Introduction
Prostate cancer is the most commonly diagnosed
cancer and the second leading cause of cancer mortality in males in
the Western world. Human prostate adenocarcinoma cell lines are
normally resistant to programmed cell death, known as apoptosis
(1).
Six transmembrane epithelial antigen of the prostate
(STEAP) (2) belongs to the six
transmembrane protein of prostate (STAMP) gene family, and is the
first characterized transmembrane gene that is enriched in the
prostate. STEAP is expressed in metastatic prostate cancer samples;
in particular, STAMP1/STEAP2 (3)
and STAMP2/STEAP4 (4) are
expressed in the androgen receptor-positive prostate cancer cell
line LNCaP, and androgen receptor-mediated regulation of STAMP2 has
previously been demonstrated (4).
The role of STAMP2 in metabolic disease and its function in the
prevention of excessive inflammation and protection of adipocyte
insulin sensitivity and systemic glucose homeostasis has been
reported in mice (5). Other
members of the STAMP family include pHyde, a rat protein that has
been implicated in the apoptosis of prostate cancer cells (6), and its human homolog, tumor
suppressor-activated pathway 6 (TSAP6), also known as STEAP3, a
p53-inducible gene, involved in apoptosis and the cell cycle in
prostate cancer and HeLa cells (7).
It is hypothesized that STAMP/STEAP family genes may
have similar functions, with roles in the normal biology and
pathophysiology of prostate cancer. Activation of extracellular
signal-regulated kinase (ERK), which has previously been implicated
in prostate cancer progression, was reported with ectopic
expression of STAMP1 in DU145 cells and, conversely, was strongly
downregulated in LNCaP cells following STAMP1 knockdown (8). The promoter regions of STAMP genes
have been analyzed, and tumor suppressor gene p53 response elements
and nuclear factor κB (NFκB) response elements identified and
confirmed in the promoter region of STAMP genes (Gonen-Korkmaz
et al, unpublished data). In the present study, tumor
necrosis factor α (TNFα)-induced apoptosis in the LNCaP (human
prostate adenocarcinoma lymph node metastasis) cell line was
investigated by amplifications conducted using a panel of
apoptosis-related gene primers. The LNCaP cell line expresses
STAMP1 and STAMP2. Another prostate cancer cell line, DU145, which
is derived from brain metastasis, was transfected with STAMP1 and
STAMP2 and then induced by TNFα. The apoptosis/survival
equilibrium, which is determined by NFκB, was investigated by
western blot analysis of the two cell lines.
Materials and methods
Cell culture
LNCaP cells were cultured in RPMI-1640 (Gibco-BRL,
Gaithersburg, MD, USA) with 10% fetal bovine serum (FBS), while
DU145 cells were cultured in Dulbecco’s modified Eagle’s medium
(DMEM)-Ham’s F12 (Gibco-BRL) with 5% FBS, 1% L-glutamine and 1 U/ml
each of penicillin/streptomycin. Cells were incubated at 37°C with
5% CO2 in a humidified atmosphere. The cell lines were
purchased from ATCC (Manassas, VA, USA).
Primer design, plasmid construction and
transfection
The full-length open reading frames of STAMP1 and
STAMP2 were amplified using primers (10 pmol of each), designed
using Light Cycler Probe Design Software 2 (Roche Diagnostics,
Mannheim, Germany). The PCR product was cloned into
pcDNA4-HisMax-TOPO (Invitrogen Life Technologies, Carlsbad, CA,
USA) vector, in accordance with the manufacturer’s instructions.
The inserts were verified by PCR amplifications. All transfections
including small interfering RNA (siRNA) were performed using FuGENE
HD (Roche Diagnostics) transfection reagent, in accordance with the
manufacturer’s instructions. Briefly, cells were seeded in 6-well
plates one day prior to transfection. The following day, the
transfection solution was prepared in a 1.5-ml tube with 100 μl
pre-warmed RPMI-1640 (without antibiotics), 1 μg pcDNA4-HisMax-gene
plasmid DNA was added and the solution was incubated for 5 min. A
total of 3 μl FuGENE HD transfection reagent was added dropwise
with tapping to mix, and, following a 15-min incubation at room
temperature, the transfection mix was added to the cells
dropwise.
siRNA-mediated knockdown of NFκB
LNCaP cells were transfected with either scrambled
control siRNA (sc-37007) or NFκB-specific siRNA (sc-29410),
purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The
sequences were provided by the manufacturer.
A total of 100 pmol siRNA (final concentration, 50
nM) was used to transfect cells with the aid of 10 μl FuGENE HD
transfection reagent and the cells were incubated with the siRNA
construct for 1 and 4 days, respectively, in accordance with the
manufacturer’s instructions.
Treatment of the cells
The LNCaP cells were divided into four groups. The
control group was cultured in the absence of treatment for 24 h;
the TNFα induction group was induced by TNFα (100 ng/ml; Sigma, St.
Louis, MO, USA) for 24 h. the R1881 group was treated for 24 h with
a synthetic androgen, R1881 (1×10−8 M; Sigma); and the
TNF + R1881 group was treated concomitantly with TNFα (100 ng/ml)
and R1881 (1×10−8 M) for 24 h.
DU145 cells transfected with STAMP1 or STAMP2 were
induced by TNFα (100 ng/ml) or were not induced for 24 h.
