Introduction
Colorectal cancer (CRC) is the second and third most
common malignancy in females and males, respectively, worldwide.
Colon cancer is considered to be one of the leading causes of
mortality in the world. There are extremely few therapeutic agents
capable of effectively treating colon cancer (1). According to the GLOBOCAN database
established by the World Health Organization (WHO), an estimated
12.7 million new cancer cases and 7.6 million cancer fatalities
occurred in 2008 (2). The incidence of
CRC is rising rapidly in numerous Asian countries and approaching
the incidence in developed countries (3). Studies have shown that the incidence of
malignant tumours has declined in males and females in the United
States and one of the notable contributing factors is the decreased
incidence of CRC. The results indicate that CRC occupies a
predominant position among the malignancies in the United States
(4). African-Americans have
experienced an increased incidence of CRC compared to other U.S.
populations. In addition, the prevalence of proximal CRC is higher
among African-Americans and the average age for the onset of CRC is
becoming younger (5). CRC is one of
the common malignancies in China. The mortality rate of CRC in
China is 4.54 per 100,000 population, which accounts for 4.9% of
all cancer fatalities and ranks as the fifth highest among the
malignant tumours. Notably, thus far the incidence and mortality
rate of CRC continues to exhibit a tendency to increase. The
five-year survival rates of CRC after curative resection remain
near 50% (for rectal cancer) and 70% (for colon cancer). CRC is
closely associated with the lifestyle of Western countries.
Lifestyle and dietary patterns affect the risk of colon cancer.
Among dietary factors, high intakes of red meat, fat and
carbohydrates enhance the risk of developing CRC. By contrast,
increased consumption of fruits, vegetables and fibres may reduce
the incidence of CRC (6–8). The report released by the WHO and the
Food and Agriculture Organization of the United Nations (FAO) in
2003 clearly stated that obesity is a risk factor for CRC (9). 1,2-Dimethylhydrazine (DMH) is a
carcinogenic agent widely utilised to induce CRC in rats and other
animal models. DMH is also commonly employed in chemistry-based
cancer prevention studies (10). The
present study mainly investigated the effects of a high-fat diet on
DMH-induced CRC in F344 rats.
Materials and methods
Animals
A total of 16 male 4-week-old F344 rats were
purchased from Charles River Laboratories Japan, Inc. (Kanagawa,
Japan). The animals were cared for in compliance with the
principles and guidelines of Ethical Committee for Animal Care and
Institutional Animal Ethical Committee, in accordance with the
Japan National Law on Animal Care and Use. The Ethical Committee
for Animal Care of the Prefectural University of Hiroshima
(Hiroshima, Japan) approved the experiments undertaken. The rats
were housed in a standard air-conditioned room at the Laboratory
Animal Research Center of the Prefectural University of Hiroshima.
The room provided a controlled ambient temperature of 23±2°C, a
humidity of 50±10% and a daily light period of 12 h. The rats had
free access to drinking water and were fed either a basal diet
[moderate fat (MF)] or a high-fat diet (Oriental Yeast Co., Ltd.,
Tokyo, Japan). The main composition of the high-fat diet included
lard, a fish meal, defatted soybean, vitamins and minerals
(Table I). The contents of the three
major types of nutrients in the high-fat and basal diets were
compared and summarised in Table II.
The contents of proteins, fats and carbohydrates were 60, 24.5 and
7.5 g, respectively, in 100 g of the high-fat diet and 23.6, 5.3
and 54.4 g, respectively, in the basal diet. The body weights of
the rats were recorded once every two weeks. DMH was purchased from
Tokyo Chemical Industry Co., Ltd., (Tokyo, Japan).
 | Table I.Contents of the high-fat diet. |
Table I.
Contents of the high-fat diet.
| Nutrient composition
of the high-fat diet | % |
|---|
| Lard | 58 |
| Fish meal | 30 |
| Defatted soybean | 10 |
| Vitamins, minerals
and other components | 2 |
 | Table II.Contents of the three major types of
nutrients in the basal and high-fat diets (100 g). |
Table II.
