Introduction
Infections that occur in the wound created by an
invasive surgical procedure are generally referred to as surgical
site infections (SSIs). SSI is one of the most common and serious
postoperative complications, occurring in up to 40% of patients
undergoing abdominal surgery and 5% of all patients undergoing
other surgery, depending on the degree of contamination (1,2). SSI
is one of the most significant causes of healthcare-associated
infection, and may prolong hospitalization by 5–20 days and
substantially increase the cost of healthcare (3–4). It
is associated with substantial morbidity and it has been reported
that over one-third of postoperative mortality is associated, at
least in part, with serious SSI (5).
However, prevalence studies tend to underestimate
SSI, since the majority of these infections occur after the patient
has been discharged from hospital. It should be noted that the
diagnosis covers a large variety of clinical conditions ranging
from a relatively trivial wound discharge with no other
complications to a life-threatening condition. Other complications
of SSI include scars that are cosmetically unacceptable, such as
keloid scars, itching, persistent pain and a significant impact on
emotional wellbeing (6).
Recent national guidelines concerning the prevention
and treatment of SSI have been issued by the National Institute for
Health and Clinical Excellence of the UK (7). The poor clinical outcomes of SSI
require extra nursing care, intervention and re-operation, and
consequently have a high direct or indirect cost. However, various
strategies for the treatment of SSI have been tested and employed
in the clinic, and the consequences of these treatments have not
always been satisfactory for patients or surgeons (3,4). A
persistent effort has been made to find a more effective and safer
method to treat SSI, such as debridement or drainage (4,6). In
this study, we conduct a randomized control trial to evaluate the
effectiveness and safety of endoscopy for the treatment of SSI
compared with conventional surgery therapy.
Materials and methods
Patients
From May 2005 to June 2012, we enrolled patients
with different types of SSI at the Tianjin Binhai New Area Dagang
Hospital (Tianjin, China) in a prospective, randomized trial to
compare clinical results and complications between the endoscopy
procedure and traditional open surgery for the debriding of the
infected wound.
The inclusion criteria were as follows: SSI patients
i) who had undergone surgery >30 days ago; and ii) in which
non-surgical treatment was ineffective after SSI. The exclusion
criteria were as follows: i) <18 years old; ii) immune system
disorder-related disease; and iii) prosthetic implant in the
surgical site. Full ethical approval was obtained from Tianjin
Binhai New Area Dagang Hospital and all participants provided
written informed consent.
To match the two groups, randomization was
stratified according to the body regions of the primary surgery
(Table I). Subsequently,
randomization was performed when the patient was in the anesthetic
room immediately before surgery by using the sealed envelope
method. The surgical team in theatre were aware of group
allocation, but only following the induction of anesthesia.
Information on group allocation was not recorded in the clinical or
surgical notes, and clinicians undertaking the follow-up of
postoperative wounds were fully blinded to the group
assignments.
 | Table IBaseline characteristics of the two
groups of patients. |
Table I
Baseline characteristics of the two
groups of patients.
| Characteristics | Group A | Group B | P-value |
|---|
| Number of
patients | 57 | 49 | |
| Age (years) | 35.5±5.5 | 34.8±7.2 | 0.95 |
| Male:female
ratio | 37:20 | 30:19 | 0.90 |
| Body mass index | 30.4±3.2 | 28.3±4.4 | 0.87 |
| Primary surgery | | | 0.76 |
| Chest surgery | 5 | 3 | |
| Abdomen surgery | 33 | 27 | |
| Limb surgery | 7 | 7 | |
| Lumbar surgery | 8 | 6 | |
| Other | 4 | 6 | |
| Size of infected
site, cm | | | 0.67 |
| ≤5 | 10 | 8 | |
| 5–10 | 26 | 23 | |
| 10–15 | 13 | 10 | |
| ≥15 | 8 | 8 | |
Interventions (surgical techniques)
In the endoscopy procedure group (group A), the
surgical field was sterilized conventionally using sterile towels.
A section of the wound approximately 6 mm long was opened along the
original incision to permit the entry of the choledochoscope
(Olympus T20, Tokyo, Japan). The wound was washed and cleaned with
sterile saline. The necrotic tissue and infected suture were
completely removed under visualization. For sinuses too small to
accommodate the choledochoscope, the necrosis was removed by biopsy
clip. Instead of inserting a drainage tube for several days, a
saline gauze was used to drain the wound for no longer than 24
h
In the traditional surgery group (group B), the
surgical field was sterilized conventionally, as in group A, using
sterile towels. Subsequently, the wound was opened through the
original incision, washed and cleaned with sterile saline, then
drained adequately via negative pressure drainage. A suture was
performed 2 days later. In both groups, an antibiotic prophylaxis
was administered intravenously 30 min before the surgical
incision.
