sensitively
regulated by hypoxia, and mediates the adaptation of cells to low
oxygen states (30,31). Thus, in our experiment,
CoCl2 was used to mimic hypoxia, at least in part
(32). The hypoxia-induced increase
in HIF-1α was blocked by the addition of DP at the protein level
but not at the mRNA level, which is in accordance with previous
studies (33,34), and which is presumably linked to
increasing HIF-1α translation (35). Given the findings, it is concluded
that DP suppressed HIF-1α-mediated VEGF expression.
The PI3K/Akt/mTOR signaling pathway plays a critical
role in regulating cellular properties, such as proliferation,
motility, survival and angiogenesis (36). The activation of the PI3K/Akt/mTOR
pathway in tumor cells increases the excretion of VEGF by both
HIF-1-dependent and -independent mechanisms, and the induction of
HIF-1α inhibits mTOR activation and stimulates the VEGF-VEGFR1
angiogenic pathway, which is turned off as hypoxia subsides
(37,38). In contrast, the inhibition of this
pathway by LY294002 and mTOR inhibitor rapamycin was found to
reduce the accumulation of HIF-1α in MCF-7 and prostate cancer cell
lines (39). We identified that DP
interrupted the PI3K/Akt/mTOR cascade. Moreover, it has been
reported that phosphorylated Akt unleashes mTORC1 by inhibiting
activation of TSC1 and TSC2 (40).
Indeed, in the present study, the DP-induced increase in TSC1 and
TSC2 was blocked by addition of wortmannin, which together suggest
that DP downregulates VEGF and HIF-1α by interrupting the PI3K
pathway.
Concerning clinical development, the complexity of
the PI3K/Akt/mTOR pathway and its extensive crosstalk with other
kinase cascades, will result in challenges in identifying patients
who will benefit most from breast cancer treatments (41,42).
DP may be a potential anti-VEGF and -HIF-1α drug for breast cancer.
Yet, its potential side-effects require careful investigation.
Acknowledgments
We gratefully acknowledge the support from members
of the Department of Medicinal Chemistry and Biomacromolecules. The
present study was supported by the Natural Science Foundation of
China (grant no. 81374021).
References
|
1
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomao F, Papa A, Zaccarelli E, Rossi L,
Caruso D, Minozzi M, Vici P, Frati L and Tomao S: Triple-negative
breast cancer: New perspectives for targeted therapies. Onco
Targets Ther. 8:177–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kubota Y: Tumor angiogenesis and
anti-angiogenic therapy. Keio J Med. 61:47–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu HY, Pan YM, Chen ZW, Lin Y, Wang LH,
Chen YH, Jie TT, Lu YY and Liu JC: 12-Deoxyphorbol 13-palmitate
inhibit VEGF-induced angiogenesis via suppression of
VEGFR-2-signaling pathway. J Ethnopharmacol. 146:724–733. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goel HL and Mercurio AM: VEGF targets the
tumour cell. Nat Rev Cancer. 13:871–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kimbro KS and Simons JW: Hypoxia-inducible
factor-1 in human breast and prostate cancer. Endocr Relat Cancer.
13:739–749. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zbytek B, Peacock DL, Seagroves TN and
Slominski A: Putative role of HIF transcriptional activity in
melanocytes and melanoma biology. Dermatoendocrinology. 5:239–251.
2013. View Article : Google Scholar
|
|
8
|
Li M and Kim WY: Two sides to every story:
The HIF-dependent and HIF-independent functions of pVHL. J Cell Mol
Med. 15:187–195. 2011. View Article : Google Scholar :
|
|
9
|
Thomas GV, Tran C, Mellinghoff IK, Welsbie
DS, Chan E, Fueger B, Czernin J and Sawyers CL: Hypoxia-inducible
factor determines sensitivity to inhibitors of mTOR in kidney
cancer. Nat Med. 12:122–127. 2006. View
Article : Google Scholar
|
|
10
|
Liu L, Ning X, Han S, Zhang H, Sun L, Shi
Y, Sun S, Guo C, Yin F, Qiao T, et al: Hypoxia induced HIF-1
accumulation and VEGF expression in gastric epithelial mucosa cell:
Involvement of ERK1/2 and PI3K/Akt. Mol Biol. 42:459–469. 2008.In
Russian. View Article : Google Scholar
|
|
11
|
Paplomata E and O'Regan R: The
PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and
biomarkers. Ther Adv Med Oncol. 6:154–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gomez-Pinillos A and Ferrari AC: mTOR
signaling pathway and mTOR inhibitors in cancer therapy. Hematol
Oncol Clin North Am. 26:483–505. vii2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moavero R, Coniglio A, Garaci F and
Curatolo P: Is mTOR inhibition a systemic treatment for tuberous
sclerosis? Ital J Pediatr. 39:572013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang J and Manning BD: The TSC1-TSC2
complex: A molecular switchboard controlling cell growth. Biochem
J. 412:179–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuckar D, Dekanić A, Stifter S, Mustać E,
Krstulja M, Dobrila F and Jonjić N: VEGF expression is associated
with negative estrogen receptor status in patients with breast
cancer. Int J Surg Pathol. 14:49–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahluwalia A and Tarnawski AS: Critical
role of hypoxia sensor - HIF-1α in VEGF gene activation.
