Introduction
Approximately 644,000 head and neck squamous cell
carcinoma (HNSCC) cases are diagnosed worldwide each year (1) and although surgery, radiotherapy and
chemotherapy improve disease control, its 5-year survival rate
remains ~60% (2), partly because
acquired chemoresistance limits its efficiency.
Interleukin (IL)-6 is a multifunctional cytokine
produced by various cell types involved in a wide range of
biological activities, including cellular growth and apoptosis
(3,4). In general, IL-6 binds to the
non-signaling alpha-receptor (IL-6R/CD126) that dimerizes with the
membrane bound signaling transducer receptor, gp130 and activates
receptor-associated kinases. The intracellular signaling pathways
induce phosphorylation of the transcription factor: signal
transducer and activator of transcription 3 (STAT3). The
cytoplasmic phosphorylated STAT3 subsequently translocates to the
nucleus where it regulates genes involved in apoptosis (e.g.,
Bcl-xL, XIAP and Fas). In addition, IL-6 initiates the
PI3K/AKT and RAS/RAF/MEK/ERK pathways, which regulate cell
proliferation (5).
Increased serum IL-6 levels predict poor prognosis
in several carcinoma types including colorectal, ovarian,
pancreatic, mammary and gastric carcinomas, which has been related
to IL-6-induced tumor cell proliferation, apoptosis inhibition and
tumor angiogenesis (6). However, to
what extent IL-6 influences HNSCC prognosis remains controversial
due to conflicting results obtained by different methods. Whereas
positive IL-6 mRNA in situ hybridization signals are
associated with favorable prognosis (7), tumorous IL-6 immunoreactivity and IL-6
serum levels have been associated with poor prognosis (8–11).
IL-6 may induce cisplatin resistance in oral
carcinomas similar to that reported in ovarian, lung and prostate
carcinoma cell lines, where IL-6 increases expression of
anti-apoptotic factors such as Bcl-2, Bcl-xL and cIAP-2 and/or
induces cell proliferation (12–14).
Moreover, IL-6 gene knock-down reverses cisplatin resistance in
esophageal carcinoma cell lines (15) and increased IL-6 production is
associated with resistance to other chemotherapy drugs, such as
fluorouracil, doxorubicin and VP-16 (6,10).
Finally, a single in vitro cisplatin challenge induces high
IL-6 mRNA levels in surviving HNSCC cells and increases their tumor
potential in a xenograft murine model (16), suggesting that IL-6 participates in
rescuing cells from cisplatin-induced apoptosis.
The aim of the current study was to evaluate whether
increased cancerous IL-6 mRNA expression had a prognostic value in
HNSCC, and whether IL-6 influenced cisplatin resistance. We used
high-throughput RNA-sequencing and clinical data of 399 HNSCC
patients in the cancer genomic atlas database (TCGA, http://cancergenome.nih.gov/) and investigated how
IL-6 gene expression was related to patient prognosis in general
and in patient subgroups. In order to examine IL-6 induced
cisplatin resistance, we furthermore tested five HNSCC cell lines,
including two in-house acquired cisplatin-resistant cell lines of
both basaloid and conventional HNSCC types, for cisplatin
sensitivity and IL-6 expression.
Materials and methods
Clinical data and RNA expression
analysis
Clinical data and mRNA expression profiles from 498
HNSCC patients were collected from the TCGA database: (https://tcga-data.nci.nih.gov/tcgafiles/ftp_auth/distro_ftpusers/anonymous/tumor/hnsc/bcr/biotab/clin/).
All patients, diagnosed and treated during 1997–2014, were followed
up until September 30, 2014. For detailed tumor sample acquisition,
see reference (17). Briefly,
biospecimens were collected from diagnosed patients with HNSCC at
the time of surgical resection. The patients had received no prior
treatment for their disease including chemotherapy or radiotherapy.
Cases were staged according to the American Joint Committee on
Cancer (AJCC), Seventh Edition. mRNA expression profiles were
estimated by normalizing raw counts of mapped RNA-sequences reads
to human reference genes, and mRNA levels measured as fragments per
kilobase per million mapped reads (FPKM). Patients without
follow-up data or who died within two months were excluded, and
finally 399 patients, 284 (71%) men and 115 (29%) women, median 61
years (range 19–90 years) were included.
Cell lines and cell culture
Three human HNSCC lines were used in the study.
PE/CA-PJ49 clone E10 (male, 55 years) were established from tongue
tissue; PE/CA-PJ34 clone C12 (male, 60 years) and PE/CA-PJ41 clone
D2 (female, 68 years) were derived from the oral cavity and the
oral squamous epithelium, respectively. The cell lines (a kind gift
from Dr A. Berndt and Dr H. Kosmehl, Friedrich-Schiller University,
Germany) were cultured under standard condition as previously
described (18).
Establishing the cisplatin-resistant C12
(C12cis) and D2 (D2cis) HNSCC cell lines
Two primary cisplatin sensitive HNSCC cell lines,
the basaloid squamous cell carcinoma (BSCC) C12 and the
conventional squamous cell carcinoma (CSCC) D2 cell lines, were
cultured to acquire cisplatin resistance. Cells were initially
treated with their 50% inhibitory concentration (IC50)
(3 µM) of cisplatin (Sigma-Aldrich, St. Louis, MO, USA) at
80% confluence. The conditioned medium was discarded and fresh
medium was added after 24-h incubation. The cells were then treated
with gradually increasing concentrations of cisplatin ranging from
3 to 10 µM at weekly intervals for eight months. The
parental C12 and D2 cells were cultured in parallel using
cisplatin-free medium.
Cell viability assay
Cells were seeded (4×103 cells/well) in
96-well microtiter plates (Nunc, Wiesbaden-Biebrich, Germany) in
100 µl IMDM with 10% FBS in quintuplicate. After 24 h,
culture medium was changed to IMDM with 10% FBS and different
concentrations of drugs or inhibitors. For drug IC50
detection, cells were treated with different dosages of cisplatin,
5-FU or docetaxel directly. Cells were also cultured in the
presence of human recombinant IL-6 or human IL-6R/gp130
neutralizing antibody (all from R&D Systems, Minneapolis, MN,
USA) for 24 h, followed by 5 µM cisplatin treatment to
examine changes in drug resistance. Cells were further grown for 72
h, before incubated in 50 µl XTT labeling mixture (Roche
Molecular Biochemicals, Mannheim, Germany) for 4 h, and then
scanned at 450 nm. IC50 was calculated using GraphPad
Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
RNA isolation and microarray
analysis
C12, C12cis, D2 and D2cis cells were lysed and total
RNA was extracted using RNeasy kit (Qiagen, USA), and
concentrations were measured using the NanoDrop 2000c
spectrophotometer (Thermo Scientific, Wilmington, DE, USA). The
integrity of samples was assessed using the Agilent 2100
Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Microarray was performed by the Department of Tumor Biology,
Institute for Cancer Research, Norwegian Radium Hospital. Briefly,
500 ng of total RNA for each individual sample was used with the
Illumina TotalPrep Amplification kit (Ambion) to make
biotin-labelled, amplified cRNA. Thereafter, 750 ng cRNA was
hybridized to HumanHT-12 v4 Expression BeadChip (Illumina) enabling
profiling of >48,000 transcripts. The Illumina arrays were
scanned with the BeadArray reader, and data extraction and initial
quality control were performed in GenomeStudio version 2011.1 using
Gene Expression module v.1.9.0 (Illumina). Raw data were log2
transformed and analyzed using lumi package in R (version
3.2.2).
