Introduction
Replacement therapy with stem cells has become a
novel method for the treatment of type 1 diabetes mellitus (T1DM).
As confirmed by in vitro experiments, embryonic stem cells
(1,2), induced pluripotent stem cells (skin
fibroblasts, pancreatic cells, spermatogonia) (3,4),
hepatic stem cells (hepatic oval cells, bile duct cells, white
blood cells) (5–7), pancreatic stem cells (pancreatic duct
cells, islet cells, exocrine cells) (8,9), and
mesenchymal stem cells (MSCs) (including bone marrow, fat,
umbilical cord and cord blood) (10,11)
can be induced to differentiate into cells with insulin secretion
function. However, when applied in clinical practice, the
sufficiency of the cell source and a series of problems, including
ethics, immunogenicity and subculture, should also be considered.
Clearly, the umbilical cord-derived MSCs have irreplaceable
advantages, and are attracting increasing clinical attention.
Thus far, selecting appropriate methods to
transplant stem cells is one study foci. In animal experiments, the
common stem cell transplantation methods include orthotopic
transplantation (12), renal
subcapsular transplantation (13),
subcutaneous transplantation (14), intravenous transplantation
(15), transplantation in the
portal vein (16) and
transplantation in the testes (17). However, these methods will
encounter a number of difficulties when applied in clinical
practice. Due to the progress of interventional technology, the
small arteries, including the dorsal pancreatic artery, can be
selected for intervention. If the stem cells are injected though
the dorsal pancreatic artery, they may be induced to differentiate
into cells with an insulin secretion function under the pancreas
microenvironment. This has attracted increasing clinical attention,
and has provided a combining site of basic research and clinical
application.
In the present study, the human umbilical
cord-derived MSCs (hUC-MSCs) were co-cultured with rat pancreatic
cells. Their induced differentiation into islet-like cells was
observed. These cells were transplanted into diabetic rats with
diabetes mellitus, and their effects on blood glucose in rats were
investigated. The objective was to provide a novel proposal for
applying stem cells to the treatment of T1DM.
Materials and methods
Materials
The experiment was performed at the Bethune
International Peace Hospital (Shijiazhuang, China) between January
2009 and December 2010. The umbilical cord was obtained from the
first delivery healthy parturient, pregnancy at term, and only when
the tests for hepatitis B, syphilis or acquired immunodeficiency
syndrome were negative. The study was conducted in accordance with
the Declaration of Helsinki and with approval from the Ethics
Committee of Beijing Traditional Chinese Medicine Hospital
Affiliated to Capital Medical University (Beijing, China). Written
informed consent was obtained from all the participants.
Pancreatic cells were obtained from 60 male Sprague
Dawley (SD) rats, weighing 220 g. Another 30 male rats, aged 8
weeks, weighing 180–220 g, were prepared. All the animals were
supplied by Hebei Experimental Animal Center (Shijiazhuang, China;
certificate no. 911141).
Cell culture
Cord blood was washed with stroke-physiological
saline solution, the umbilical vein and artery were removed
followed by cutting into 1.0 to 2.0-cm segments in the laminar flow
chamber. The Wharton’s Jelly was separated, smashed into 1×1×1-mm
size and cultured with Dulbecco’s modified Eagle’s medium
(DMEM)/F12 medium in an aseptic culture flask at 37°C in a 5%
CO2 incubator. One week later, the supernatant was
removed and the medium was replaced every 3–4 days. When the
adhered cells reached 80%–90% confluency, trypsin/EDTA was added to
digest and passage the cells. The cells were passaged for 11
generations.
hUC-MSCs surface markers
The 3–5 generation adhered cells were digested with
2.5 g/l trypsin, and when the cells were nearly round under an
inverted microscope, DMEM/F12 medium containing 10% fetal bovine
serum (FBS) was added to terminate the digestion, followed by 5 min
centrifugation at 840 × g for 5 min and twice washing with
phosphate-buffered saline (PBS). PBS-balanced solution (100 μl) was
added to prepare the cell suspension. Mouse-anti-human monoclonal
and negative-control antibodies were added (all purchased from
Sigma-Aldrich, St. Louis, MO, USA): i) Cluster of differentiation
105-phycoerythrin (CD105-PE) and immunoglobulin G (IgG)1k-PE. ii)
CD29-fluorescein isothiocyanate (FITC) and IgG2a-FITC. iii)
CD44-FITC and IgG1-FITC. iv) CD14-FITC and IgG2ak-FITC. v) CD34-PE
and IgG1k-PE. vi) CD45-PE-cyanine 5 (PECY5) and IgG1-PECY5. The
cells were incubated for 30 min at room temperature, washed twice
with PBS following centrifugation, followed by the addition of FITC
cross-linked rat-anti-mouse second antibody (1:400). Subsequent to
culture for 45 min, the cells were resuspensed with 3 ml PBS,
washed with PBS at 840 × g for 5 min and resuspended in 300 μl PBS.
The cell suspension was filtered and analyzed using flow cytometry.
All the procedures were repeated three times.
MTT
The passages of three, five, seven, nine and 11
generations of hUC-MSCs were incubated in 96-well culture plates
with a 100 μl density and 1×104 cells per well. Each
passage of cells was cultured in six wells, and the culture medium
was set for the blank control. All the cells were routinely
cultured in the incubator. MTT (10 μl; 5 g/l) was added and the
cells were cultured for another 4 h. The supernatant was removed,
followed by the addition of 150 μl dimethylsulfoxide, shocking for
10 min and measuring the absorbance value (A) at 492 nm. The
cell growth curve was drawn with time as the X-axis and A
value as the Y-axis. A value was expressed as the mean ±
standard deviation.
Cells separation
Male SD rats, weighing 220 g, were anesthetized
using 10 g/l pentobarbital with 45 mg/kg. At the dorsal position,
an arrow-shaped incision was performed at the abdominal section and
the abdominal cavity was opened. The common bile duct was ligated
at the approaching duodenum site using no. 1 silk suture. A no. 4.5
intravenous needle was inserted into the common bile duct near the
hepatic porta and fixed using a silk suture. The rats were
sacrificed by opening the hearts and bloodletting. A total of 8 ml
pre-cooled collagenase V solution (0.5 g/l) was injected into the
pancreas to make it expand. The harvested pancreas was placed in
the digestion bottle with 6 ml D-Hank’s solution. The blood vessel,
fat and lymph node were removed, and the pancreas was smashed,
followed by incubation in a 30°C incubator for 10 min. Following
the removal of the digested tissues, the remaining tissues were
continuously digested with collagenase V. Digestion of all the
digested tissues was terminated by the addition of 10 ml 4°C FBS
and 30 ml 4°C D-Hank’s solution. Following filtration, the cell
suspensions were centrifuged with a 50 ml centrifuge tube for 3 min
at 728 × g, discharging the supernatant and washing with 4°C normal
saline. The procedure was repeated. The deposition was resuspended
with low glucose (LG)-DMEM, and 2 ml dithizone was added. Finally,
the staining of the cells was observed under an inverted microscope
(Olympus CX41; Olympus, Tokyo, Japan).