Contents of the three major types of
nutrients in the basal and high-fat diets (100 g).
| Nutrients | Basal diet | High-fat diet |
|---|
| Protein, g | 23.6 | 24.5 |
| Fat, g | 5.3 | 60 |
| Carbohydrate, g | 54.4 | 7.5 |
| Total energy,
kcal | 360 | 640 |
Experimental protocol
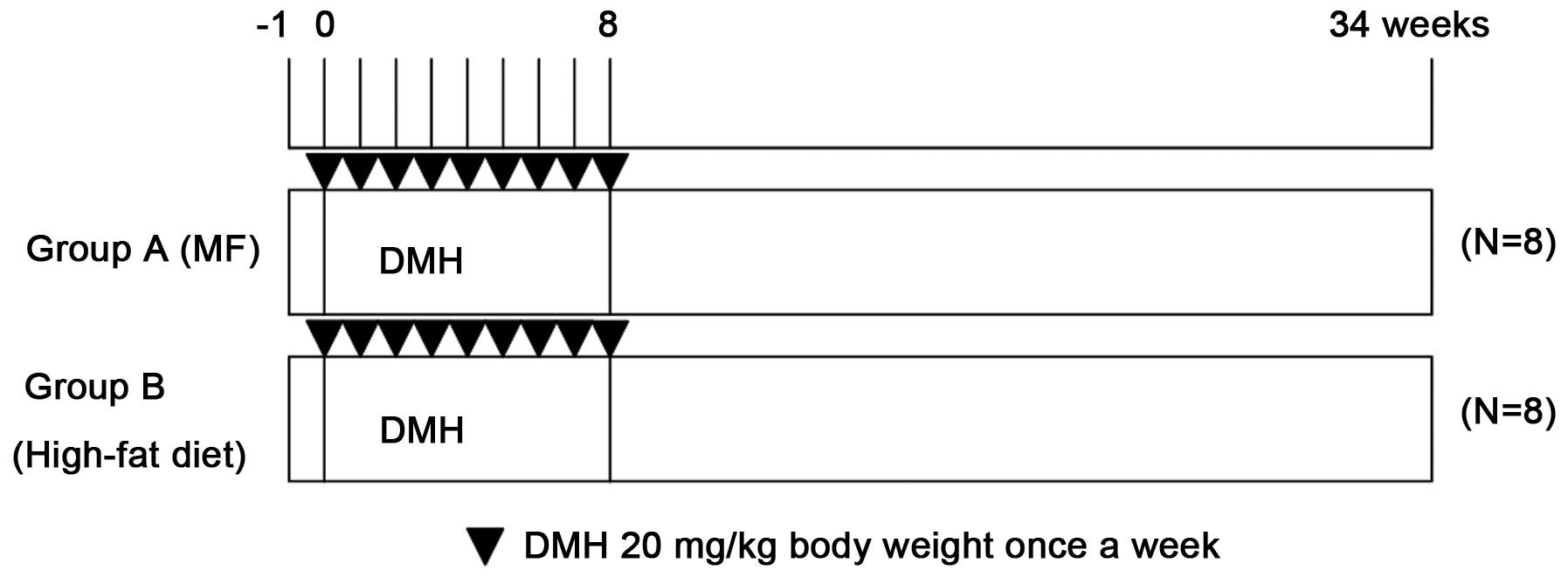
The experimental design is shown in Fig. 1. The 16 male rats were randomly divided
into two groups (8 rats/group) based on their diets: Group A, basal
diet; group B, high-fat diet. DMH was dissolved in 0.9% sodium
chloride and the pH value was adjusted to 6.5 with
NaHCO3. Upon reaching 5 weeks of age, the experimental
rats were injected subcutaneously with DMH (20 mg/kg body weight)
once a week for 8 consecutive weeks. All the rats were sacrificed
34 weeks after the first DMH injection and dissected to obtain
samples of colorectal tissues. The tissues were examined under a
microscope for the presence of aberrant crypt foci (ACFs) and
subjected to histopathological analysis.