Quantitative real-time reverse
transcriptase polymerase chain reaction (qRT-PCR)
After RNA isolation, complementary DNA (cDNA) was
synthesized by RT-RTCK-05 kit (Eurogentec, Berlin, Germany) and
stored at −20°C. A standard real-time PCR reaction with SYBR Green
Real Master Mix (Eppendorf, Hamburg, Germany) was performed in
duplicates using Mx3005p (Agilent Technologies) under the following
conditions: 95°C for 2 min followed by 40 cycles of 95°C for 20
sec, 60°C for 1 min and 68°C for 30 sec. The primers used were:
IL-6 forward, 5′-GCA-GAA-AAA-GGC-AAA-GAA-TC-3′ and reverse,
5′-CTA-CAT-TTG-CCG-AAG-AGC-3′; IL-6 X1 isoform forward,
5′-TCC-TCA-TTC-CCT-CAA-CTT-GG-3′ and reverse,
5′-GCA-GAA-GAG-AGC-CAA-CCA-AC-3′; and IL-6R forward,
5′-CTG-GAA-AGC-ATT-CAT-GCT-ACC-3′ and reverse,
5′-GAC-TGT-TCT-GAA-ACT-TCC-TC-3′ (all designed by Sigma-Aldrich);
TATA box binding protein (TBP): forward,
5′-CGT-GGC-TCT-CTT-ATC-CTC-ATG-A-3′ and reverse,
5′-GCC-CGA-AAC-GCC-GAA-TAT-A-3′ (designed by Eurogentec).
Dissociation curves ensured product uniformity. Expression data
were normalized to the housekeeping gene TBP. The relative
expression levels of the genes of interest were calculated using
the 2−ΔΔCt method.
Western blotting
Cells were pre-treated with 10 ng/ml human
recombinant IL-6, 40 µg/ml human IL-6R neutralizing antibody
or 60 µg/ml human gp130 neutralizing antibody for 24 h,
following by 5 µM cisplatin treatment for 6 h. For the
stimulation assay, cells were treated with 1 or 10 ng/ml IL-6 for
30 min. Cells were harvested and lysed in CelLytic M Cell Lysis
reagent (Sigma-Aldrich) with protease and phosphatase inhibitor
cocktails (Pierce Biotechnology, Rockford, IL, USA). Protein
concentrations were determined (Bio-Rad, Munich, Germany), and 50
µg proteins were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electroblotted onto PVDF membranes (Bio-Rad). After blocked with 5%
BSA for 1 h, the membranes were incubated with primary antibody to
human anti-phospho-STAT3(Tyr705) (rabbit polyclonal, 1:1,000; Cell
Signaling Technology, Beverly, MA, USA), anti-STAT3 (mouse
monoclonal, 1:5,000; R&D Systems) and anti-GAPDH (mouse
monoclonal, 1:1,000; Abcam, Cambridge, UK) overnight at 4°C. The
blots were then washed three times and incubated with alkaline
phosphatase-conjugated anti-rabbit IgG (1:10,000; Sigma-Aldrich) or
mouse IgG (1:10,000; Dako, Glostrup, Denmark) antibodies at room
temperature for 1 h, then washed three times and visualized with
ECF substrate in a scanner (Storm) (both from GE Healthcare,
Uppsala, Sweden).
Enzyme-linked immunosorbent assay
(ELISA)
Cells were seeded in duplicates in 96-well-plate at
a density of 4×103 cells/well and cultured in 200
µl medium with 10% FBS. The supernatant was harvested and
stored frozen (−70°C) prior to use. The IL-6 concentration was
determined in quadruplicates by Human IL-6 ELISA (R&D
Systems).
Statistics
Statistical analysis was performed using GraphPad
Prism 6.0. The survival distributions were compared with the
log-rank test (Kaplan-Meier method). Normally distributed data were
shown as mean ± SD, and group differences were analyzed using
paired Student's t-test; data that were not normally distributed
were shown as median ± SD, and group differences were analyzed
using Wilcoxon rank-sum test. For all in vitro assays, data
are shown of at least three experiments. p<0.05 were considered
as significant.