Differentiation
The 4–10 passage hUC-MSCs were incubated in the
Transwell plate with a density of 1×106 and cultured
with 3 ml LG-DMEM containing 10% FBS. The medium was replaced by
LG-DMEM containing 10% FBS mixed with RPMI 1640 (1:1) at 3 days
following culture. The insert, with a pore diameter of 0.4 μm, was
inserted into the plates, and islet-like cells were seeded at
1×106 density to co-culture with hUC-MSCs. The culture
medium was renewed every 3–4 days. The cell morphological changes
were observed under an inverted microscope. Identification of
pancreatic and duodenal homeobox-1 (PDX-1) expression was carried
out by cell dithizone staining.
The slides of the cells in the co-culture group and
pure-culture group were dried for 3, 7, 10 and 14 days, fixed with
40 g/l paraform for 60 min, washed three times with PBS for 5 min,
soaked in 3% hydrogen peroxide for 10–15 min, washed three times
with PBS at 5 min per time, followed by the addition of 0.1%
TritonX-100, cultured at room temperature for 10 min, washed three
times with PBS at 5 min per time, blocked with bovine serum albumin
for 10–15 min, followed by the addition of rabbit-anti-human PDX-1
polyclonal antibody (1:50), and incubated overnight in a wet box.
The following day, the cells were washed with PBS for 5 min three
times, colorized with diaminobenzidine for 5 min, washed five times
with double-distilled water, followed by re-staining with
haematoxylin for 1 min, dehydration, clearing in xylene and
embedding in paraffin. hUC-MSCs prior to and at 3, 7, 10 and 14
days after culture were dried and washed twice with PBS. Dithizone
was added and observed under an inverted microscope.
Reverse transcription polymerase chain
reaction (RT-PCR)
The expressions of PDX-1 and human insulin in
hUC-MSCs were detected prior to and at 3, 7, 10 and 14 days after
culture as follows. The cells were centrifuged, rinsed three times
in PBS and counted. The cell RNA was subsequently extracted using
TRIzol® reagent (Invitrogen, Carlsbad, CA, USA).
Reaction conditions were: 94°C for 30 sec, 94°C for 30 sec, 58°C
for 30 sec and 72°C for 30 sec repeated in total for 30 cycles,
followed by 72°C for 5 min. The primers were: Human insulin [192
base pairs (bp)] forward, 5′-TTC TTC TAC ACA CCC AAG AC-3′;
reverse, 5′-CTA GTT GCA GTA GTT CTC CA-3′; PDX-1 (305 bp)
forward, 5′-ACC CGT ACA GCC TAC ACT CG-3′; and reverse, 5′-TCA TCG
CCC TGT TGC TCG-3′.
Cells secretion
hUC-MSCs were cultured in vitro with a
density of 1×108/l in LG-DMEM/RPMI1640 (1:1) containing
82.5 mmol/l glucose. The contents of insulin and C-peptide in the
co-cultured and single group were detected by a radioimmunological
kit (Human C-Peptide and Human Insulin-Specific radioimmunoassay
kits, Linco Research, St. Charles, MO, USA) at 0, 3, 7, 10, and 14
days after culture.
Glucose-stimulated insulin secretion
(GSIS) test
Islet-like cells in the co-cultured group were
obtained at 6 days after culture, washed twice using RPMI 1640, and
serum-free LG-DMEM (containing 55 mmol/l glucose) and high glucose
(HG)-DMEM (containing 247.5 mmol/l glucose) were added,
respectively, at 2 ml per well, and incubated in a 5%
CO2 incubator at 37°C for 2 h. Subsequently, 0.5 ml
supernatant was harvested from each well, placed into an Eppendorf
tube and maintained at −20°C. Pure cultured cells at 6 days served
as controls. The secretion of insulin in the two groups was
analyzed by the radioimmunological method, and the glucose
stimulation index was calculated as: Glucose stimulation index =
HG-DMEM insulin content/LG-DMEM insulin content.
Model preparation
Male SD rats, aged eight weeks, weighing 180–220 g,
were prepared for the diabetic models according to routine methods.
Secretion of insulin and C-peptide was detected by the
radioimmunological method, and hematoxylin and eosin (HE) staining
was used to observe morphological changes of the islets. Blood
glucose of the rat caudal vein was measured every other day (Roche
Accu-Chek® Advantage blood glucose meter system; Roche,
Manheim, Germany), and the body weight was measured every week. All
the rats were randomly divided into three groups: Normal control
group (n=6), no treatment; model group (n=8), prepared for diabetic
models; and experimental group (n=16), transplanted islet-like
cells following model preparation.
Labeling rate
Culture medium for the islet-like cells was renewed
at 48 h before transplantation, and 5-bromo-2′-deoxyuridine (BrdU)
with a final concentration of 10 μmol/l was added. Trypsin/EDTA
were added after 48 h following culture to digest the cells,
followed by washing twice using PBS. Subsequently, the cells were
prepared for the slides, fixed, and incubated overnight in a wet
box with added 1:50 mouse-anti-human BrdU monoclonal antibody. The
cells were colorized by a chromogenic agent, and the positive cells
were stained blue. The coloration duration was controlled under a
microscope.
Transplantation
BrdU-labeled islet-like cells were digested by
trypsin/EDTA, centrifuged, washed twice by PBS and prepared for
single cell suspension. The cell concentration was adjusted for
5×109/l. The rats were anesthetized by 10 g/l
pentobarbital with 45 mg per kilogram, and fixed on the surgical
table in the prone position. The surgical area was sterilized using
75% ethanol. A lateropulsion incision was cut along the left costal
margin, and the skin was cut to expose the left kidney. The
prepared cell suspension (1 ml) was injected into the capsule at
the lower pole of the left kidney. The kidney was replaced, and the
skin was sutured and sterilized with 75% ethanol. All the rats were
maintained under thermal insulation until conscious.