Analysis of ACFs and histological
analysis
In rodents and humans, ACFs are believed to be the
microscopic damage formed at the earliest stage of CRC development.
ACFs may be the precancerous lesions that emerge prior to dysplasia
(11). DMH-induced intestinal mucosal
injury is a multi-step pathological process that involves the
formation of ACFs, a gradual increase in the number of ACFs and
eventually the development of CRC (12). The emergence of ACFs is closely
associated with DMH. DMH is converted into azoxymethanol in
vivo, which is further decomposed to form alkylated methyl
diazonium. The product induces the methylation of DNA, RNA and
proteins. DNA methylation results in chromosomal gene mutations and
tumour cell formation. A study conducted by Tanaka (13) has shown that the DNA sequence of
proto-oncogene K is mutated in ACFs, leading to the formation of
adenomas. Subsequently, DNA repair errors and p53 gene mutation
occur and adenomas develop further into adenocarcinomas.
After the animals were sacrificed, the entire
colorectum was quickly removed and cut open along the longitudinal
axis. The colorectal samples were rinsed clean with precooled 0.9%
NaCl solution and laid onto filter paper with the mucosal side up.
After 24 h of fixation at 4°C in 10% buffered formalin, the
colorectal samples were stained with 0.5% methylene blue for 15–30
min, washed with distilled water, placed on glass slides and
observed under an optical microscope using the 4 and 10X
objectives. The number of ACFs was recorded. After the ACFs were
recorded and calculated, the colorectal samples were fixed with 10%
buffered formalin, embedded in paraffin, sectioned at a thickness
of 4 µm, stained with hematoxylin and eosin and examined under an
optical microscope (Olympus, Tokyo, Japan). The number of tumours
was counted and tumour types and grades were investigated.