Results
High IL-6 expression predicts poor
prognosis
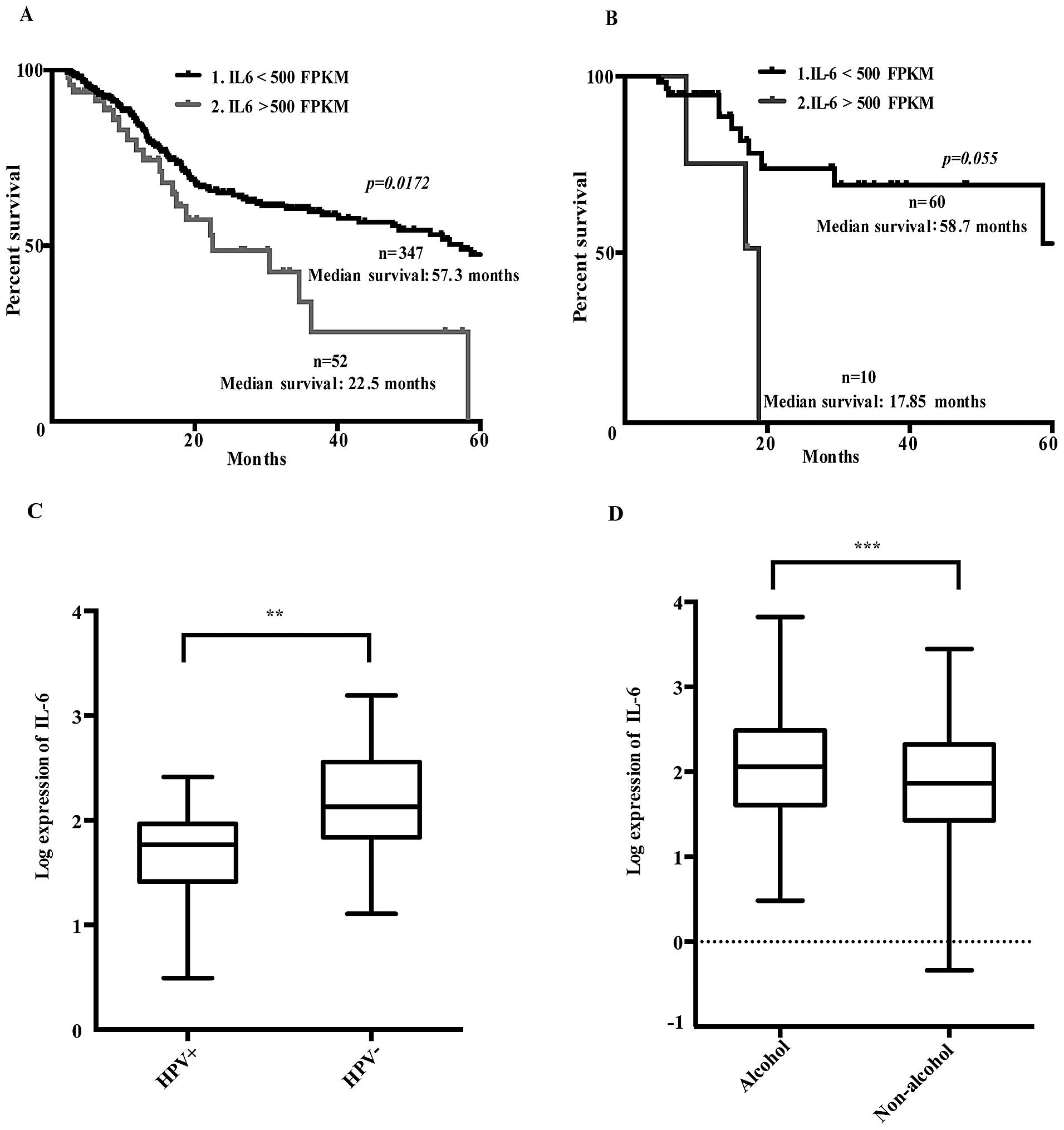
Dividing patients in high (>500 FPKM) and low
(<500 FPKM) IL-6 expression levels revealed that the high IL-6
expressing group had a significantly reduced 5-year survival rate
(Fig. 1A).
Further investigation of 70 cis-/carboplatin treated
patients showed that those with high IL-6 mRNA expression levels
tended to have a lower 5-year survival rate, suggesting reduced
response to platinum-based treatment (Fig. 1B). Thus, an increased IL-6 gene
expression in the HNSCC tumors was related to poor prognosis and
presumably also to cisplatin resistance, similar to that previously
reported in ovarian carcinomas (6).
Patients with human papillomavirus infection
(HPV+) had lower IL-6 expression levels than those
without HPV (Fig. 1C). Moreover,
IL-6 mRNA expression levels were correlated to alcohol consumption
history (Fig. 1D), but not to any
other clinical parameters or high risk factors such as tumor sites,
pathological or histological grading or smoking history.
Characterization of the
cisplatin-resistant C12 (C12cis) and D2 (D2cis) HNSCC cell
lines
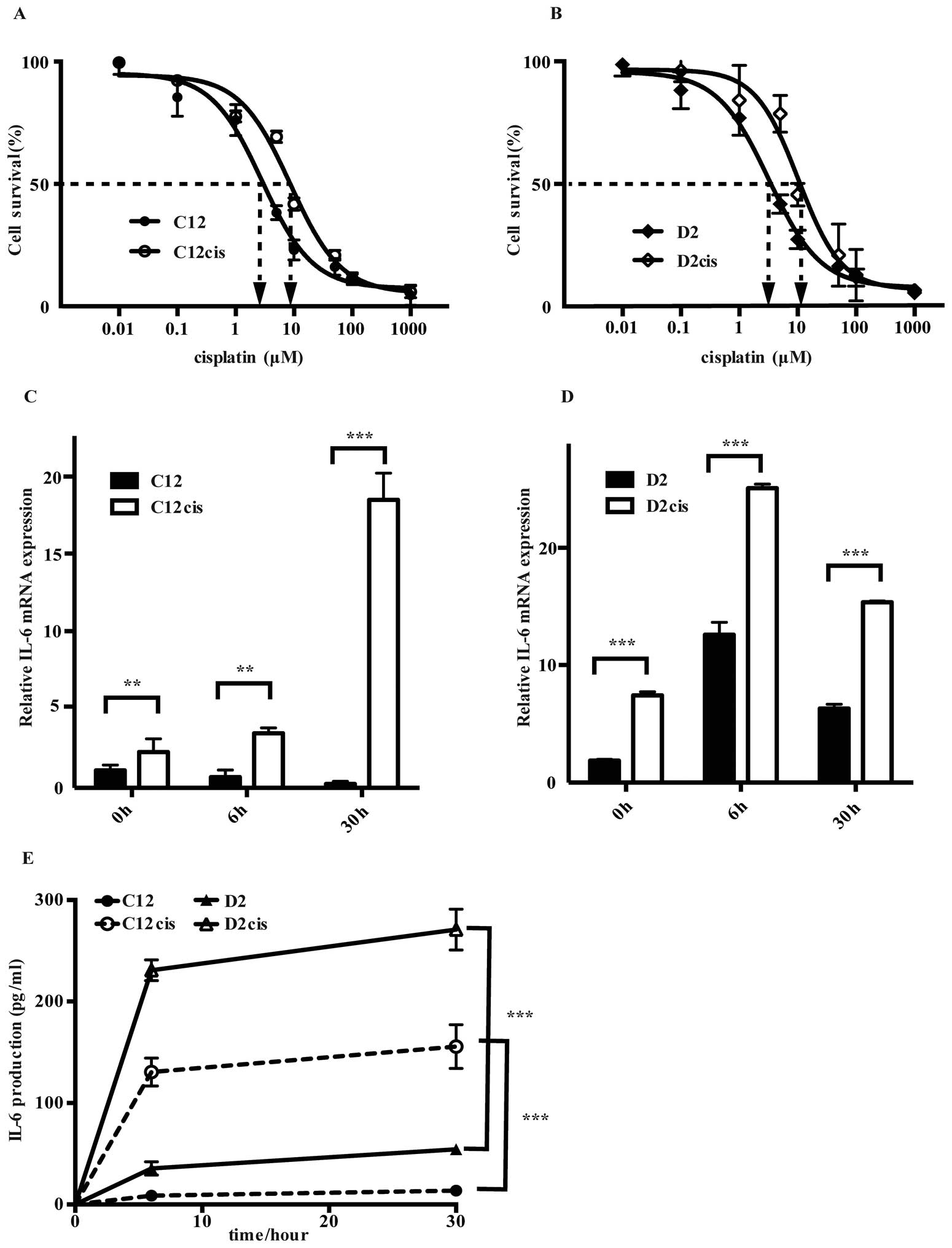
To examine whether IL-6 signaling is involved in
cisplatin resistance in HNSCC, we established cisplatin-resistant
sublines from C12 and D2 cells, as described in Materials and
Methods. The IC50 values for cisplatin treated cells
(i.e., C12cis and D2cis) were more than three times higher than in
the parental cells (Fig. 2A and B),
and it was unaltered after two months of cisplatin-free culturing,
revealing stable phenotypic changes.