Implications for angiogenesis and tissue injury healing. Curr Med
Chem. 19:90–97. 2012. View Article : Google Scholar
|
|
17
|
Mac Gabhann F, Qutub AA, Annex BH and
Popel AS: Systems biology of pro-angiogenic therapies targeting the
VEGF system. Wiley Interdiscip Rev Syst Biol Med. 2:694–707. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Serfontein J, Nisbet RE, Howe CJ and de
Vries PJ: Evolution of the TSC1/TSC2-TOR signaling pathway. Sci
Signal. 3:ra492010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weinstein IB and Joe AK: Mechanisms of
disease: Oncogene addiction - a rationale for molecular targeting
in cancer therapy. Nat Clin Pract Oncol. 3:448–457. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fakhrejahani E and Toi M: Antiangiogenesis
therapy for breast cancer: An update and perspectives from clinical
trials. Jpn J Clin Oncol. 44:197–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kerbel RS: Tumor angiogenesis: Past,
present and the near future. Carcinogenesis. 21:505–515. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reich SJ, Fosnot J, Kuroki A, Tang W, Yang
X, Maguire AM, Bennett J and Tolentino MJ: Small interfering RNA
(siRNA) targeting VEGF effectively inhibits ocular
neovascularization in a mouse model. Mol Vis. 9:210–216.
2003.PubMed/NCBI
|
|
24
|
Fox SB, Generali DG and Harris AL: Breast
tumour angiogenesis. Breast Cancer Res. 9:2162007. View Article : Google Scholar
|
|
25
|
Ventrice P, Leporini C, Aloe JF, Greco E,
Leuzzi G, Marrazzo G, Scorcia GB, Bruzzichesi D, Nicola V and
Scorcia V: Anti-vascular endothelial growth factor drugs safety and
efficacy in ophthalmic diseases. J Pharmacol Pharmacother. 4(Suppl
1): S38–S42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bausero P, Ben-Mahdi M, Mazucatelli J,
Bloy C and Perrot-Applanat M: Vascular endothelial growth factor is
modulated in vascular muscle cells by estradiol, tamoxifen, and
hypoxia. Am J Physiol Heart Circ Physiol. 279:H2033–H2042.
2000.PubMed/NCBI
|
|
27
|
Xiao H, Gu Z, Wang G and Zhao T: The
possible mechanisms underlying the impairment of HIF-1α pathway
signaling in hyperglycemia and the beneficial effects of certain
therapies. Int J Med Sci. 10:1412–1421. 2013. View Article : Google Scholar
|
|
28
|
Chen MH, Ren QX, Yang WF, Chen XL, Lu C
and Sun J: Influences of HIF-lα on Bax/Bcl-2 and VEGF expressions
in rats with spinal cord injury. Int J Clin Exp Pathol.
6:2312–2322. 2013.
|
|
29
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-α and von Hippel-Lindau protein by direct
binding to hypoxia-inducible factor-α. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y,
Jeong JW and Kim YJ: Melatonin suppresses tumor angiogenesis by
inhibiting HIF-1α stabilization under hypoxia. J Pineal Res.
48:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1α in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn GO, Seita J, Hong BJ, Kim YE, Bok S,
Lee CJ, Kim KS, Lee JC, Leeper NJ, Cooke JP, et al: Transcriptional
activation of hypoxia-inducible factor-1 (HIF-1) in myeloid cells
promotes angiogenesis through VEGF and S100A8. Proc Natl Acad Sci
USA. 111:2698–2703. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leung KW, Ng HM, Tang MK, Wong CC, Wong RN
and Wong AS: Ginsenoside-Rg1 mediates a hypoxia-independent
upregulation of hypoxia-inducible factor-1α to promote
angiogenesis. Angiogenesis. 14:515–522. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Laughner E, Taghavi P, Chiles K, Mahon PC
and Semenza GL: HER2 (neu) signaling increases the rate of
hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel
mechanism for HIF-1-mediated vascular endothelial growth factor
expression. Mol Cell Biol. 21:3995–4004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao N, Qi XY, Tang LN, Tan LL, Chen YQ
and Zhao HM: VEGF promotes cardiac stem cells differentiation into
vascular endothelial cells via the PI3K/Akt signaling pathway.
Artif Cells Nanomed Biotechnol. 42:400–405. 2014. View Article : Google Scholar
|
|
37
|
Agani F and Jiang BH: Oxygen-independent
regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in
cancer. Curr Cancer Drug Targets. 13:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SH, Jee JG, Bae JS, Liu KH and Lee YM:
A group of novel HIF-1α inhibitors, glyceollins, blocks HIF-1α
synthesis and decreases its stability via inhibition of the
PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol.
230:853–862. 2015. View Article : Google Scholar
|
|
39
|
Fang J, Ding M, Yang L, Liu LZ and Jiang
BH: PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis.
Cell Signal. 19:2487–2497. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang J, Dibble CC, Matsuzaki M and
Manning BD: The TSC1-TSC2 complex is required for proper activation
of mTOR complex 2. Mol Cell Biol. 28:4104–4115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Massacesi C, di Tomaso E, Fretault N and
Hirawat S: Challenges in the clinical development of PI3K
inhibitors. Ann NY Acad Sci. 1280:19–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
May FE: Novel drugs that target the
estrogen-related receptor α: Their therapeutic potential in breast
cancer. Cancer Manag Res. 6:225–252. 2014. View Article : Google Scholar
|