Observation
All the rats were observed for eight weeks after
transplantation. The blood glucose was measured and the animals
were sacrificed following observation. The kidney was cut into
slices, and stained by human insulin and BrdU to observe the local
transplantation outcomes.
Main outcome measures
i) Surface marker for stem cells. ii) Morphology,
dithizone and PDX-1 staining of hUC-MSCS co-cultured with rat islet
cells. iii) Changes of PDX-1 and human insulin mRNA
expression during cell differentiation. iv) Secretion of insulin
and C-peptide in co-cultured cells. v) Outcomes of the GSIS
test.
Statistics
Data are presented as mean ± standard deviation.
One-way analysis of variance (ANOVA) was used to assess
significance. The Student-Newman-Keuls method was used to assess
the mean values using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Isolation, culture and passage of
umbilical cord Wharton’s jelly-derived MSCs
Following the removal of the vein, the umbilical
cord was a type of milky white connective tissue (Fig. 1A). Subsequent to shearing and
sieving, the tissue blocks were implanted to the culture medium.
Two days later, short spindle cells appeared on the tissue blocks
(Fig. 1B). Five days later, 80–90%
cells were fused and the adherent cells were obtained, which was
the first generation of cells. These long spindle cells had a
unique shape of MSCs (Fig. 1C).
After 7 days of culture, these cells had a typical appearance of
MSCs (Fig. 1D). Subsequent to the
first passage, the growth of the cells increased, with one passage
for each 3–4 days. There were 11 passages in total. Flow cytometry
was conducted. As shown in Fig.
1E, the positive surface marker presented the expression of
CD44 (91.4%), CD29 (91.3%) and CD105 (99.2%), respectively, without
expression of CD34 (0.2%), CD45 (0.9%) or CD14 (0.6%). The 3rd,
5th, 7th, 9th and 11th generation of cells were obtained and
cultured in medium. The cell activity was determined by testing the
absorbance. There was no significant difference of absorbance among
6 days of culture (P>0.05) (Fig.
1F).
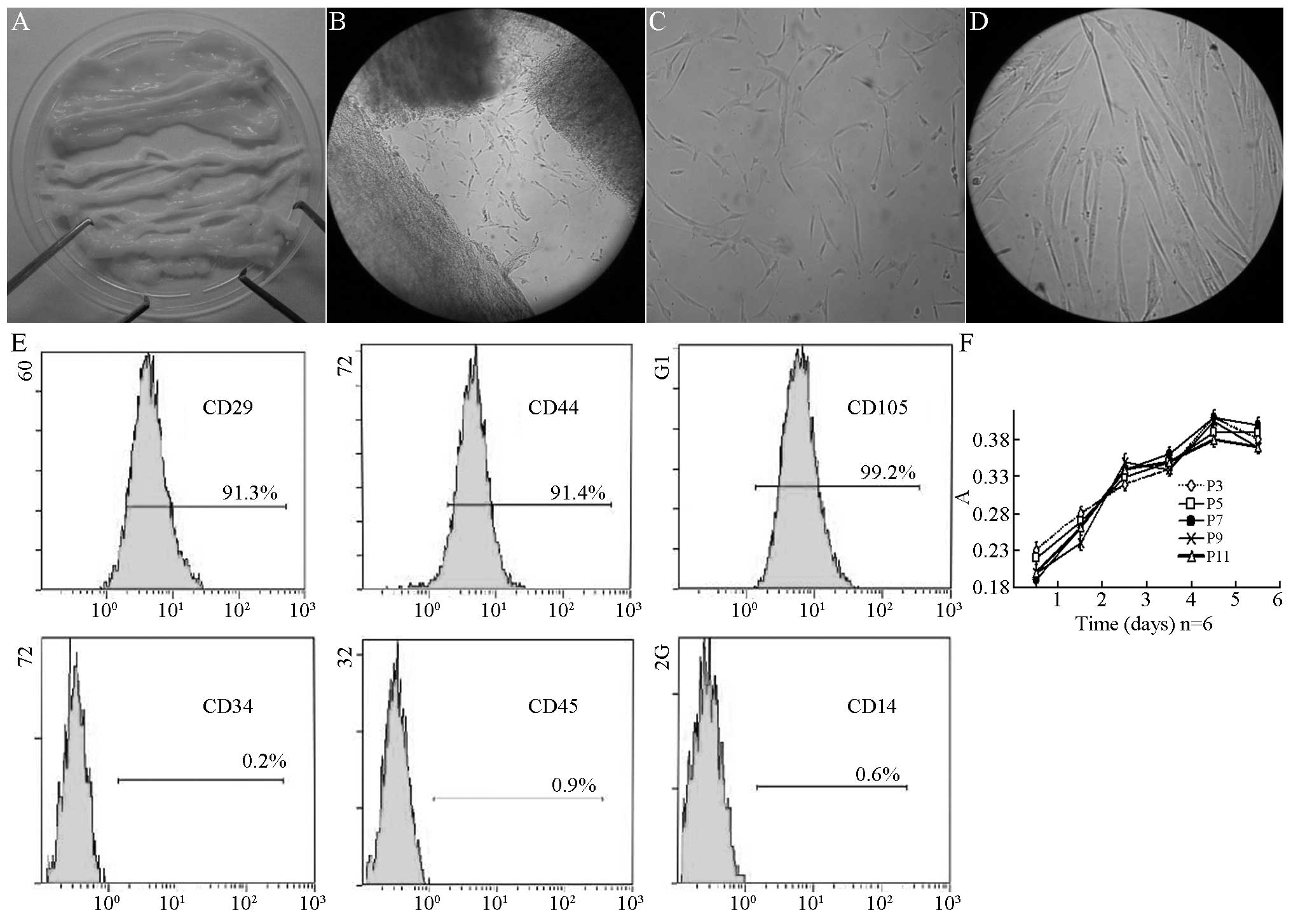 | Figure 1Passage of hUC-MSCs in Wharton’s
Jelly. (A) Wharton’s Jelly tissues without umbilical artery or
vein. (B) Cells crawled out of cultured Wharton’s Jelly 2 days
after culture (magnification, ×100). (C) Fusiform shape cells
adhered to the bottle 5 days after culture (magnification, ×100).