In another series of experiments, following NFκB
gene silencing, the LNCaP cells transfected for 1 day were induced
by TNFα (100 ng/ml) or were not induced for a further 24 h. The
cells transfected for 4 days were induced by TNFα (100 ng/ml) or
were not induced at the third day of transfection.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR) using a panel of
apoptosis-related gene primers
qPCR was performed using a Light Cycler®
480 (Roche Diagnostics) instrument and Light Cycler 480 SYBR Green
1 Master kit (Roche Diagnostics). Briefly, the reactions were
performed in a 20-μl volume with 5 pmol of each primer and 1 μl of
cDNA template derived from reverse-transcribed RNA of scrambled
siRNA (control) and NFκB siRNA-transfected cells. The primers used
are shown in Table I. GAPDH, a
human housekeeping gene, was used as an endogenous control and
reference gene for relative quantifications. The same thermal
profile was optimized for all primers: pre-incubation for 5 min at
95°C for 1 cycle, followed by 40 cycles of denaturation at 95°C for
10 sec, primer annealing at 64°C for 20 sec, and primer extension
at 72°C for 10 sec. Water was included as a no-template control.
Melting curves were derived after 40 cycles by a denaturation step
at 95°C for 10 sec, followed by annealing at 65°C for 15 sec, and a
temperature rise to 95°C with a heating rate of 0.1°C/sec and
continuous fluorescence measurement. Final cooling was performed at
37°C for 30 sec. Melting curve analyses of each sample were
performed using LightCycler 480 Software version LCS480 (Roche
Diagnostics). The analysis step of relative quantification was a
fully automated process accomplished by the software, with the
efficiency set at 2 and the cDNA of untreated cells defined as the
calibrator.
 | Table IGenes and primers used as an apoptosis
panel for quantitative polymerase chain reaction (qPCR)
analysis. |
Table I
Genes and primers used as an apoptosis
panel for quantitative polymerase chain reaction (qPCR)
analysis.
| GenBank/Symbol | Description | Gene name | Primer sequence |
|---|
| NM_005163/AKT1 | V-akt murine thymoma
viral oncogene homolog 1 | PKB/PRKBA | Forward:
TCCCCCTCAGATGATCTCTCCA
Reverse: CGGAAAGGTTAAGCGTCGAAAA |
| NM_001227/CASP7 | Caspase 7, apoptosis-
related cysteine peptidase | CMH-1/ICE-LAP3 | Forward:
AAGTGAGGAAGAGTTTATGGCAAA
Reverse: CCATCTTGAAAACAAAGTGCCAAA |
| NM_001229/CASP9 | Caspase 9, apoptosis-
related cysteine peptidase | APAF-3/APAF3 | Forward:
TCCTGAGTGGTGCCAAACAAAA
Reverse: AGTGGTTGTCAGGCGAGGAAAG |
| NM_005427/TP73 | Tumor protein
p73 | P73 | Forward:
AGCAGCCCATCAAGGAGGAGTT
Reverse: TCCTGAGGCAGTTTTGGACACA |
| NM_000546/TP53 | Tumor protein p53
(Li-Fraumeni syndrome) | CYS51STOP/P53 | Forward:
AGATGGGGTCTCACAGTGTTGC
Reverse: ATGTTGACCCTTCCAGCTCCAC |
| NM_002392/MDM2 | MDM2 proto-oncogene,
E3 ubiquitin ligase | HDMX/MGC71221 | Forward:
GGGTTCGCACCATTCTCCTG
Reverse: GGCAGATGACTGTAGGCCAAGC |
|
NM_152999.3/STAMP1 | STEAP family member
2, metalloreductase (STEAP2), transcript variant 1 | STEAP2/STAMP1 | Forward:
ATAGGAAGTGGGGATTTTGC
Reverse: AGATGTCTCAGGTCCCACAA |
|
NM_024636.3/STAMP2 | STEAP family member 4
(STEAP4), transcript variant 1 | STEAP4/STAMP2 | Forward:
GCACTTACACTGCTTGC
Reverse: CAGTGGTCAAGCCAGTC |
| NM_002046/GAPDH |
Glyceraldehyde-3-phosphate
dehydrogenase | G3PD, GAPD | Forward:
CATTGCCCTCAACGACCACTTT
Reverse: GGTGGTCCAGGGGTCTTACTCC |
Cell lysis, protein extraction and
western blot analysis
For protein extraction, cells were grown on 60-mm
culture dishes (Orange Scientific, Braine-l’Alleud, Belgium) and
washed once with phosphate-buffered saline (PBS) prior to cell
lysis. Cells were resuspended in 250 μl modified
radioimmunoprecipitation assay (RIPA) lysis buffer (10 mM Tris Cl,
pH 8.0; 1% Triton X-100; 0.1% SDS; 0.1% Na deoxycholate; 1 mM EDTA;
1 mM EGTA; 140 mM NaCl) containing protease and phosphatase
inhibitors. Cells were collected from culture plates using a cell
scraper and were transferred to Eppendorf tubes. Cells were
incubated on ice for 1 h (with pipetting up/down every 10 min),
centrifuged at 14,000 × g for 30 min and the cleared supernatants
were then collected. The protein concentration was determined using
the Qubit Protein assay kit (Invitrogen Life Technologies) where
appropriate. SDS-PAGE and western blot analysis was performed under
standard conditions using 20 μg lysate per lane. Proteins were
separated on a 10% gel and transferred to a polyvinylidene
difluoride (PVDF) membrane (Amersham Pharmacia Biotech, Amersham,
UK) using a semi-dry transfer blotter (VWR International Ltd.,
Lutterworth, UK). The PVDF membrane was blocked with 10% dry milk
in PBS solution containing 0.1% Tween 20 (PBS-T) for 10 min.