Interestingly, cisplatin treatment induced
cross-resistance for two other common drugs used in HNSCC treatment
(19), fluorouracil (5-FU) and
docetaxel by increasing their IC50 values by 50–100%
(Table I).
 | Table ICharacterization of HNSCC cell
lines.a |
Table I
Characterization of HNSCC cell
lines.a
| Diameter
(µM)c | Doubling time time
(h)d | IC50 for
cisplatin (µM) | IC50 for
5-FU (µM) | IC50 for
docetaxel (nM) |
|---|
| C12 | 13.70±2.78 | 35±2,41 | 2.8±0,21 | 1.2±0,31 | 1.4±0,29 |
| C12cis | 13.59±2.90 | 31±1,57b | 8.7±0,43b | 2.5±0,54b | 2.6±0,23b |
| D2 | 15.08±2.23 | 32±1,72 | 3.2±0,76 | 5.2±0,81 | 0.9±0,14 |
| D2cis | 14.45±2.26 | 41±2,21b | 10.3±0,84b | 7.9±0,47b | 2.2±0,33b |
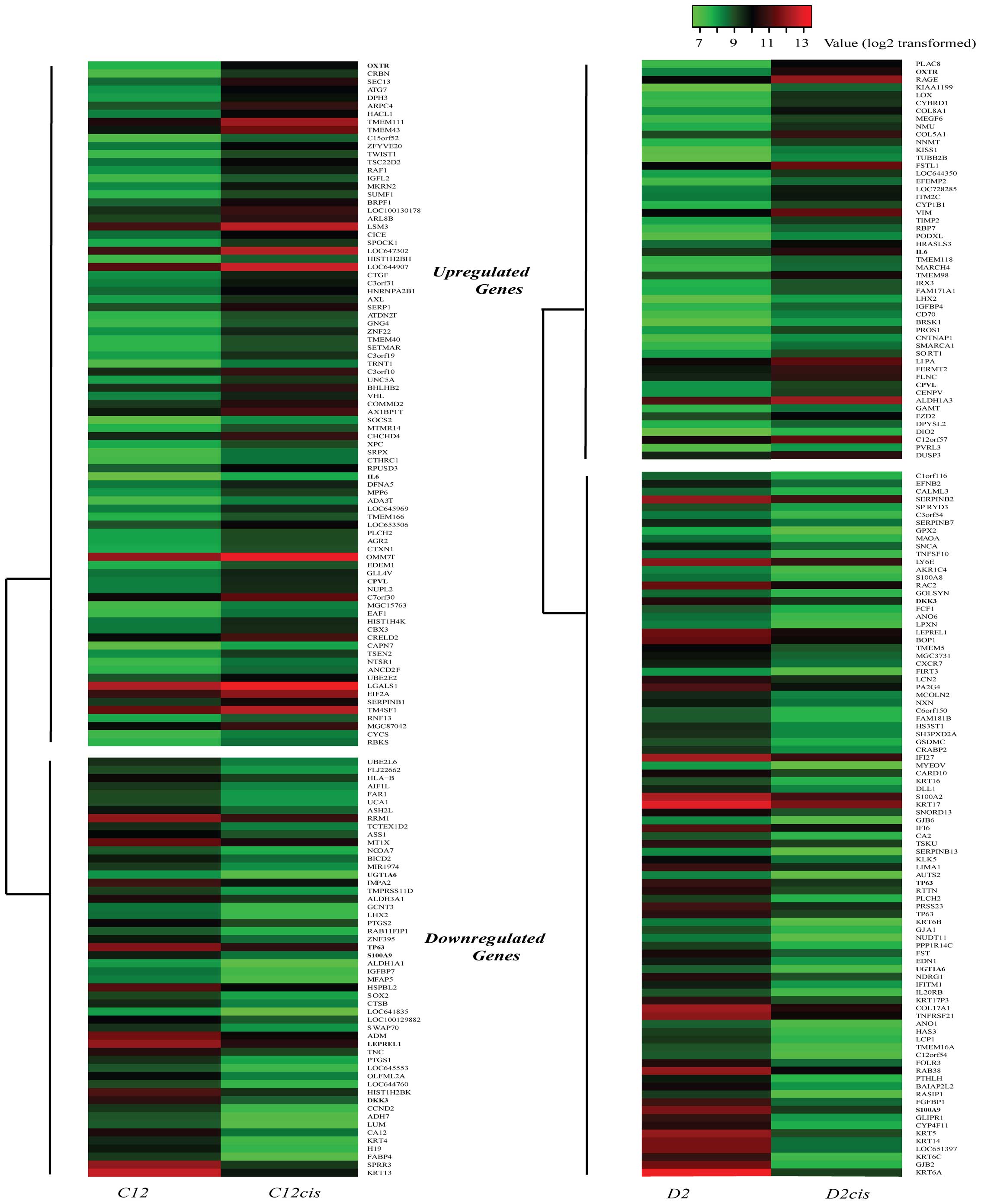
Differentially expressed genes in C12cis
and D2cis cell lines
We compared the mRNA expression profiles of the
cisplatin-resistant cell lines (C12cis and D2cis) and parental cell
lines (C12 and D2) by using microarray analysis, and found that 137
genes were differentially expressed (increased or decreased 100%)
in C12cis cell lines and 141 genes in D2cis cell lines (Fig. 3). Among them, only 3 genes, OXTR
(oxytocin receptor), CPVL (probable a serine carboxypeptidase) and
IL-6 were found upregulated in both cell lines (Table II). Since IL-6 is associated with
poor prognosis and cisplatin resistance in several different
carcinomas, we hypothesized that IL-6 mediated cisplatin resistance
in current cell lines.
 | Table IIExpression levels of genes exhibiting
>2-fold expression changes in both C12cis and D2cis cell
lines. |
Table II
Expression levels of genes exhibiting
>2-fold expression changes in both C12cis and D2cis cell
lines.