(D) Typical hUC-MSCs could be observed 7 days after culture
(magnification, ×400). (E) The surface marker of human MSCs in
Wharton’s Jelly of the human umbilical cord expressed CD44 (91.4%),
CD29 (91.3%), CD105 (99.2%), but not CD34 (0.2%), CD45 (0.9%) or
CD14 (0.6%). (F) Growth curves of human umbilical cord derived MSCs
at 3, 5, 7, 9 and 11 passages. hUC-MSCs, human umbilical cord
mesenchymal stem cells; CD, cluster of differentiation. |
Dithizone and PDX-1 immunohistochemical
staining in co-culture of hUC-MSCs with rat islet cells
The digestive enzyme was inversely injected into the
common bile duct by intubation (Fig.
2A), for obtaining more rat islet cells. The cytoplasm was
brownish red under dithizone staining, indicating strong positive
staining (Fig. 2F). Fig. 2K showed that before culture, the
hUC-MSCs were long fiber-shaped or fusiform-shaped. In the first
few days of co-culture with the rat islet cells, hUC-MSCs rapidly
became spherical (Fig. 2B). The
dithizone (Fig. 2G) and PDX-1
staining (Fig. 2L) were negative,
and there was almost no brown staining in the cytoplasm. In the
7–10 days of co-culture, the spherical cells gradually accumulated,
and became a large cell mass (Fig. 2C
and D). The dithizone staining presented as a strong
brownish-red (Fig. 2H and I), with
strong brown in cytoplasm by PDX-1 staining (Fig. 2M and N). After 14 days, the cells
aggregated and became a larger cell mass (Fig. 2E), the stained cells were
comparatively rare in dithizone staining (Fig. 2J) and PDX-1 staining (Fig. 2O).
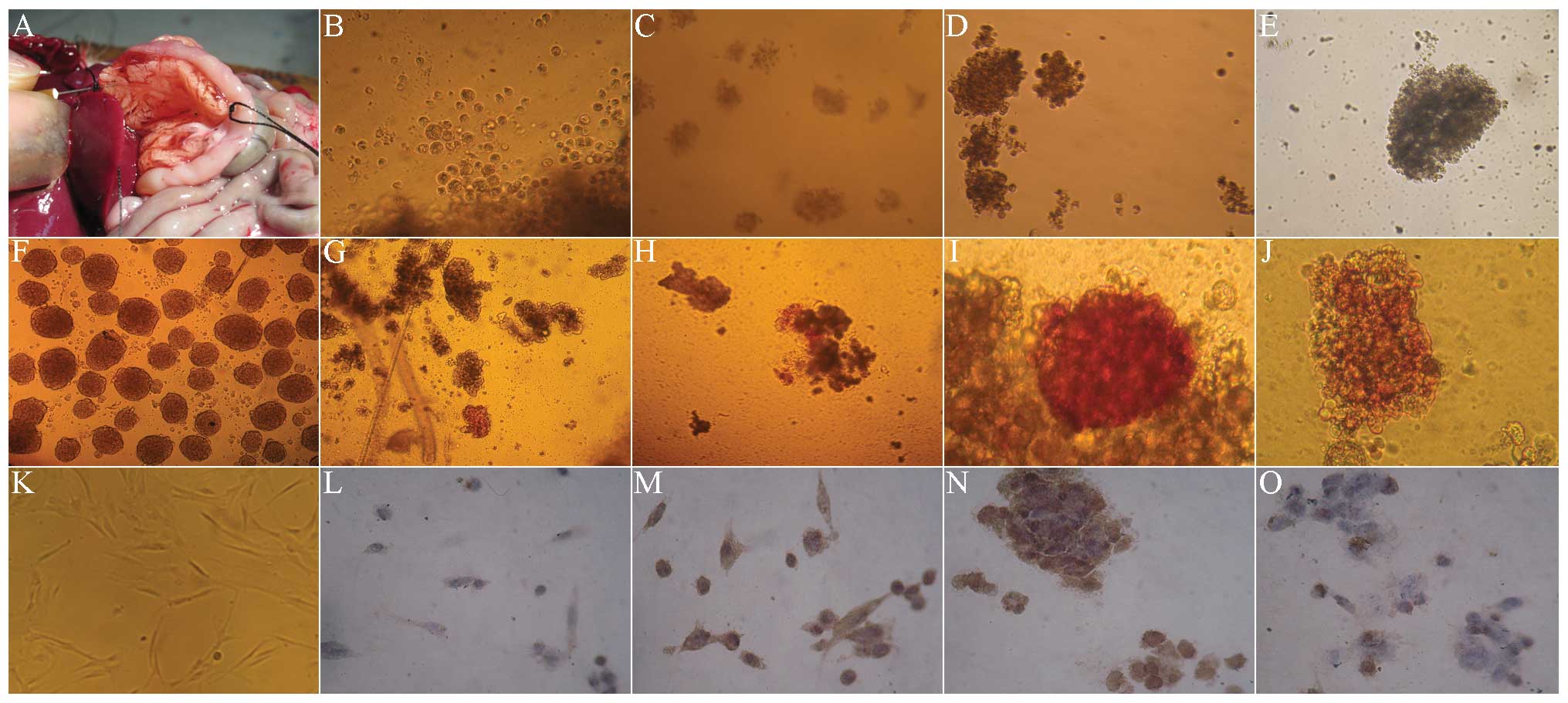 | Figure 2Morphology, dithizone and PDX-1
staining of hUC-MSCs in Wharton’s Jelly co-cultured with rat’s
islet cells (magnification, ×100). (A) Extraction of rat islet
cells. (B) In the first few days of co-culture with the rat islet
cells, hUC-MSCs rapidly became spherical. (C) In the 7 days of
co-culture, the spherical cells gradually accumulated and became a
large cell mass. (D) In the 10 days of co-culture, the spherical
cells gradually accumulated and became a large cell mass. (E) After
14 days, the cells aggregated and became a larger cell mass. (F)
The cytoplasm was brownish-red under dithizone staining, indicating
strong positive. (G) At day 3 of co-culture with the rat islet
cells, the dithizone staining of hUC-MSCs was negative. (H) At day
7 of co-culture with the rat islet cells, the dithizone staining
presented strong brownish red. (I) At day 10 of co-culture with the
rat islet cells, the dithizone staining presented strong brownish
red. (J) At day 14 of co-culture with the rat islet cells, the
stained cells were comparatively rare in dithizone staining. (K)
Prior to culture, the hUC-MSCs were long fiber-shaped or
fusiform-shaped. (L) At day 3 of co-culture with the rat islet
cells, the PDX-1 staining of hUC-MSCs was negative. (M) At day 7 of
co-culture with the rat islet cells, the PDX-1 staining presented
strong brownish. (N) At day 10 of co-culture with the rat islet
cells, the PDX-1 staining presented strong brownish. (O) At day 14
of co-culture with the rat islet cells, the stained cells were