Statistical analysis
The Statcel software package (KaleidaGraph version
4.1) was used for analysis. The Fisher's exact test and t-test
(Statcel, the useful add in forms on Excel; 2nd edition) were used
to compare data between the two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Body weight and visceral fat
content
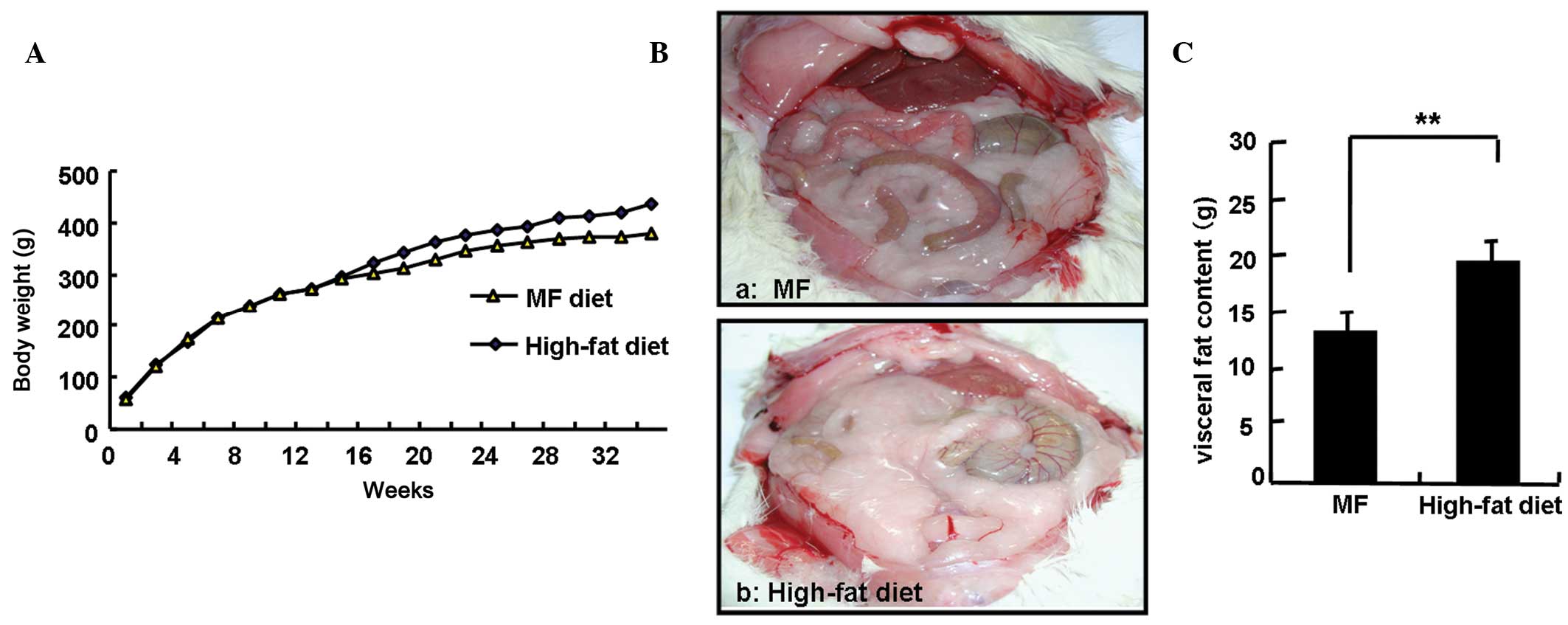
Fig. 2A shows no
significant differences in body weight between the two groups of
rats within the first 14 weeks of the experiment. However, compared
to the rats in the basal diet group, the rats in the high-fat diet
group experienced significantly greater weight gains after 14
weeks. At the end of the 34-week experiment, body weights of the
rats in the high-fat diet group were significantly higher compared
to the rats in the basal diet group (418.6±14.9 vs. 381.9±20.9 g;
P<0.05). For the purpose of the study, the amount of visceral
fat was calculated as the sum of the amount of body fat deposited
in the greater omentum and testicles. The amounts of visceral fat
in the two groups of rats were determined and are shown in Fig. 2B. The amount of visceral fat was
significantly increased in the high-fat diet group compared to that
of the basal diet group (19.2±1.9 vs. 13.1±1.9 g; P<0.01). The
average daily energy intake in each rat from the high-fat diet
group was ~1.3-fold higher compared to that of the basal diet group
and the average daily fat intake in each rat from the high-fat diet
group increased ~8-fold compared to that of the basal diet group
(data not shown).
Colonic ACFs
The effect of the tested diets on the growth and
development of DMH-induced ACFs in rats is shown in Fig. 3. All the rats treated with DMH showed a
100% incidence. The high-fat diet group showed a significantly
higher average number of ACFs than that of the MF diet group.
Colon tumours