Primary and secondary antibody incubations were performed using
PBS-T containing 0.5% dry milk at 4°C overnight. Membranes were
developed using enhanced chemiluminescence (ECL) plus reagent
(Amersham Pharmacia Biotech) for 5 min, and images were captured
using a FX7 dark room chemiluminescence camera (Vilber Lourmat,
Marne-la-Vallée, France). The antibodies used were mouse anti-human
NFκB (p50/p105) monoclonal antibody (sc-166588; Santa Cruz
Biotechnology) used at a dilution of 1:1,000 and mouse anti-human
β-actin monoclonal antibody (A5316; Sigma) used at a dilution of
1:20,000. The secondary antibody was mouse anti-rabbit IgG-HRP
polyclonal antibody (sc-2357; Santa Cruz Biotechnology) used at a
dilution of 1:10,000.
Statistical analysis
All results represent one of at least three
independent experiments with similar outcomes. All data are
expressed as the mean ± standard error of mean. One-way analysis of
variance (ANOVA) and Tukey post hoc test were used to compare
groups of data. P≤0.05 was considered to indicate a statistically
significant result. GraphPad Software, Version 4.03 (San Diego, CA,
USA) was used for the statistical analysis.
Results
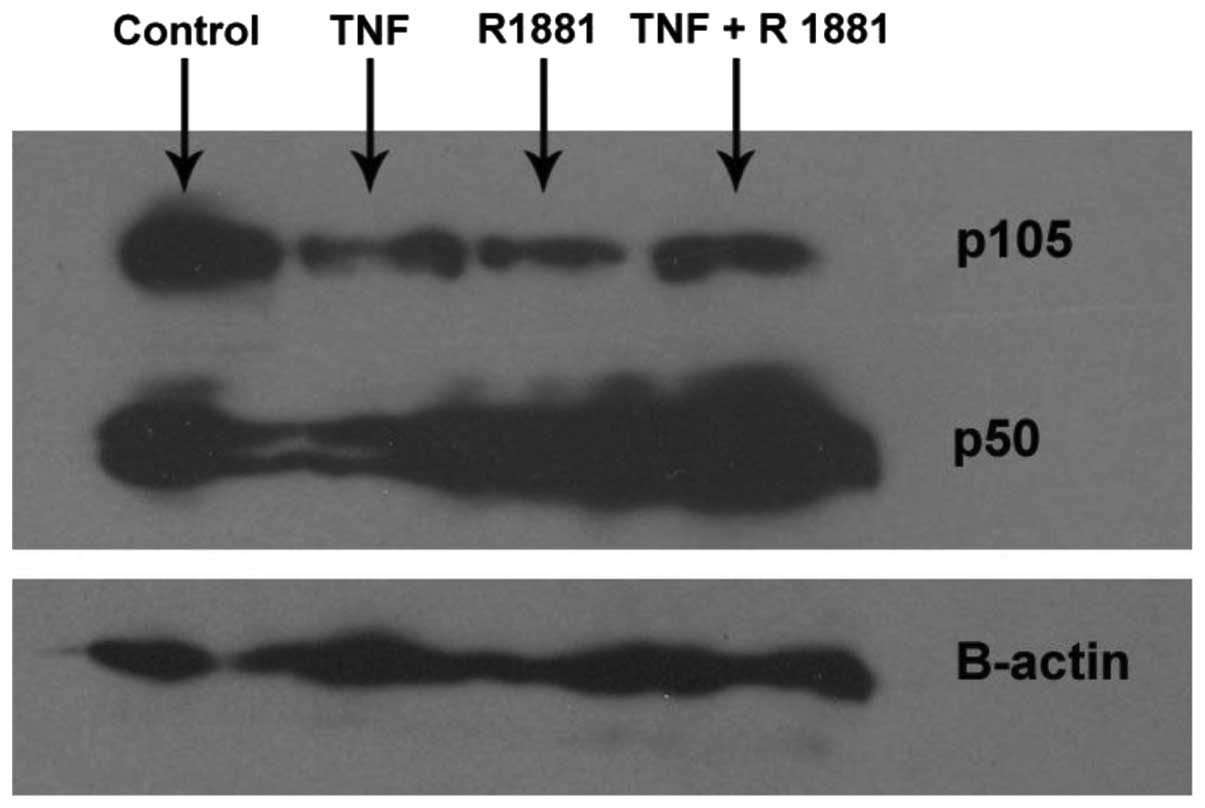
Effects of TNFα induction with or without
R1881 treatment on the expression of p50 and p105 in LNCaP
cells
LNCaP cells were treated with TNFα in the presence
or absence of R1881, which is a synthetic androgen analog. TNFα
induction, R1881 treatment and TNFα induction plus R1881 treatment
led to reductions in p105 expression levels. Treatment with TNFα
alone caused a slight reduction in the p50 expression level,
whereas R1881 treatment increased the protein expression level of
p50 in the presence or absence of TNFα (Fig. 1).