comparatively rare in PDX-1 staining. PDX-1, pancreatic and
duodenal homeobox-1; hUC-MSCs, humal umbilical cord mesenchymal
cells. |
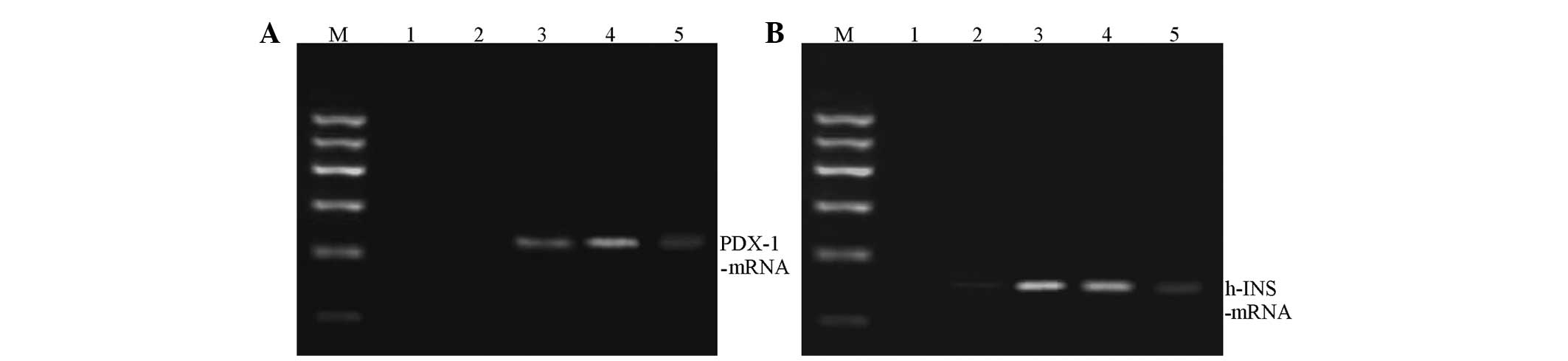
Changes of PDX-1 and human insulin mRNA
expressions during induced differentiation in co-culture
During co-culture, the PDX-1 and human
insulin mRNA expressions in hUC-MSCs at various time points were
determined. The results showed that the PDX-1 and human
insulin mRNA expression was essentially the same at each time
point. The expression was lower in the first 3 days, and
significantly increased at 7–10 days. At day 14, the expression
levels were significantly reduced (Fig. 3).
Change of insulin and C-peptide level in
culture medium during culture
During co-culture, the insulin and C-peptide levels
of culture supernatant were detected. As shown in Table I, for MSCs with no co-culture (only
in culture medium; pure culture group), the insulin and C-peptide
levels did not clearly change during culture (P>0.05). During
the co-culture (co-culture group), the insulin concentration in the
culture supernatant increased from the initial 2.0254 μIU/ml to
141.1400 μIU/ml at day 7. At day 10 it was 105.7600 μIU/ml and
decreased to 57.4533 μIU/ml at day 14, with significant differences
among the various time points (P<0.01). There were similar
results of C-peptide level. At day 3 of culture, the C-peptide
concentration increased by ~20 times, and continued to rise at the
first 7–10 day, then decreased gradually. At day 14, the C-peptide
concentration remained high, with significant differences among
time points (P<0.01).
 | Table IComparison of insulin secretion levels
between the two groups at different times following induction by
radioimmunoassay (n=6). |
Table I
Comparison of insulin secretion levels
between the two groups at different times following induction by
radioimmunoassay (n=6).
| Pure cultured
group | Co-cultured
group |
|---|
|
|
|
|---|
| Time, days | Insulin, mU/l | C-peptide,
nmol/l | Insulin, mU/l | C-peptide,
nmol/l |
|---|
| 0 | 2.03±0.75 | 0.02±0.01 | 2.02±0.56 | 0.02±0.01 |
| 3 | 2.03±0.80 | 0.01±0.01 | 65.46±17.30 | 0.28±0.09 |
| 7 | 2.17±0.76 | 0.01±0.01 | 141.14±20.14 | 0.60±0.15 |
| 10 | 1.98±0.74 | 0.01±0.01 | 105.76±18.68 | 0.44±0.08 |
| 14 | 2.17±0.46 | 0.02±0.00 | 57.45±17.06 | 0.20±0.08 |
| F | | | 26.775 | 17.424 |
| P-value | >0.05 | >0.05 | <0.01 | <0.01 |
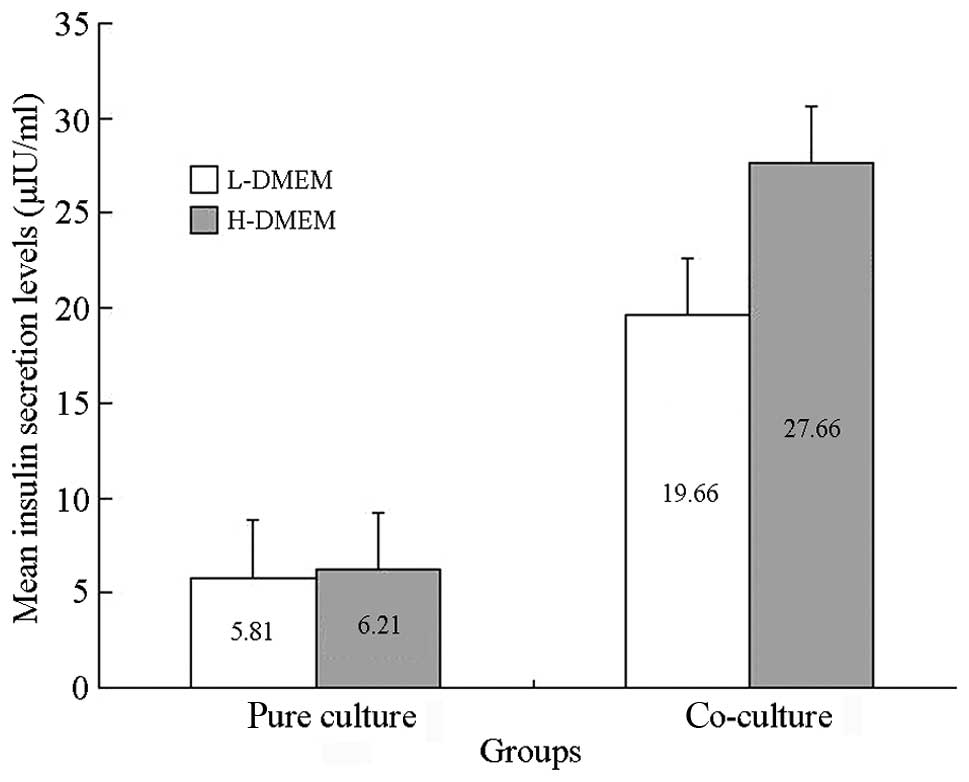
Results of the glucose-stimulated insulin
release experiment
In the co-culture group, the insulin secretion in
LG-DMEM and HG-DMEM was 19.6025±5.9516 μIU/ml and 27.6617±7.1483
μIU/ml, respectively. The secretion significantly increased with an
increase of glucose concentration (P<0.01). In the pure culture
group, the amount of insulin secretion for the simple culture cells
in LG-DMEM and HG-DMEM was 5.8000±1.006 μIU/ml and 6.2100±1.1746
μIU/ml, respectively, with no significant difference between them
(P>0.05). This indicated that at day 7 of co-culture, the
islet-like cells reacted to the stimulation of glucose, and the
glucose stimulation index was 1.4372±0.1390 μIU/ml (Fig. 4).