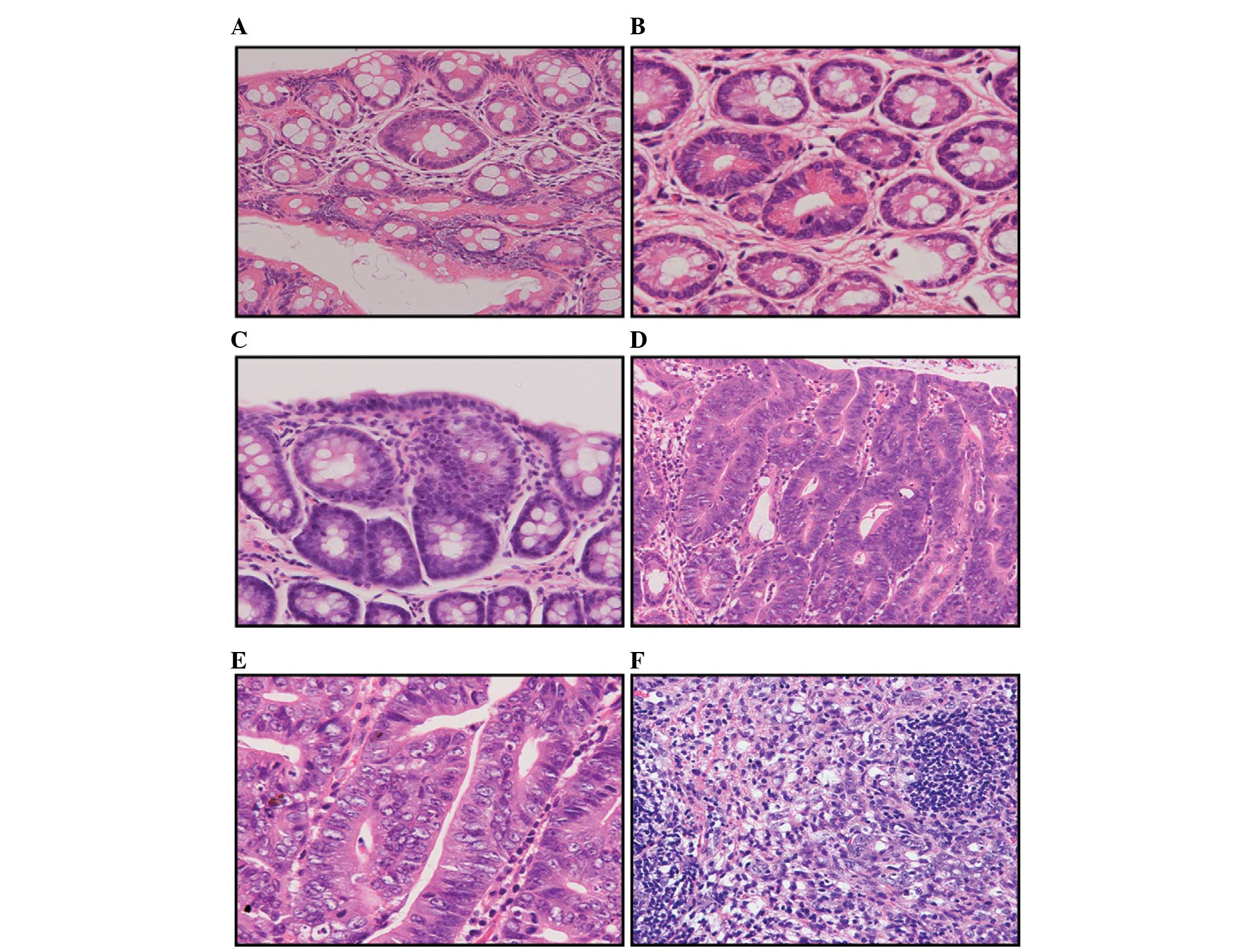
The colorectal tissue sections were subjected to
histopathological investigation and the results are summarised in
Tables III and IV, and Fig. 4.
Tables III and IV show that at the 34th week of the
experiment, the incidence of colorectal adenomas and
adenocarcinomas was significantly higher in the high-fat diet group
compared to that of the basal diet group. A total of 119 adenomas
were detected in the basal diet group, including 103 low-grade and
16 moderate-grade adenomas. The average number of adenomas
developed in each rat was 14.8±8.5. By contrast, 175 adenomas were
detected in the high-fat diet group, including 141 low-grade, 30
moderate-grade and 4 high-grade adenomas. The average number of
adenomas observed in each rat was 21.9±7.2. The differences in the
adenoma incidence between the two groups of rats were statistically
significant (P<0.01). In addition, the numbers of highly,
moderately and poorly differentiated adenocarcinomas found in the
basal diet group were 4, 1 and 3, respectively. An average of
1.0±0.8 adenocarcinomas were developed in each rat. By contrast,
the numbers of highly, moderately and poorly differentiated
adenocarcinomas detected in the high-fat diet group were 16, 3 and
1, respectively. An average of 2.5±1.1 adenocarcinomas were
developed in each rat. The differences in the adenocarcinoma
incidence between the two groups of rats were also statistically
significant (P<0.01).
 | Table III.Number of colon adenomas. |
Table III.
Number of colon adenomas.
|
| No. of colon
adenomas |
|
|---|
|
|---|
| Group | Mild-grade
dysplasia | Moderate-grade
dysplasia | Severe-grade
dysplasia | Total | No. of colon adenomas
per rat |
|---|
| A | 103 | 16 | 0 | 118 |
14.8±8.54a |
| B | 141 | 30 | 4 | 175 | 21.9±7.19 |
 | Table IV.Number of colon adenocarcinomas per
rat. |
Table IV.