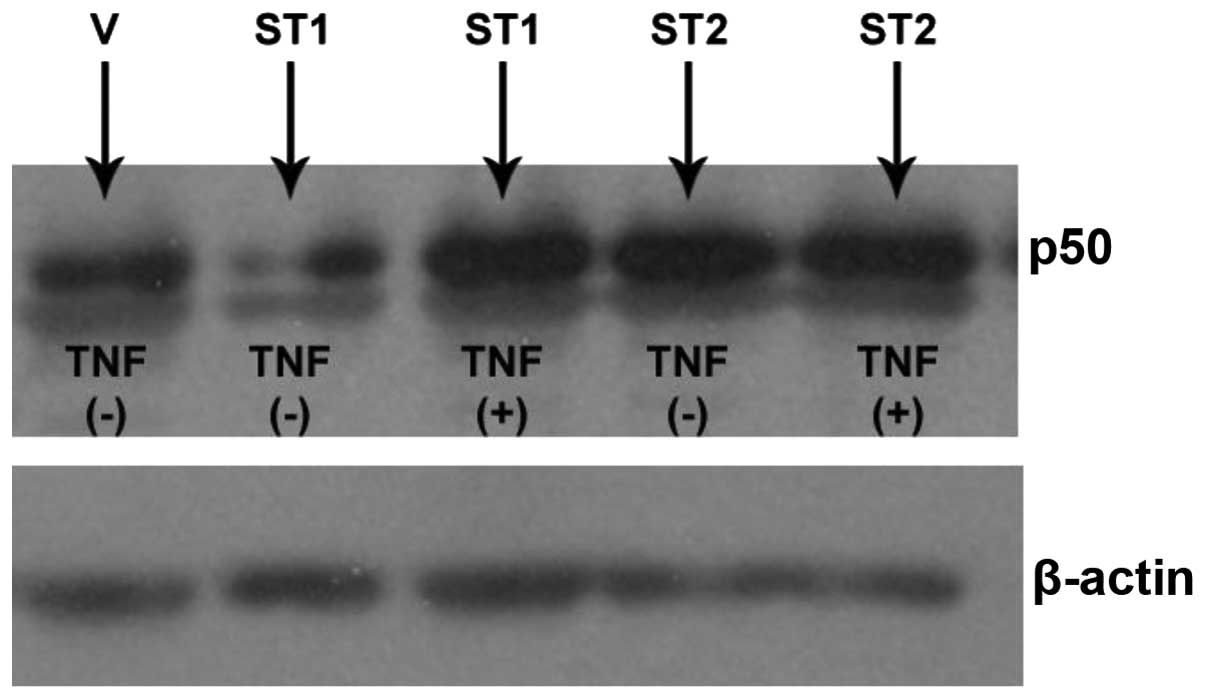
Effects of STAMP1 and STAMP2
transfections with or without TNFα induction on the expression of
p50 in DU145 cells
DU145 cells were transfected with HisMax-vector,
HisMax-STAMP1 or HisMax-STAMP2. The transfected cells were then
either induced by TNFα or were not induced. HisMAX-STAMP1
transfection decreased the expression level of p50. However, TNFα
induction following HisMAX-STAMP1 transfection led to an increase
in the expression level of p50. By contrast, HisMAX-STAMP2
transfection increased the expression level of p50, and TNFα
induction had no effect on the expression level of p50 in cells
transfected with HisMAX-STAMP2 (Fig.
2).
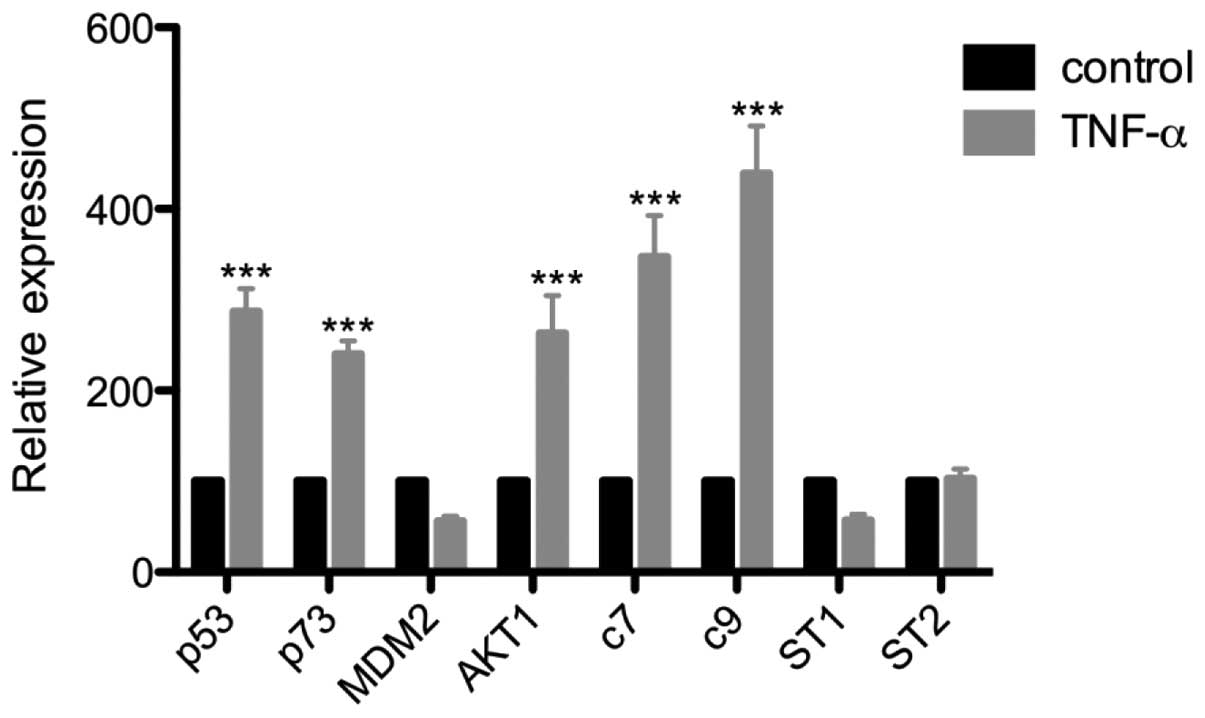
Effects of TNFα induction on
apoptosis-related gene expression in LNCaP cells
RT-qPCR amplifications were performed with a panel
of apoptosis-related primers following the induction of LNCaP cells
with TNFα. Induction with TNFα led to increases in the mRNA levels
of the apoptosis-related genes p53, p73, caspase 7 and caspase 9,
and the survival-related gene AKT1. Conversely, TNFα induction
tended to decrease the mRNA levels of MDM2 and STAMP1; however, the
reductions were not significant. The mRNA levels of STAMP2 were
unaffected by TNFα induction (Fig.