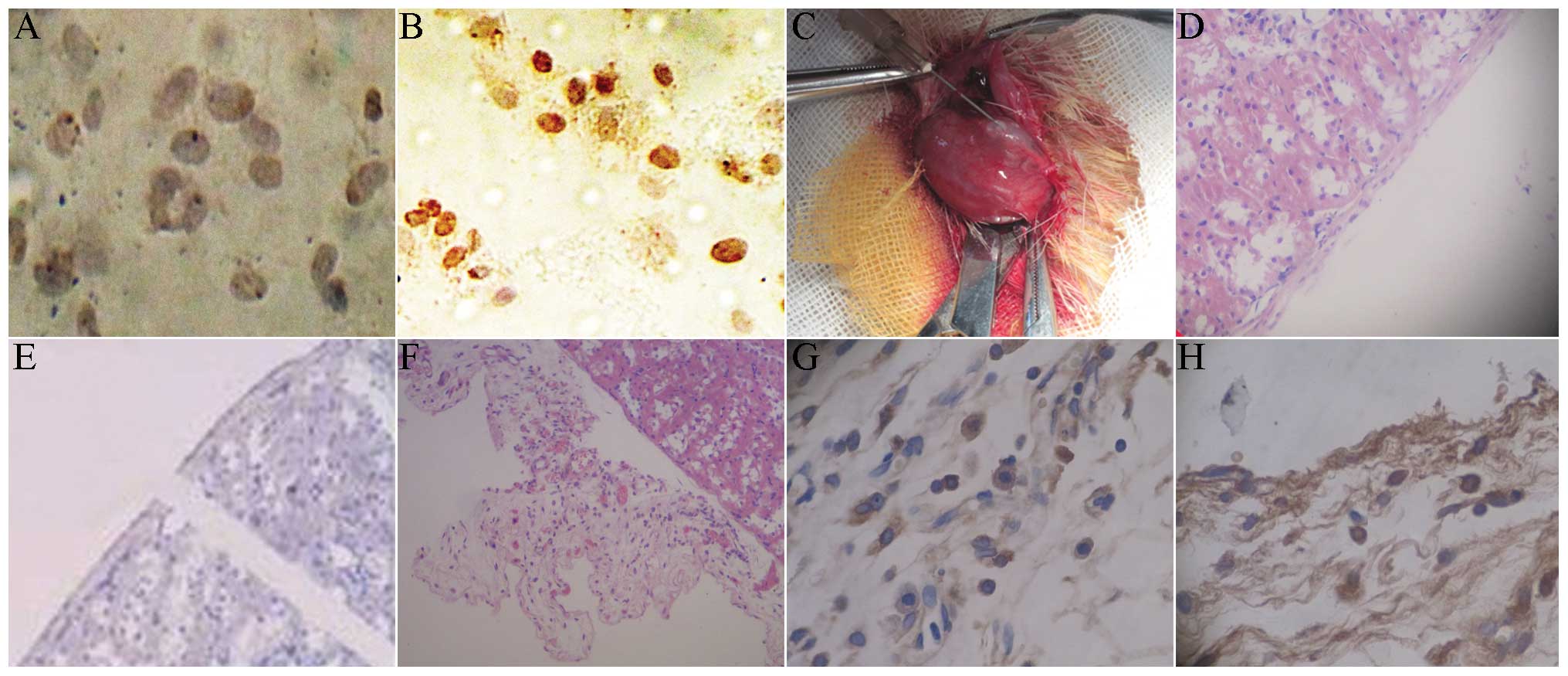
Results of BrdU and HE staining
Under the 250× microscope, 10 visual fields were
randomly selected to count the number of positive and non-positive
cells. The positive rate of BrdU was calculated as: Number of
positive cells/(number of positive cells + non-positive cells). The
mark rate of BrdU was 52%. For the cells without BrdU marking,
there was no brown staining in the cytoplasm (Fig. 5A), with light brown staining for
BrdU-marked cells (Fig. 5B). Under
sterile conditions, the micro injector was used for local injection
to the renal capsule, followed by induced transplantation. The
induced islet-like cell clusters were transplanted into the renal
capsule in rats (Fig. 5C). The HE
staining of normal rat kidney showed an integral renal capsule
edge, with clear morphology and cell structure (Fig. 5D). The immunohistochemistry of the
normal rat kidney showed that the insulin and BrdU staining of the
renal capsule were negative (Fig.
5E). Following transplantation with induced islet-like cells,
the HE staining of the renal cells showed that there were a large
number of survived cells between renal capsule and the renal
cortex, with fracture of renal capsule (Fig. 5F). The kidney immunohistochemical
staining showed insulin-positive cells between the renal capsule
and renal cortex (Fig. 5G). The
immunohistochemical staining indicated BrdU-positive cells between
the renal capsule and renal cortex, with brown nuclei (Fig. 5H).