Number of colon adenocarcinomas per
rat.
|
| No. of colon
adenocarcinomas |
|
|---|
|
|---|
| Group | Well-differentiated
adenocarcinomas | Moderately
differentiated adenocarcinomas | Poorly
differentiated adenocarcinomas | Total | No. of colon
adenocarcinomas per rat |
|---|
| A | 4 | 1 | 3 | 8 |
1.0±0.83a |
| B | 16 | 3 | 1 | 20 | 2.5±1.07 |
Discussion
Obesity is one of the leading causes of numerous
types of cancer. The report released by the WHO and the FAO in 2003
clearly stated that obesity is a risk factor for CRC (9). Investigators in the U.S. have conducted
studies on the potential link between obesity and cancer and found
that in males and females, kidney cancer and cancers of the
digestive system, such as oesophageal cancer, CRC, hepatobiliary
and pancreatic cancers, are associated with obesity, as defined by
high body mass index values (14).
Relevant studies have shown that obese populations with high blood
sugar levels have almost double the risk of developing
obesity-related cancers, while obese populations with normal blood
sugar levels only carry a 50% increased risk of developing cancers.
In addition, metabolic dysfunction also increases the risk of
cancer in obese individuals (15). In
the present study, CRC was induced in rats using DMH. The rats were
fed a high-fat diet and the effect of the high-fat diet-induced
obesity on rat CRC was investigated. The results showed that after
14 weeks, the rats in the high-fat diet group experienced a
significantly greater body weight gain compared to the rats in the
basal diet group. At the end of the 34-week experiment, the average
difference in body weights between the two groups of rats was ~40
g. The level of visceral fat was significantly higher in rats from
the high-fat diet group compared to the rats from the basal diet
group. In addition, the incidence of ACF, adenomas and
adenocarcinomas was markedly elevated in the high-fat diet group
compared to the basal diet group. The above results indicate that a
high-fat diet promotes the development and progression of CRC in
rats.
The mechanisms by which obesity induces CRC may be
associated with insulin-resistant hyperinsulinaemia and
insulin-like growth factors (IGFs) (14,16). To
maintain normal blood sugar levels and carbohydrate metabolism,
patients with insulin resistance require a large amount of insulin.
A variety of conditions, such as obesity, inflammation and
hyperlipidaemia, may result in the loss of normal insulin function.
Obesity causes insulin resistance and hyperinsulinaemia. Shortly
following the inhibition of insulin, the secretion and expression
of IGF binding protein are altered, which increases the amount of
free IGFs. Insulin and IGF-1 are growth factors that promote cell
proliferation and inhibit apoptosis in cancer cells, resulting in
tumour development and progression. Blood insulin levels measured
in CRC patients and healthy individuals before breakfast and at 90
minutes after breakfast or lunch have shown that an increased IGF-1
level is associated with the incidence of CRC (17). In addition, the levels of C-peptide
have been analysed in CRC patients and healthy individuals. The
group with the highest C-peptide value exhibits a 3.2-fold increase
in CRC risk compared to the group with the lowest C-peptide value.
Higher C-peptide values indicate a greater risk of CRC (17,18).
In addition to insulin resistance, high-fat
diet-induced bile acid secretion also promotes CRC development.
Increased animal fat intake enhances the levels of secreted bile
acids and cholesterol in bile. Bile acids and cholesterol are
converted to secondary bile acids and steroids by bacteria in the
colon, which are further metabolised by colonic bacteria into
carcinogens (19). Animal experiments
have shown that a high-fat diet increases bile acid secretion. Bile
acids exhibit no direct carcinogenic effects; however, they
increase the rate of cancer induction by carcinogens through
accelerating cell turnover (20). This
study showed that the consumption of a high-fat diet significantly
increased the incidence of colorectal adenomas and adenocarcinomas,
indicating that the cancer-promoting effect of a high-fat diet is
associated with the increased secretion of bile acids. In addition,
bile acids induce the expression of cyclooxygenase-2 (Cox-2) in CRC
cells (21). Cox-2 is rarely expressed
in the majority of normal tissues but is highly expressed in cancer
cells. Cox-2 inhibits apoptosis in cancer cells and promotes
angiogenesis (21–23). In addition, secondary bile
(docosahexaenoic acid) triggers the production of the receptor of
urokinase-type activating factor and is associated with the
increased invasiveness of CRC cells (24).