| Gene symbol | Full name | Fold change in
C12cis/C12a | Fold change in
D2cis/D2a |
|---|
| Upregulated |
| OXTR | Oxytocin
receptor | 2.19 | 2.13 |
| IL6 | Interleukin 6 | 1.20 | 1.24 |
| CPVL | Probable serine
carboxypeptidase | 1.12 | 1.07 |
| Downregulated |
| DKK3 | Dickkopf-related
protein 3 | −1.84 | −1.11 |
| LEPREL1 | Prolyl
3-hydroxylase 2 | −1.40 | −1.16 |
| S100A9 | S100
calcium-binding protein A9 | −1.16 | −2.46 |
| TP63 |
Transformation-related protein 63 | −1.16 | −1.47 |
| UGT1A6 |
UDP-glucuronosyltransferase 1–6 | −1.09 | −1.61 |
Acquired cisplatin-resistant cells
express more IL-6
Whereas cisplatin exposure decreased IL-6 mRNA
expression in the parental cisplatin sensitive C12 cell line, it
increased markedly, reaching 71 times higher expression in the
resistant C12cis cell line than former 30 h after cisplatin
treatment (Fig. 2C). Similarly,
although IL-6 mRNA expression increased after cisplatin treatment
in both the sensitive D2- and the resistant D2cis cell lines, the
basal and cisplatin induced IL-6 mRNA expression was two times
higher in the latter (Fig. 2D).
Importantly, the IL-6 mRNA increase was accompanied with high IL-6
production in both the acquired resistant cell lines (Fig. 2E).
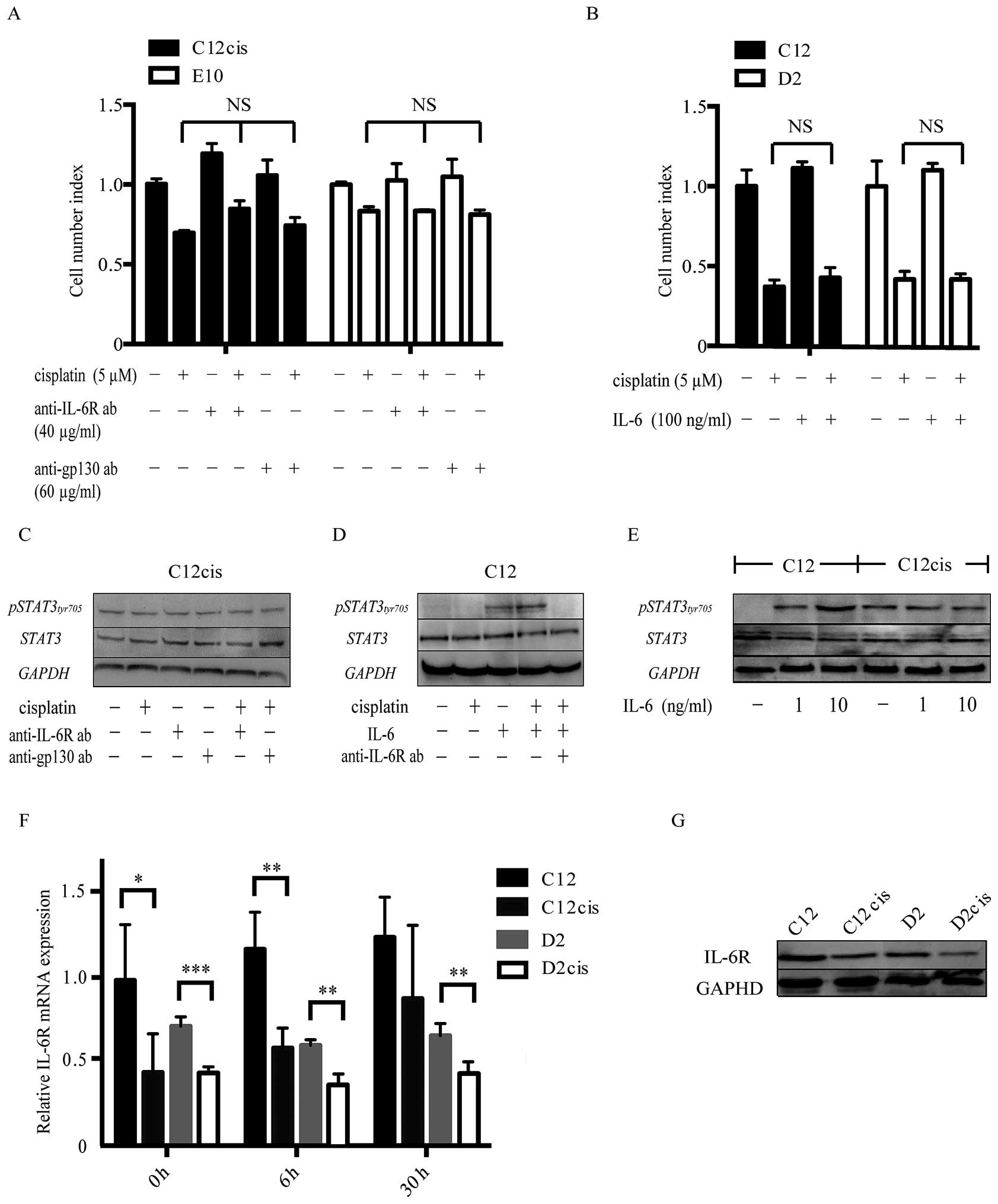
Cisplatin resistance is not affected by
IL-6 receptor inhibitors or exogenous IL-6
Since IL-6 was highly upregulated in the
cisplatin-resistant cell lines, we examined whether cisplatin
resistance could be blocked by IL-6 receptor inhibitors.
Interestingly, neither IL-6R nor gp130 neutralizing antibody
altered cisplatin resistance in the acquired cisplatin-resistant
cell lines or the intrinsic cisplatin-resistant cell line E10
(Fig. 4A). Moreover, STAT3 was
constantly phosphorylated in these cell lines, with no decrease
after anti-IL-6R/gp130 inhibition (Fig.
4C). Despite cisplatin induced IL-6 production,
p-STAT3Tyr705 was not further increased in the resistant
cell lines after cisplatin treatment, suggesting IL-6 independent
STAT3 activation. Further experiment revealed that the IL-6/STAT3
pathway was diminished in the resistant cell lines (Fig. 4E), as exogenous IL-6 induced less
STAT3Tyr705 phosphorylation in the cisplatin-resistant
than in the cisplatin- sensitive parental cell lines.
Furthermore, exogenous IL-6 did not increase
cisplatin resistance in the sensitive cell lines regardless of
different dosages (10–100 ng/ml; Fig.