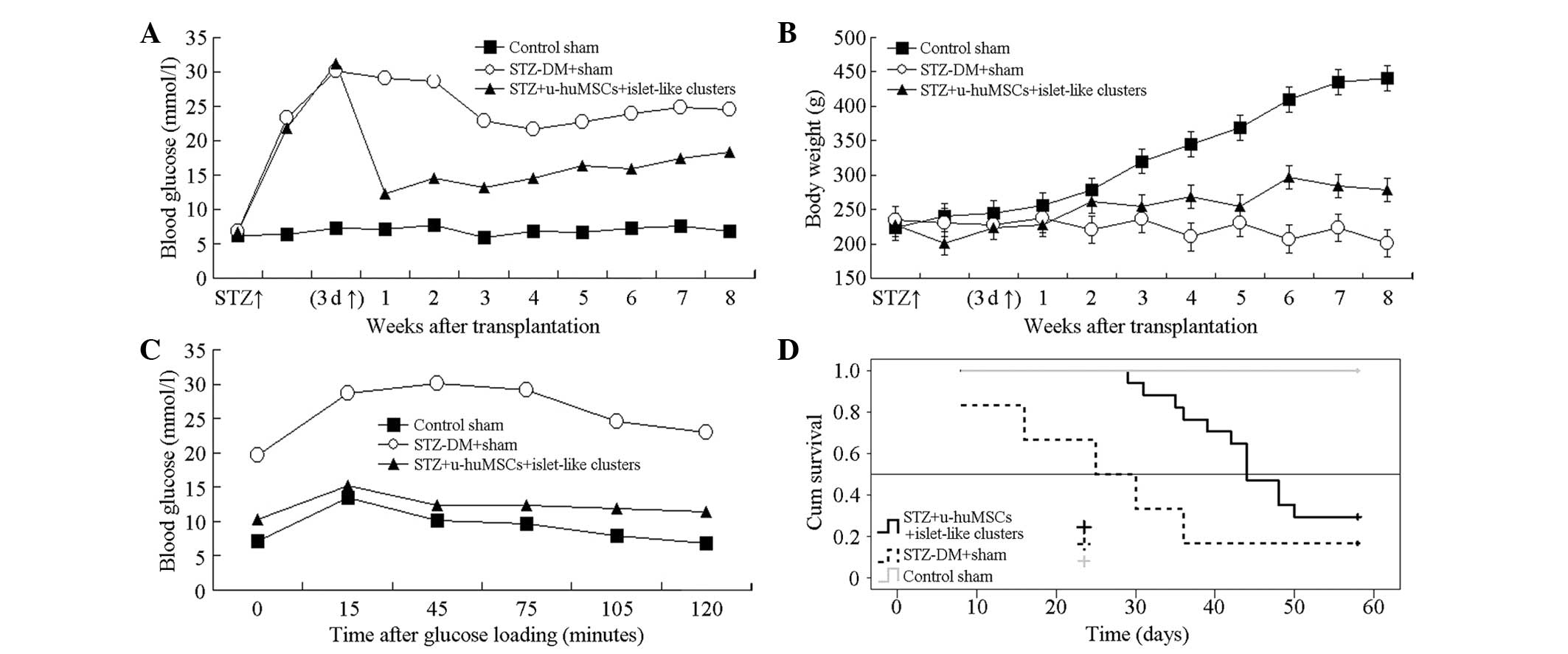
Blood glucose and body weight change,
glucose-stimulated test results and survival curve
The Roche blood glucose meter detection results
(Fig. 6A) showed that in the first
week, the blood glucose level in the STZ-experimental group
decreased, with significant difference compared to the normal and
STZ-control groups (P<0.01). Fig.
6B showed that following treatment the body weight in the
normal group significantly increased, while in the STZ-normal group
the weight decreased gradually. In the STZ-experimental group, the
body weight gradually increased with a significant difference
compared to the normal and STZ control groups in the 4, 6, 7 and 8
weeks after transplantation (P<0.01). Kaplan-Meier survival
curve of each group following cell transplantation is shown in
Fig. 6C. The survival time of the
diabetic rats in the STZ-experimental and STZ-control groups was
46.56±2.29 and 28.83±6.49 days, respectively, with a significant
difference between them (P=0.026). Fig. 6D shows that the survival rate in
the STZ-experimental group was higher compared to the STZ-control
group.
Discussion
Wharton’s Jelly is a type of mucin-like connective
tissue in the umbilical cord that contains two umbilical arteries
and an umbilical vein. During cell culture, there are long
fiber-like or fusiform-shaped adherent cells, which express CD44,
CD29 and CD105, but not CD34, CD45 or CD14. From the morphology and
cell surface markers, these cells have typical characteristics of
MSCs. Therefore, these cells can be regarded as hUC-MSCs.
There are a number of approaches for the
differentiation of MSCs into insulin secretion cells, which include
in vitro induction (18),
transgene induction (19) and
micro-environmental methods such as low-sugar environment culture
(20) and pancreatic extract
co-culture (21). Transwell
co-culture plate has 0.4 μm microporous semi-permeable membrane
chamber. The selective membrane only allows the passage of small
molecules from the medium, but not insulin, C-peptide or other
large molecules. The isolated rat pancreatic cells were co-cultured
with hUC-MSCs, so that the cell cytokine in the islet
microenvironment can act with hUC-MSCs through the semi-permeable
membrane. This can simulate the microenvironment in the human
pancreas more efficiently.
In the present study, the morphological observation,
immunocytochemistry, RT-PCR and radioimmunoassay were conducted to
observe the potential and functional change trends of hUC-MSCs
differentiating into islet-like cells with different in
vitro induction terms. In the first few days of induction in
the islet microenvironment, the shape of hUC-MSCs changed from long
fusiform into round shapes. These cells aggregated into clusters,
with a semi-suspended state growth, which is similar with in
vitro cultured islet.
Under physiological conditions, PDX-1 is a critical
transcription factor for the early growth of pancreatic tissue.
PDX-1 can promote the directional differentiation of pancreatic
precursor cells to pancreatic tissue, and promote the cell to
secret insulin and C-peptide. With the growth and maturation of the
pancreas, the expression of PDX-1 gradually decreases. The basic
principle of hUC-MSCS differentiating into islet-like stem cells is
that it initiates the expression of PDX-1. In the present study,
after 7 and 10 days of induction, the PDX-1 expression in the
islet-like cells in the co-culture group was strong positive, and
in the pure culture group it was negative. This indicates that in
the short induction term (one week), there is PDX-1 expression in
cells. In the co-culture system under the microenvironment provided
by the rat islet cells, the hUC-MSCs can differentiate to early
islet cells. In the co-culture group, the radioimmunoassay detected
high-level insulin and C-peptide in the culture supernatant in the
day 7 and 10 cells, indicating that the induced cells have the
ability to synthesize and secrete insulin, and have reactivity to
glucose stimulation with an insulin stimulation index of
1.4372±0.1390. In the pure culture group, only a small amount of
insulin was detected in the supernatant. With the prolonged in
vitro culture time, the secretion amount of insulin had no
evident change. This may be caused by the effect of
low-concentration glucose in medium on the differentiation of
hUC-MSCs. After 10 days of in vitro induced culture, the
insulin and C-peptide secretion function of hUC-MSCs gradually
decreased. At day 14, there was only an extremely small amount of
insulin secretion and PDX-1 expression. This is consistent with the
RT-PCR results.