3).
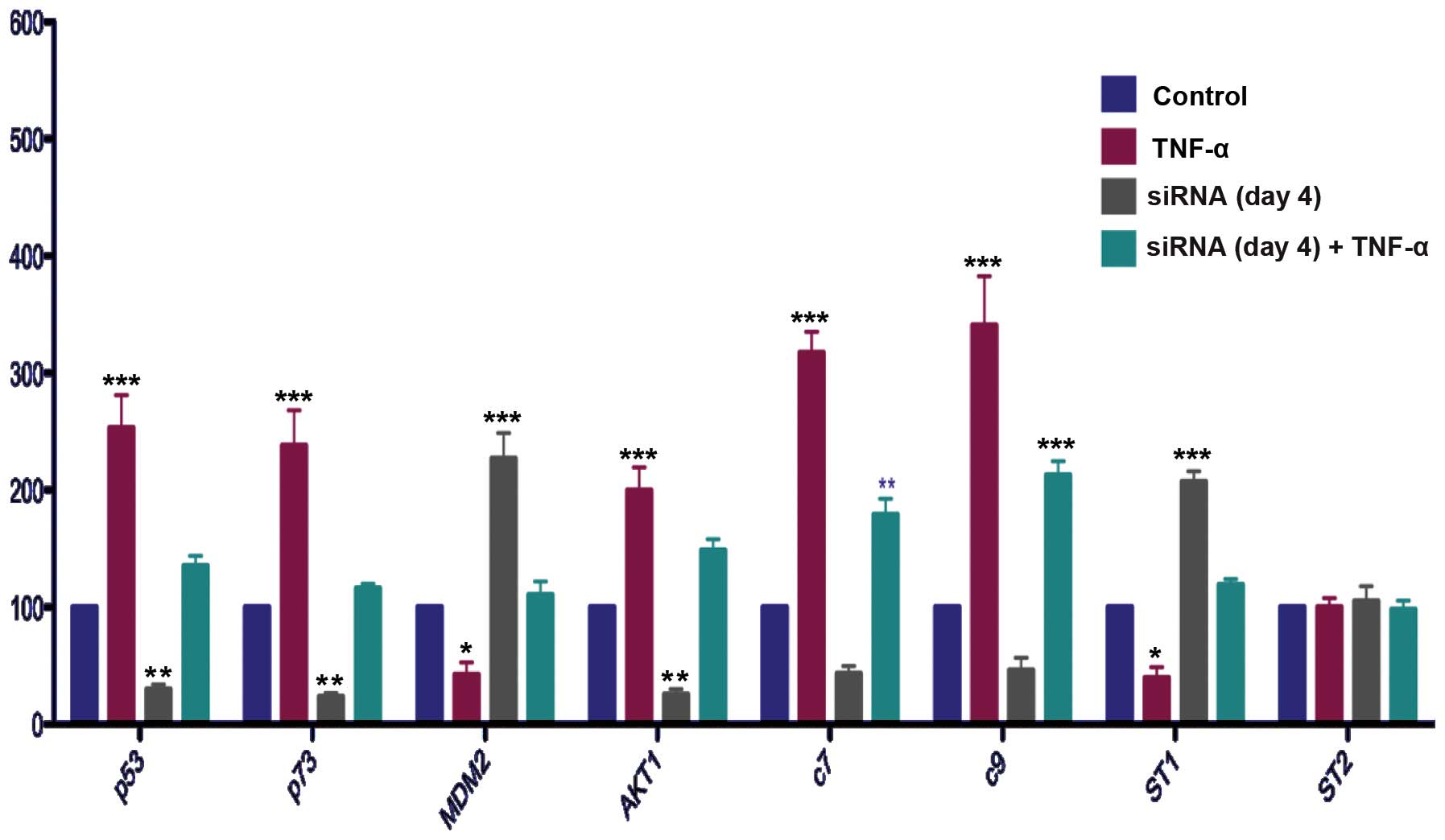
Effect of NFκB gene silencing with or
without TNFα induction on apoptosis-related gene expression in
LNCaP cells
Cells were transfected with siNFκB construct or
scrambled control for 1 or 4 days. The cells transfected for 1 day
were induced by TNFα or were not induced for a further 24 h in
serum medium. The cells transfected for 4 days were induced by TNFα
or not induced for 24 h at the third day of transfection.