Obesity is a strong risk factor for CRC. Adiponectin
(APN) is a lipid factor secreted by adipose tissue that exhibits an
immune-modulatory, anticancer effect in carbohydrate and lipid
metabolism in CRC. A number of studies have shown that obesity is
associated with APN and the incidence of CRC (25–29). Wei
et al (30) found that the
level of APN was reduced in obese individuals. APN deficiency
induces chronic inflammation of the large intestine, which may
further develop into CRC and increase the risk of developing
cancer. Saxena et al (31) have
investigated DMH-induced CRC under the condition of APN deficiency
and identified that experimental animals deficient in APN clearly
exhibit the clinical symptoms of carcinogenesis.
The consumption of a high-fat diet induces insulin
resistance and increases bile secretion. The products of lipid
metabolism exhibit mutagenic effects on DNA and RNA. In addition,
the APN level is reduced in individuals with a high body fat level.
Therefore, a high-fat diet promotes the development of CRC.
Exercise may have a preventive effect against CRC. It is also
necessary to maintain a nutritionally balanced diet and avoid
excess fat intake. The present study also showed that the
consumption of a high-fat diet promoted the development and
progression of CRC. Therefore, controlling fat intake may prevent
CRC.
Acknowledgements
The present study was supported in part by the
Important Research Grant from the Prefectural University of
Hiroshima, Hiroshima Tsuchiya General Hospital. The authors thank
them for their technical and materials assistance.
References
|
1
|
Misra S, Ghatak S, Vyas A, et al:
Isothiocyanate analogs targeting CD44 receptor as an effective
strategy against colon cancer. Med Chem Res. 23:3836–3851. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung SJ, Kim YS, Yang SY, Song JH, Park
MJ, Kim JS, Jung HC and Song IS: Prevalence and risk of colorectal
adenoma in asymptomatic Koreans aged 40–49 years undergoing
screening colonoscopy. J Gastroenterol Hepatol. 25:519–525. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xicola R, Gagnon M, Clark JR, et al:
Excess of proximal microsatellite-stable colorectal cancer in
African Americans from a multiethnic study. Clin Cancer Res.
20:4962–4970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan AT and Giovannucci EL: Primary
prevention of colorectal cancer. Gastroenterology. 138:2029–2043.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Willett WC, Stampfer MJ, Colditz GA,
Rosner BA and Speizer FE: Relation of meat, fat, and fiber intake
to the risk of colon cancer in a prospective study among women. N
Engl J Med. 323:1664–1672. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Macdonald RS and Wagner K: Influence of
dietary phytochemicals and microbiota on colon cancer risk. J Agric
Food Chem. 60:6728–6735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amine EK, Baba NH, Belhadj M, et al: Diet,
nutrition and the prevention of choronic diseases. World Health
Organ Tech Rep Ser. 916:1–149. 2003.
|
|
10
|
Baskar AA, Ignacimuthu S, Paulraj GM and
Al Numair KS: Chemopreventive potential of beta-Sitosterol in
experimental colon cancer model - an in vitro and in vivo study.
BMC Complement Altern Med. 10:242010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Inamine M, Suzui M, Morioka T, Kinjo T,
Kaneshiro T, Sugishita T, Okada T and Yoshimi N: Inhibitory effect
of dietary monoglucosylceramide
1-O-beta-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two
different categories of colon preneoplastic lesions induced by
1,2-dimethylhydrazine in F344 rats. Cancer Sci. 96:876–881. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rodrigues MA, Silva LA, Salvadori DM, De
Camargo JL and Montenegro MR: Aberrant crypt foci and colon cancer:
Comparison between a short- and medium-term bioassay for colon
carcinogenesis using dimethylhydrazine in Wistar rats. Braz J Med
Biol Res. 35:351–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tanaka T: Development of an
inlammation-associated colorectal cancer model and its application
for research on carcinogenesis and chemoprevention. Int J Inflamm.