The present study shows that in the microenvironment
of the co-culture with rat pancreatic cells, the hUC-MSCs can
survive, proliferate and be induced to differentiate to islet-like
cells. At week 8 after transplantation to the kidney capsule, the
cells remained alive. The dithizone staining showed that the cells
produced insulin and decreased the blood glucose in diabetic rats.
In addition, there was no clear immunological rejection associated
with transplantation. The induction does not bring other chemicals
and genes. Simultaneously, the hUC-MSCs have superiority in number
compared to other stem cells, which fulfills the clinical
requirement. The study has provided the experimental basis for the
further application of dorsal pancreatic artery injection of
hUC-MSCs to the treatment of T1DM.
Acknowledgements
This study was supported by grants from the Beijing
Municipal Administration of Traditional Chinese Medicine (nos.
WZF2012-13 and WZF2012-02), Beijing Municipal Health Bureau Talent
Project (no. 2012-18), Beijing Municipal Health System High-level
Health Technicians Project (no. 2013-3-080), Beijing Science and
Technology Committee Capital Special Project (no. Z121107001012103)
and the Beijing Traditional Chinese Medicine Technology Development
Fund (no. JJ2013-03).
References
|
1
|
Kroon E, Martinson LA, Kadoya K, et al:
Pancreatic endoderm derived from human embryonic stem cells
generates glucose-responsive insulin-secreting cells in vivo. Nat
Biotechnol. 26:443–452. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S, Borowiak M, Fox JL, et al: A small
molecule that directs differentiation of human ESCs into the
pancreatic lineage. Nat Chem Biol. 5:258–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alipio Z, Liao W, Roemer EJ, et al:
Reversal of hyperglycemia in diabetic mouse models using
induced-pluripotent stem (iPS)-derived pancreatic beta-like cells.
Proc Natl Acad Sci USA. 107:13426–13431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bar-Nur O, Russ HA, Efrat S and Benvenisty
N: Epigenetic memory and preferential lineage-specific
differentiation in induced pluripotent stem cells derived from
human pancreatic islet beta cells. Cell Stem Cell. 9:17–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cardinale V, Wang Y, Carpino G, et al: The
biliary tree - a reservoir of multipotent stem cells. Nat Rev
Gastroenterol Hepatol. 9:231–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Liu Y, Wang H, et al: Direct
differentiation of hepatic stem-like WB cells into
insulin-producing cells using small molecules. Sci Rep.
3:11852013.PubMed/NCBI
|
|
7
|
Yechoor V, Liu V, Espiritu C, et al:
Neurogenin3 is sufficient for transdetermination of hepatic
progenitor cells into neo-islets in vivo but not
transdifferentiation of hepatocytes. Dev Cell. 16:358–373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smukler SR, Arntfield ME, Razavi R, et al:
The adult mouse and human pancreas contain rare multipotent stem
cells that express insulin. Cell Stem Cell. 8:281–293. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Q, Brown J, Kanarek A, et al: In vivo
reprogramming of adult pancreatic exocrine cells to beta-cells.
Nature. 455:627–632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ho JH, Tseng TC, Ma WH, et al: Multiple
intravenous transplantations of mesenchymal stem cells effectively
restore long-term blood glucose homeostasis by hepatic engraftment
and β-cell differentiation in streptozocin-induced diabetic mice.
Cell Transplant. 21:997–1009. 2012.PubMed/NCBI
|
|
11
|
Kim SJ, Choi YS, Ko ES, et al:
Glucose-stimulated insulin secretion of various mesenchymal stem
cells after insulin-producing cell differentiation. J Biosci
Bioeng. 113:771–777. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang C, Wang X, Niu D, et al: Mesenchymal
stem cells adopt beta-cell fate upon diabetic pancreatic
microenvironment. Pancreas. 38:275–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin G, Wang G, Liu G, et al: Treatment of
type 1 diabetes with adipose tissue-derived stem cells expressing
pancreatic duodenal homeobox 1. Stem Cells Dev. 18:1399–1406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fumimoto Y, Matsuyama A, Komoda H, et al:
Creation of a rich subcutaneous vascular network with implanted
adipose tissue-derived stromal cells and adipose tissue enhances
subcutaneous grafting of islets in diabetic mice. Tissue Eng Part C
Methods. 15:437–444. 2009. View Article : Google Scholar
|
|
15
|
Lee J, Wen J, Park JY, et al: Reversal of
diabetes in rats using GLP-1-expressing adult pancreatic duct-like
precursor cells transformed from acinar to ductal cells. Stem Cells
Dev. 18:991–1002. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Trivedi HL, Vanikar AV, Thakker U, et al:
Human adipose tissue-derived mesenchymal stem cells combined with
hematopoietic stem cell transplantation synthesize insulin.
Transplant Proc. 40:1135–1139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gabr MM, Sobh MM, Zakaria MM, Refaie AF
and Ghoneim MA: Transplantation of insulin-producing clusters
derived from adult bone marrow stem cells to treat diabetes in
rats. Exp Clin Transplant. 6:236–243. 2008.PubMed/NCBI
|
|
18
|
Li Y, Zhang R, Qiao H, et al: Generation
of insulin-producing cells from PDX-1 gene-modified human
mesenchymal stem cells. J Cell Physiol. 211:36–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen LB, Jiang XB and Yang L:
Differentiation of rat marrow mesenchymal stem cells into
pancreatic islet beta-cells. World J Gastroenterol. 10:3016–3020.
2004.PubMed/NCBI
|
|
20
|
Yang L, Li S, Hatch H, et al: In vitro
trans-differentiation of adult hepatic stem cells into pancreatic
endocrine hormone-producing cells. Proc Natl Acad Sci USA.
99:8078–8083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woodbury D, Schwarz EJ, Prockop DJ and
Black IB: Adult rat and human bone marrow stromal cells
differentiate into neurons. Neur Sci Res. 61:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|