4B), despite de novo STAT3Tyr705
phosphorylation which could be blocked by IL-6R inhibitor (Fig. 4D).
Expression of IL-6 splicing variants and
its receptor
Since IL-6/STAT3 pathway was impaired in the
acquired cisplatin-resistant cell lines, we examined the expression
of IL-6 isoforms with putative antagonistic effects, and IL-6
receptor expression in the cell lines.
The expression levels of IL-6 splicing variants
(based on NCBI RefSeq gene annotation - release 69) were evaluated
using qRT-PCR. Only one alternative transcript variant (X1,
accession: XM_005249745) was expressed and at similar, low levels
in both the parental and the cisplatin-resistant cell lines
(expression ratio of IL-6 X1 isoform/wild type = 1/20), which was
also confirmed by analyzing the IL-6 isoform expression levels in
patient samples from the TCGA database. Thus, no known competitive
IL-6 isoforms were expressed in our HNSCC cell lines.
However, unlike IL-6, the mRNA and protein levels of
its receptor were downregulated in both C12cis and D2cis cells in
comparison with the parental cells (Fig. 4F and G).
Discussion
Analysis of 399 HNSCC patients revealed that
patients with high tumorous IL-6 mRNA expression (>500 FPKM) had
a significant reduced 5-year survival. However, this association
became less statistically evident when median IL-6 expression level
(~100 FPKM) was used as discriminator. Similar results were
obtained by separate de novo analysis of two other databases
using SurvExpress (20), which
showed that IL-6 expression tended to be associated with poor
prognosis (bordline non-significant) as median expression was
automatically used as discriminator. Thus, the association between
IL-6 expression and poor prognosis may be more intriguing in HNSCC
than reported for other carcinoma types (6), which may be reflected in previous
studies (7,9,11).
Besides, although Chen et al (8) observed the association between
increased IL-6 expression and poor prognosis in male patients only,
a case-control study design revealed no gender specific differences
in the current HNSCC patients (not shown), and the gender
differences may have been due to less advanced disease in Chen
et al female patients. An IL-6 associated poor prognosis
would explain why serum and salivary IL-6 levels were increased in
more aggressive HNSCC grades (11)
with higher recurrence rates (9,21).
Although the mechanism for why high IL-6 expression
is associated with poor prognosis is not fully understood, IL-6 is
known to inhibit cellular apoptosis and induce
epithelial-mesenchymal transition (EMT) (22), both of which increase drug
resistance, cellular invasiveness and metastatic potential
(6,23). In particular, the IL-6 induced
anti-apoptotic effects may prevent cisplatin treated, DNA-damaged
cells to undergo apoptosis, which actually may facilitate the
development of mutation-induced drug resistance (12,13,15).
It is, therefore, intriguing that HNSCC cells which survived a
single cisplatin dosage in vitro, had increased IL-6
expression and increased tumor forming capacity in a xenograft
mouse model (16), suggesting that
cisplatin induced IL-6 expression is an important factor to reduce
cisplatin sensitivity. Survival analysis of the 70 patients who had
been treated with cisplatin or carboplatin, further suggested an
IL-6 associated reduction of cisplatin cytotoxicity, as IL-6 was
associated with poor prognosis in these patients as well. However,
although IL-6 may reduce cisplatin cytotoxicity and increase cell
proliferation in prostate (13,24,25)
and ovarian carcinomas (26),
neither anti-IL-6 receptor antibodies nor exogenous IL-6 affected
cell proliferation or cisplatin toxicity. In fact, the
cisplatin-resistant cell line D2cis, despite having higher IL-6
expression, grew slower than the parental, cisplatin sensitive cell
line D2 (Table I).
IL-6 may generally suppress cisplatin-induced
apoptosis through STAT3 induced upregulation of anti-apoptotic
factors (27,28). Both IL-6R and p-STAT3 are highly
expressed in OSCC patients with poor response to chemoradiotherapy,
suggesting that activation of IL-6/STAT3 signaling may be involved
in modulation of chemosensitivity to anticancer drugs (10). In our study, p-STAT3 was observed in
unstimulated cisplatin-resistant cell lines (C12cis, D2cis and
E10). However, this was independent of IL-6/IL6R/gp130 signaling
despite increased IL-6 production in the resistant cell lines, as
p-STAT3 was not reduced after IL-6 receptor inhibition. Besides,
higher IL-6 dosages, which induced p-STAT3 phosphorylation in
sensitive cell lines, did not induce cisplatin resistance in the
parental cell lines nor increase expression of any of the apoptosis
inhibitors, illustrating that cisplatin resistance was not mediated
through IL-6/STAT3 activation. Further investigation in six
additional HNSCC cell lines revealed, moreover, that neither IL-6
gene expression nor protein production correlated to cisplatin
resistance (p>0.05, data not shown). Although this is in
contrast to cisplatin resistance mechanisms in other carcinomas,
similar results have been noted in myeloma and lymphoma cell lines:
although chemotherapy resistance correlated to IL-6 secretion, IL-6
blocking antibodies did not reverse the resistance (29).
A few alternative spliced IL-6 variants exist in
human, some of which have antagonistic activities and a
tissue-specific expression pattern, similar to IL-4 (30). For example, lung tissue, renal
tissue, renal carcinomas and fibroblasts produce three IL-6
inhibitory variants lacking either exon 4 (IL-6δ4), exon 2 (IL-6δ2)
or both exons (IL-6δ2δ4) (31,32).
Human fetal tissues express these variants in a tissue-specific
manner (33). However, there was no
IL-6δ2 or IL-6δ4 expression either in patient samples or in our
cell lines. Only one variant, IL-6X1 was detected in HNSCC patients
and cell lines. Although its function is still unknown, the mRNA
level was 5% of the wild-type IL-6, reducing the possibility that
it had any significant inhibitory function. Thus, the impaired
IL-6/STAT3 pathway may rather have been due to decreased IL-6R
expression in the resistant cell lines (Fig. 4F and G).
Dysregulated microRNA (34) expression is common in various
malignancies where miRNAs regulate cell proliferation, apoptosis
and invasion by controlling downstream target genes (35). Some inhibitory miRNAs (i.e.,
miR-200b, miR-200c, miR-203 and miR-205), were negatively
associated with IL-6 mRNA expression in the current HNSCC patients
(p<0.001, not shown), suggesting that IL-6 overexpression may
result from demethylation of the IL-6 promoter triggered by heat
shock factor protein 1 (HSF1) due to reduction of miR-200c
(36). Similar phenomena were
observed in tongue and lung carcinoma where reduced miR-200b and
miR-200c expression inhibited cell proliferation, apoptosis and
cisplatin cytotoxicity (37,38).