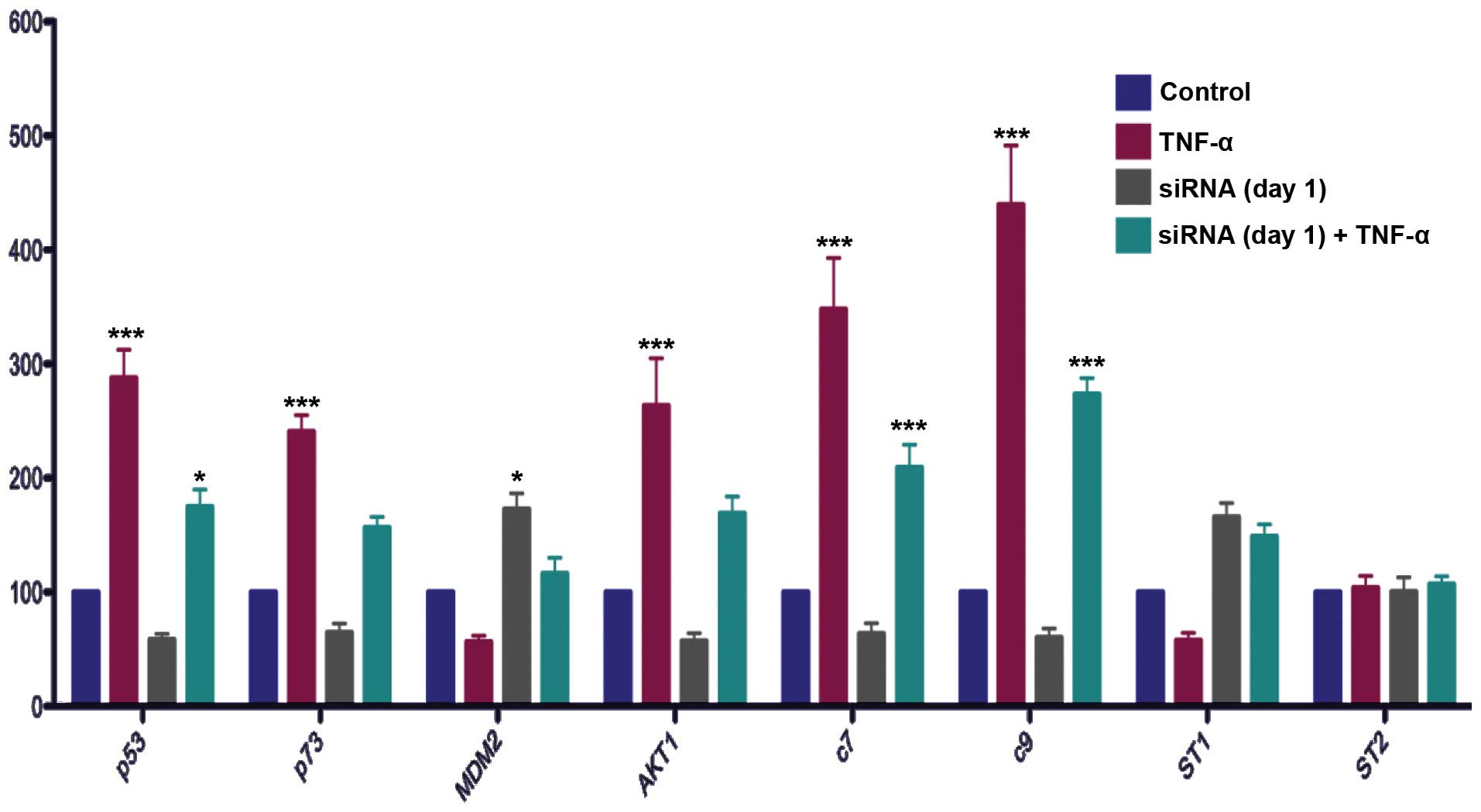
TNFα induction increased the mRNA levels of p53,
p73, AKT1 and caspases 7 and 9, and also tended to decrease the
mRNA levels of MDM2 and STAMP1 in the LNCaP cells transfected with
scrambled control (Figs. 4 and
5).
Silencing of the NFκB gene decreased the mRNA levels
of p53 (Fig. 5). NFκB gene
silencing also attenuated the effect of TNFα induction on the mRNA
levels of p53 at day 1 (Fig. 4).
Silencing of the NFκB gene inhibited the effect of TNFα induction
on the mRNA levels of p53 at day 4 (Fig 5).
The effects of NFκB gene silencing on p73 were
similar to those on p53. Specifically, NFκB gene silencing
decreased the mRNA levels of p73 and these results showed a
statistically significant difference between the scrambled control
and NFκB gene-silenced groups on day 4 (Fig. 5). In addition, NFκB gene silencing
inhibited the effect of NFκB induction on the mRNA levels of p73
(Figs. 4 and 5).
Notably, silencing the NFκB gene decreased the mRNA
levels of AKT1, which is known to be a survival gene, at day 4. In
addition, it inhibited the effect of TNFα induction on the mRNA
levels of AKT1 (Figs. 4 and
5).
Comparison of the MDM2 mRNA levels between the
scrambled control and NFκB gene-silenced groups showed that
silencing the NFκB gene increased the mRNA levels of MDM2 at both
transfection times (Figs. 4 and
5). Silencing the NFκB gene
inhibited the effect of TNFα on the mRNA levels of MDM2 on days 1
and 4 (Figs. 4 and 5).
The effects of NFκB gene silencing in the presence
or absence of NFκB induction on caspase 7 and 9 were also
investigated. Silencing the NFκB gene tended to decrease the mRNA
levels of caspase 7 and 9, although the reductions were not
statistically significant (Figs. 4
and 5). NFκB silencing decreased
the mRNA levels of caspase 7 and 9 in the TNFα-induced cells
(Figs. 4 and 5).
NFκB gene silencing increased the mRNA levels of
STAMP1 at day 4, and reversed the inhibitory effect of TNFα
induction on the mRNA levels of STAMP1 at day 4 (Fig. 5).