2012:1–16. 2012. View Article : Google Scholar
|
|
14
|
Frezza EE, Wachtel MS and
Chiriva-Internati M: Influence of obesity on the risk of developing
colon cancer. Gut. 55:285–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore LL, Chadid S, Singer MR, Kreger BE
and Denis GV: Metabolic health reduces risk of obesity-related
cancer in framingham study adults. Cancer Epidemiol Biomarkers
Prev. 23:2057–2065. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giovannucci E: Insulin, insulin-like
growth factors and colon cancer: A review of the evidence. J Nutr.
131 (Suppl 11):3109S–3120S. 2001.PubMed/NCBI
|
|
17
|
Otani T, Iwasaki M, Sasazuki S, et al:
Plasma C-peptide, insulin-like growth factor-I, insulin-like growth
factor binding proteins and risk of colorectal cancer in a nested
case-control study: The Japan public health center-based
prospective study. Int J Cancer. 120:2007–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powell AA, LaRue JM, Batta AK and Martinez
JD: Bile acid hydrophobicity is correlated with induction of
apoptosis and/or growth arrest in HCT116 cells. Biochem J.
356:481–486. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hill MJ and Aries VC: Faecal steroid
composition and its relationship to cancer of the large bowel. J
Pathol. 104:129–139. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ryser HJP: Chemical carcinogenesis. N Engl
J Med. 285:721–734. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oshio H, Abe T, Onogawa T, et al:
Peroxisome proliferator-activated receptor alpha activates
cyclooxygenase-2 gene transcription through bile acid transport in
human colorectal cancer cell lines. J Gastroenterol. 43:538–549.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang D, Wang H, Shi Q, Katkuri S, Walhi W,
Desvergne B, Das SK, Dey SK and DuBois RN: Prostaglandin E(2)
promotes colorectal adenoma growth via transactivation of the
nuclear peroxisome proliferator-activated receptor delta. Cancer
Cell. 6:285–295. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsujii M, Kawano S, Tsuji S, et al:
Cyclooxygenase regulates angiogenesis induced by colon cancer
cells. Cell. 93:705–716. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baek MK, Park JS, Park JH, Kim MH, Kim HD,
Bae WK, Chung IJ, Shin BA and Jung YD: Lithocholic acid upregulates
uPAR and cell invasiveness via MAPK and AP-1 signaling in colon
cancer cells. Cancer Lett. 290:123–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Larsson SC and Wolk A: Obesity and colon
and rectal cancer risk: A meta-analysis of prospective studies. Am
J Clin Nutr. 86:556–565. 2007.PubMed/NCBI
|
|
26
|
Gunter MJ and Leitzmann MF: Obesity and
colorectal cancer: Epidemiology, mechanisms and candidate genes. J
Nutr Biochem. 17:145–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calle EE and Kaaks R: Overweight, obesity
and cancer: Epidemiological evidence and proposed mechanisms. Nat
Rev Cancer. 4:579–591. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Birmingham JM, Busik JV, Hansen-Smith FM
and Fenton JI: Novel mechanism for obesity-induced colon cancer
progression. Carcinogenesis. 30:690–697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bianchini F, Kaaks R and Vainio H:
Overweight, obesity, and cancer risk. Lancet Oncol. 3:565–574.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei EK, Giovannucci E, Fuchs CS, Willett
WC and Mantzoros CS: Low plasma adiponectin levels and risk of
colorectal cancer in men: A prospective study. J Natl Cancer Inst.
97:1688–1694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saxena A, Chumanevich A, Fletcher E,
Larsen B, Lattwein K, Kaur K and Fayad R: Adiponectin deficiency:
Role in chronic inflammation induced colon cancer. Biochim Biophys
Acta. 1822:527–536. 2012. View Article : Google Scholar : PubMed/NCBI
|