The reduced miR-205 expression, which may induce increased IL-6
expression, was associated with poor prognosis in head and neck
cancer (39). miRNA regulates
several downstreams gene targets, e.g., miR-203 may affect
expression of more than 100 genes involved in EMT and other
cellular processes (40). The IL-6
associated poor prognosis may therefore either be an epiphenomenon
due to miRNA induced co-regulation of genes more directly related
to cancer survival and/or cisplatin resistance, or involved in
paracrine signaling, inducing tumor surrounding fibroblasts to
become cancer-associated fibroblasts (CAFs) which support
carcinomas growth, survival, and metastatic potential (41).
Cisplatin cytotoxicity is mediated by several
transcription factors and downstream pathways, such as
RAS/RAF/MEK/ERK and PI3K/AKT and may involve many different signal
transduction pathways and gene regulatory networks (42). In comparison with previous studies
in other cancer types, IL-6 signaling pathway appeared not to be
critical for cisplatin resistance in the current HNSCC cell lines.
Additionally, the acquired cisplatin-resistant cell lines tended to
gain cross-resistance to two other chemotherapy drugs, 5-FU and
docetaxel, suggesting induction of anti-apoptotic proteins
(43). Although the conventional
cisplatin-resistant SCC cell line D2cis had an increased expression
of the cellular Inhibitor of Apoptosis-1 and -2 (c-IAP1, c-IAP2),
there was no increased expression of any apoptosis inhibitors in
the cisplatin-resistant BSCC cell line C12cis (microarray data,
confirmed by qRT-PCR, not shown). This is in contrast to previous
reports on HNSCC, which in particular focused on the importance of
increased XIAP expression for cisplatin resistance (44). Thus, further investigation is needed
to reveal which major mechanisms BSCC and conventional SCC may use
to overcome cisplatin cytotoxicity.
Patients with HPV infection had lower IL-6 mRNA
levels (this study), and the HPV-positive HNCSS patients had
noticeably better prognosis as shown by Cancer Genome Atlas Network
(17). Such IL-6 regulation is
probably mediated by E6 and E7 proteins, and may contribute to
maintenance of the viral genome and to escape the immune activity
in HPV-related cancers (45).
In conclusion, high tumor IL-6 transcription levels
were associated with poor prognosis and acquired cisplatin
resistance in HNSCC, but IL-6 did not itself mediate cisplatin
resistance. Thus, inhibiting IL-6 signaling may not reduce
cisplatin resistance in HNSCC.
Acknowledgments
The clinical results in the study are based upon
data generated by the TCGA Research Network: http://cancergenome.nih.gov/. We acknowledge Solvig
Stig in the Department of Oral Biology at the University of Oslo
for technical help and valuable discussions.
References
|
1
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirano T, Yasukawa K, Harada H, Taga T,
Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K,
Iwamatsu A, et al: Complementary DNA for a novel human interleukin
(BSF-2) that induces B lymphocytes to produce immunoglobulin.
Nature. 324:73–76. 1986. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuzhalin A and Kutikhin A: Interleukins in
Cancer Biology: Their Heterogeneous Role. Elsevier; 2014
|
|
5
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo Y, Xu F, Lu T, Duan Z and Zhang Z:
Interleukin-6 signaling pathway in targeted therapy for cancer.
Cancer Treat Rev. 38:904–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang YF, Chang SY, Tai SK, Li WY and Wang
LS: Clinical significance of interleukin-6 and interleukin-6
receptor expressions in oral squamous cell carcinoma. Head Neck.
24:850–858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen CJ, Sung WW, Lin YM, Chen MK, Lee CH,
Lee H, Yeh KT and Ko JL: Gender difference in the prognostic role
of interleukin 6 in oral squamous cell carcinoma. PLoS One.
7:e501042012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy SA, Taylor JM, Terrell JE, Islam M,
Li Y, Fowler KE, Wolf GT and Teknos TN: Interleukin-6 predicts
recurrence and survival among head and neck cancer patients.
Cancer. 113:750–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jinno T, Kawano S, Maruse Y, Matsubara R,
Goto Y, Sakamoto T, Hashiguchi Y, Kaneko N, Tanaka H, Kitamura R,
et al: Increased expression of interleukin-6 predicts poor response
to chemoradiotherapy and unfavorable prognosis in oral squamous
cell carcinoma. Oncol Rep. 33:2161–2168. 2015.PubMed/NCBI
|
|
11
|
Mojtahedi Z, Khademi B, Hashemi SB, Abtahi
SM, Ghasemi MA, Fattahi MJ and Ghaderi A: Serum interleukine-6
concentration, but not interleukine-18, is associated with head and
neck squamous cell carcinoma progression. Pathol Oncol Res.
17:7–10. 2011. View Article : Google Scholar
|
|
12
|
Cohen S, Bruchim I, Graiver D, Evron Z,
Oron-Karni V, Pasmanik-Chor M, Eitan R, Bernheim J, Levavi H,
Fishman A, et al: Platinum-resistance in ovarian cancer cells is
mediated by IL-6 secretion via the increased expression of its
target cIAP-2. J Mol Med Berl. 91:357–368. 2013. View Article : Google Scholar
|
|
13
|
Pu YS, Hour TC, Chuang SE, Cheng AL, Lai
MK and Kuo ML: Interleukin-6 is responsible for drug resistance and
anti-apoptotic effects in prostatic cancer cells. Prostate.
60:120–129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan HQ, Huang XB, Ke SZ, Jiang YN, Zhang
YH, Wang YN, Li J and Gao FG: Interleukin 6 augments lung cancer
chemotherapeutic resistance via ataxia-telangiectasia
mutated/NF-kappaB pathway activation. Cancer Sci. 105:1220–1227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suchi K, Fujiwara H, Okamura S, Okamura H,
Umehara S, Todo M, Furutani A, Yoneda M, Shiozaki A, Kubota T, et
al: Overexpression of interleukin-6 suppresses cisplatin-induced
cytotoxicity in esophageal squamous cell carcinoma cells.
Anticancer Res. 31:67–75. 2011.PubMed/NCBI
|
|
16
|
Poth KJ, Guminski AD, Thomas GP, Leo PJ,
Jabbar IA and Saunders NA: Cisplatin treatment induces a transient
increase in tumorigenic potential associated with high
interleukin-6 expression in head and neck squamous cell carcinoma.
Mol Cancer Ther. 9:2430–2439. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lawrence MS, Sougnez C, Lichtenstein L,
Cibulskis K, Lander E, Gabriel SB, Getz G, Ally A, Balasundaram M,
Birol I, et al: Cancer Genome Atlas Network: Comprehensive genomic
characterization of head and neck squamous cell carcinomas. Nature.