Neither silencing the NFκB gene nor TNFα induction
had any effect on the mRNA levels of STAMP2 (Figs. 4 and 5).
Discussion
Since, inflammation and cancer are closely related
disorders (9), NFκB is a topic of
particular interest to researchers (10). The activation of NFκB is generally
achieved by chronic exposure to TNFα (11). The activation results in the
altered expression of various genes (12), and also the constant expression of
TNF receptors R1 and R2 (data not shown). Altered gene expression
has been reported in the following cell lines: DU145, which has
constitutive NFκB expression, and LNCaP, which has TNF-inducible
NFκB expression (13). NFκB is a
heterodimeric or homodimeric complex formed from five different
subunits that are known as: RelA (p65), Rel B, c-Rel, NFκB1 (p50)
and NFκB2 (p52). p50 and p52 subunits are derivatives of large
precursor units p105 and p100, respectively. The classical NFκB
heterodimer consists of p65 and p50 (14). In the present study we focused on
p105 and one of its subunit, p50 (NFκB1). TNFα induction decreased
p105 and p50 expression. This result is consistent with previous
studies. The LNCaP cell line has androgen receptor expression and
is therefore responsive to R1881, an androgen analog. R1881
treatment decreased p105 expression, whereas it increased p50
expression. The DU145 cell line does not have an androgen receptor
and ST1 transfection decreased p50 expression. However, TNFα
diminished the effect of STAMP1 transfection and STAMP2
transfection increased p50 expression. Furthermore, TNFα induction
has no additional effect on p50 in ST2 transfected cells. These
results may indicate that further studies may reveal the
correlation between androgen stimulation and the survival gene NFκB
in prostate cancer. Additionally, STAMP1 and STAMP2 genes may have
different and opposite roles on NFκB signaling.
In order to investigate the potential interactions,
RT-qPCR amplifications using a panel of primers specific for
intrinsic apoptosis were conducted in the present study. Following
induction with TNFα, the mRNA levels of p53, which is a tumor
suppressor (15), and p73, which
is both a suppressor and supporter of cell growth (16), were found to increase. TNFα
induction also increased mRNA levels of AKT1, a survival gene.
Expression level changes of AKT1 have been previously revealed in
prostate cancer cell lines (17).
By contrast, the mRNA levels of MDM2, a ubiquitin ligase for p53
(18) and of STAMP1, identified as
a p53 negative regulator (unpublished data), were reduced. Caspases
7 and 9 each have distinct roles during intrinsic apoptosis
(19,20), and it was observed in the present
study that the mRNA levels of caspase 7 and 9 were increased by
treatment with TNFα. Silencing of NFκB almost completely inhibited
the effects of TNFα induction on the expression of apoptosis
related genes. This result implied that NFκB may play an important
role on the regulation of apoptosis-related genes in prostate
cancer. The activation of NFκB may cause chemoresistance in
chemotherapy regimens (21);
therefore, alternative reagents for inhibiting NFκB have been
investigated (22,23). Besides, the mRNA expression of
STAMP1 was also decreased by TNFα induction. To the best of our
knowledge, the present study is the first to reveal effect of TNFα
induction on STAMP1. Interestingly, NFκB gene silencing increased
STAMP1 expression. Regulation of STAMP1 gene expression may be
related to the NFκB pathway. Conversely, STAMP2 amplification was
not changed by either TNFα induction or NFκB silencing.
The androgen receptor (AR) is a member of the
steroid receptor superfamily and a transcription factor. The
response elements of prostate specific antigen (PSA) and NFκB are
located at the AR promoter region (24), and suggest that NFκB may effect AR
expression. The activation of AKT and NFκB is reported to be
involved in the progression of prostate cancer from androgen
dependence to independence (25,26).
These findings, in combination with previous observations (27) indicate that the effective
inhibition of NFκB may be critical in providing a targeted pathway
for the prevention of prostate cancer.
Acknowledgements
This study was supported by grants from The
Scientific and Technological Research Council of Turkey (TUBITAK)
to CGK (Grant no: 106S295) and The Turkish Academy of Sciences
(TUBA) to CGK (GEBIP-2007).
Abbreviations:
|
MDM2
|
E3 ubiquitin ligase
|
|
TNF
|
tumor necrosis factor
|
|
NFκB
|
nuclear factor κB
|
|
STEAP
|
six transmembrane epithelial antigen
of prostate
|
|
STAMP
|
six transmembrane protein of
prostate
|
References
|
1
|
Lorenzo PI, Arnoldussen YJ and Saatcioglu
F: Molecular mechanisms of apoptosis in prostate cancer. Crit Rev
Oncog. 13:1–38. 2007. View Article : Google Scholar
|
|
2
|
Hubert RS, Vivanco I, Chen E, Rastegar S,
Leong K, Mitchell SC, Madraswala R, et al: STEAP: a
prostate-specific cell-surface antigen highly expressed in human
prostate tumors. Proc Natl Acad Sci USA. 96:14523–14528. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korkmaz KS, Elbi C, Korkmaz CG, Loda M,
Hager GL and Saatcioglu F: Molecular cloning and characterization
of STAMP1, a highly prostate-specific six transmembrane protein
that is overexpressed in prostate cancer. J Biol Chem.
277:36689–36696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korkmaz CG, Korkmaz KS, Kurys P, Elbi C,
Wang L, Klokk TI, Hammarstrom C, et al: Molecular cloning and
characterization of STAMP2, an androgen-regulated six transmembrane
protein that is overexpressed in prostate cancer. Oncogene.