517:576–582. 2015. View Article : Google Scholar
|
|
18
|
Husvik C, Bryne M and Halstensen TS: c-Jun
N-terminal kinase negatively regulates epidermal growth
factor-induced cyclooxy-genase-2 expression in oral squamous cell
carcinoma cell lines. Eur J Oral Sci. 117:663–668. 2009. View Article : Google Scholar
|
|
19
|
Haddad R, Wirth L and Posner M: Emerging
drugs for head and neck cancer. Expert Opin Emerg Drugs.
11:461–467. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aguirre-Gamboa R, Gomez-Rueda H,
Martínez-Ledesma E, Martínez-Torteya A, Chacolla-Huaringa R,
Rodriguez- Barrientos A, Tamez-Peña JG and Treviño V: SurvExpress:
An online biomarker validation tool and database for cancer gene
expression data using survival analysis. PLoS One. 8:e742502013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sato J, Ohuchi M, Abe K, Satoh T, Abe T,
Yamazaki Y, Satoh A, Notani K and Kitagawa Y: Correlation between
salivary interleukin-6 levels and early locoregional recurrence in
patients with oral squamous cell carcinoma: Preliminary study. Head
Neck. 35:889–894. 2013. View Article : Google Scholar
|
|
22
|
Yadav A, Kumar B, Datta J, Teknos TN and
Kumar P: IL-6 promotes head and neck tumor metastasis by inducing
epithelial-mesenchymal transition via the JAK-STAT3-SNAIL signaling
pathway. Mol Cancer Res. 9:1658–1667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Yang J, Qian J, Li H, Romaguera
JE, Kwak LW, Wang M and Yi Q: Role of the microenvironment in
mantle cell lymphoma: IL-6 is an important survival factor for the
tumor cells. Blood. 120:3783–3792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borsellino N, Bonavida B, Ciliberto G,
Toniatti C, Travali S and D'Alessandro N: Blocking signaling
through the Gp130 receptor chain by interleukin-6 and oncostatin M
inhibits PC-3 cell growth and sensitizes the tumor cells to
etoposide and cisplatin-mediated cytotoxicity. Cancer. 85:134–144.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lou W, Ni Z, Dyer K, Tweardy DJ and Gao
AC: Interleukin-6 induces prostate cancer cell growth accompanied
by activation of stat3 signaling pathway. Prostate. 42:239–242.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ
and Li LZ: Autocrine production of interleukin-6 confers cisplatin
and paclitaxel resistance in ovarian cancer cells. Cancer Lett.
295:110–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ara T, Nakata R, Sheard MA, Shimada H,
Buettner R, Groshen SG, Ji L, Yu H, Jove R, Seeger RC, et al:
Critical role of STAT3 in IL-6-mediated drug resistance in human
neuroblastoma. Cancer Res. 73:3852–3864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Li PK, Li C and Lin J: Inhibition
of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in
human liver cancer cells. J Biol Chem. 285:27429–27439. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gougelet A, Mansuy A, Blay JY, Alberti L
and Vermot-Desroches C: Lymphoma and myeloma cell resistance to
cytotoxic agents and ionizing radiations is not affected by
exposure to anti-IL-6 antibody. PLoS One. 4:e80262009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Arinobu Y, Atamas SP, Otsuka T, Niiro H,
Yamaoka K, Mitsuyasu H, Niho Y, Hamasaki N, White B and Izuhara K:
Antagonistic effects of an alternative splice variant of human
IL-4, IL-4delta2, on IL-4 activities in human monocytes and B
cells. Cell Immunol. 191:161–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alberti L, Bachelot T, Duc A, Biota C and
Blay JY: A spliced isoform of interleukin 6 mRNA produced by renal
cell carcinoma encodes for an interleukin 6 inhibitor. Cancer Res.
65:2–5. 2005.PubMed/NCBI
|
|
32
|
Bihl MP, Heinimann K, Rüdiger JJ,
Eickelberg O, Perruchoud AP, Tamm M and Roth M: Identification of a
novel IL-6 isoform binding to the endogenous IL-6 receptor. Am J
Respir Cell Mol Biol. 27:48–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yatsenko OP, Silkov AN, Khrapov EA,
Filipenko ML, Kozlov VA and Sennikov SV: Tissue-specific expression
of splice variants of human IL-4 and IL-6 gene mRNA. Bull Exp Biol
Med. 152:329–332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stransky N, Egloff AM, Tward AD, Kostic
AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C,
McKenna A, et al: The mutational landscape of head and neck
squamous cell carcinoma. Science. 333:1157–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jansson MD and Lund AH: MicroRNA and
cancer. Mol Oncol. 6:590–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rokavec M, Wu W and Luo JL: IL6-mediated
suppression of miR-200c directs constitutive activation of
inflammatory signaling circuit driving transformation and
tumorigenesis. Mol Cell. 45:777–789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ceppi P, Mudduluru G, Kumarswamy R, Rapa
I, Scagliotti GV, Papotti M and Allgayer H: Loss of miR-200c
expression induces an aggressive, invasive, and chemoresistant
phenotype in non-small cell lung cancer. Mol Cancer Res.
8:1207–1216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M,
Zhang W, Chen W, Pan C, Liu Q, et al: miR-200b and miR-15b regulate
chemotherapy-induced epithelial-mesenchymal transition in human
tongue cancer cells by targeting BMI1. Oncogene. 31:432–445. 2012.
View Article : Google Scholar
|
|
39
|
Childs G, Fazzari M, Kung G, Kawachi N,
Brandwein-Gensler M, McLemore M, Chen Q, Burk RD, Smith RV,
Prystowsky MB, et al: Low-level expression of microRNAs let-7d and
miR-205 are prognostic markers of head and neck squamous cell
carcinoma. Am J Pathol. 174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Taube JH, Malouf GG, Lu E, Sphyris N,
Vijay V, Ramachandran PP, Ueno KR, Gaur S, Nicoloso MS, Rossi S, et
al: Epigenetic silencing of microRNA-203 is required for EMT and
cancer stem cell properties. Sci Rep. 3:26872013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Heneberg P: Paracrine tumor signaling
induces transdifferentiation of surrounding fibroblasts. Crit Rev
Oncol Hematol. 97:303–311. 2016. View Article : Google Scholar
|
|
42
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar
|
|
43
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar
|
|
44
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guerrera IC, Quetier I, Fetouchi R, Moreau
F, Vauloup-Fellous C, Lekbaby B, Rousselot C, Chhuon C, Edelman A,
Lefevre M, et al: Regulation of interleukin-6 in head and neck
squamous cell carcinoma is related to papillomavirus infection. J
Proteome Res. 13:1002–1011. 2014. View Article : Google Scholar : PubMed/NCBI
|