24:4934–4945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wellen KE, Fucho R, Gregor MF, Furuhashi
M, Morgan C, Lindstad T, Vaillancourt E, et al: Coordinated
regulation of nutrient and inflammatory responses by STAMP2 is
essential for metabolic homeostasis. Cell. 129:537–548. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steiner MS, Zhang X, Wang Y and Lu Y:
Growth inhibition of prostate cancer by an adenovirus expressing a
novel tumor suppressor gene, pHyde. Cancer Res. 60:4419–4425.
2000.PubMed/NCBI
|
|
7
|
Passer BJ, Nancy-Portebois V, Amzallag N,
Prieur S, Cans C, Roborel de Climens A, et al: The p53-inducible
TSAP6 gene product regulates apoptosis and the cell cycle and
interacts with Nix and the Myt1 kinase. Proc Natl Acad Sci USA.
100:2284–2289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang L, Jin Y, Arnoldussen YJ, Jonson I,
Qu S, Maelandsmo GM, Kristian A, et al: STAMP1 is both a
proliferative and an antiapoptotic factor in prostate cancer.
Cancer Res. 70:5818–5828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hussain SP and Harris CC: Inflammation and
cancer: an ancient link with novel potentials. Int J Cancer.
121:2373–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morgan MJ and Liu Z: Crosstalk of reactive
oxygen species and NF-κB signaling. Cell Res. 21:103–115. 2011.
|
|
11
|
Morgan MJ, Kim YS and Liu ZG: TNFalpha and
reactive oxygen species in necrotic cell death. Cell Res.
18:343–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jablonska E, Piotrowski L and Grabowska Z:
Serum levels of IL-1β, IL-6, TNF-α, sTNFR1 and CRP in patients with
oral cavity cancer. Pathol Oncol Res. 3:126–129. 1997.
|
|
13
|
Mukhopadhyay A, Bueso-Ramos C, Chatterjee
D, Pantazis P and Aggarwal BB: Curcumin downregulates cell survival
mechanisms in human prostate cancer cell lines. Oncogene.
20:7597–7609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Kandasamy K and Srivastava RK:
Differential roles of RelA (p65) and c-Rel subunits of nuclear
factor kappa B in tumor necrosis factor-related apoptosis-inducing
ligand signaling. Cancer Res. 63:1059–1066. 2003.PubMed/NCBI
|
|
15
|
Galluzzi L, Morselli E, Kepp O, Tajeddine
N and Kroemer G: Targeting p53 to mitochondria for cancer therapy.
Cell Cycle. 7:1949–1955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vikhanskaya F, Toh WH, Dulloo I, Wu Q,
Boominathan L, Ng HH, Vousden KH and Sabapathy K: p73 supports
cellular growth through c-Jun-dependent AP-1 transactivation. Nat
Cell Biol. 9:698–705. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deeb D, Jiang H, Gao X, Al-Holou S,
Danyluk AL, Dulchavsky SA and Gautam SC: Curcumin
[1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione;
C21H20O6] sensitizes human
prostate cancer cells to tumor necrosis factor-related
apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing
nuclear factor-kappaB via inhibition of the prosurvival Akt
signaling pathway. J Pharmacol Exp Ther. 321:616–625. 2007.
|
|
18
|
Wadgaonkar R and Collins T: Murine double
minute (MDM2) blocks p53-coactivator interaction, a new mechanism
for inhibition of p53-dependent gene expression. J Biol Chem.
274:13760–13767. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamkanfi M and Kanneganti TD: Caspase-7: a
protease involved in apoptosis and inflammation. Int J Biochem Cell
Biol. 42:21–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brentnall M, Rodriguez-Menocal L, Ladron
De Guevara R, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bharti AC and Aggarwal BB: Nuclear
factor-kappa B and cancer: its role in prevention and therapy.
Biochem Pharmacol. 64:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bharti AC and Aggarwal BB: Chemopreventive
agents induce suppression of nuclear factor-kappaB leading to
chemosensitization. Ann NY Acad Sci. 973:392–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casanelles E, Gozzelino R,
Marqués-Fernández F, Iglesias-Guimarais V, Garcia-Belinchón M,
Sánchez-Osuna M, Solé C, et al: NF-κB activation fails to protect
cells to TNFα-induced apoptosis in the absence of Bcl-xL, but not
Mcl-1, Bcl-2 or Bcl-w. Biochim Biophys Acta. 1833:1085–1095.
2013.
|
|
24
|
Zhang L, Charron M, Wright WW, Chatterjee
B, Song CS, Roy AK and Brown TR: Nuclear factor-kappaB activates
transcription of the androgen receptor gene in Sertoli cells
isolated from testes of adult rats. Endocrinology. 145:781–789.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murillo H, Huang H, Schmidt LJ, Smith DI
and Tindall DJ: Role of PI3K signaling in survival and progression
of LNCaP prostate cancer cells to the androgen refractory state.
Endocrinology. 142:4795–4805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kikuchi E, Horiguchi Y, Nakashima J,
Kuroda K, Oya M, Ohigashi T, Takahashi N, et al: Suppression of
hormone-refractory prostate cancer by a novel nuclear factor kappaB
inhibitor in nude mice. Cancer Res. 63:107–110. 2003.PubMed/NCBI
|
|
27
|
Sun HZ, Yang TW, Zang WJ and Wu SF:
Dehydroepiandrosterone-induced proliferation of prostatic
epithelial cell is mediated by NFKB via PI3K/AKT signaling pathway.
J Endocrinol. 204:311–318. 2010. View Article : Google Scholar : PubMed/NCBI
|