Outcome measures
The primary outcome of interest was the wound
healing time. The secondary outcomes of interest were duration of
surgery, blood loss, pain level 7 days after surgery [measured by
the visual analog scale (VAS)], volume of irrigation saline, rate
of skin transplantation and length of hospital stay.
Statistical analysis
All analyses were performed using SPSS statistical
software (version 17.0). Student’s t-test and the χ2
test were performed to analyze the data and determine whether there
were differences between the two groups. P<0.05 was considered
to indicate a statistically significant difference.
Results
Study participants
From May 2005 to June 2012, we enrolled 106 patients
with serious SSI in our study and randomized 57 to the endoscopy
procedure group (group A) and 49 to the traditional surgery group
(group B). No patients were lost during the follow-up period.
Patients’ baseline demographical characteristics (age and gender
ratio), primary surgery characteristics and size of the infected
sites did not differ substantially between the two groups (Table I).
Outcomes
No significant difference between the groups was
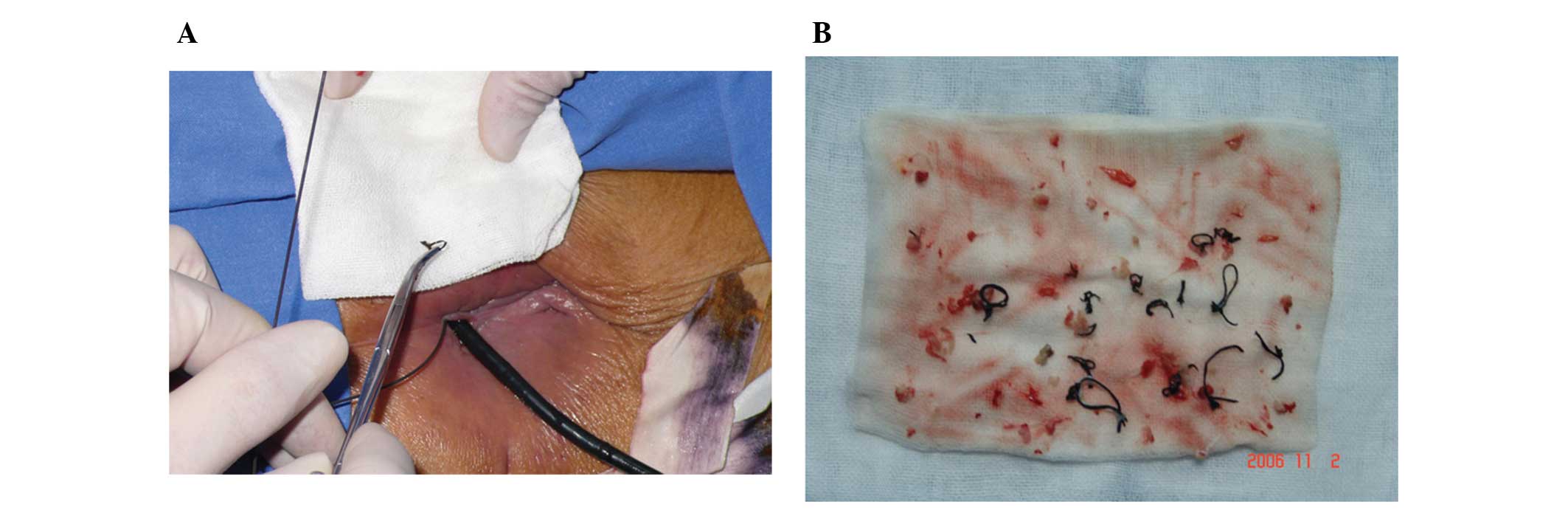
noted with respect to the duration of surgery (P=0.06). Three
patients in group A (Fig. 1) and
six in group B required a skin transplant for skin defects, but the
rate of skin transplantation was not statistically different
between the two groups (P=0.21; Table
II). Patients in group A had a mean intra-operative blood loss
of 327±89 ml, which was significantly reduced [mean difference
(MD), −78.00; 95% CI, −132.68 to −23.32; P=0.005] when compared
with that of group B (405±177 ml). The intra-operative volume of
irrigation saline was lower in group A compared with that in group
B, and the difference was statistically significant (MD, −770.00;
95% CI, −1082.82 to −457.18; P<0.00001).
 | Table IIOutcomes of the treatment groups. |
Table II
Outcomes of the treatment groups.
| Outcomes | Group A | Group B | MD and 95% CI | P-value |
|---|
| Duration of surgery
(min) | 128±56 | 104±72 | 24.00 (−0.85 to
48.85) | 0.06 |
| Blood loss (ml) | 327±89 | 405±177 | −78.00 (−132.68 to
−23.32) | 0.005 |
| Wound healing
(days) | 10.0±2.5 | 19.4±5.2 | −9.40 (−10.99 to
−7.81) | <0.00001 |
| Rate of skin
transplantation | 3/57 | 6/49 | 0.40 (0.09 to
1.68) | 0.21 |
| VAS 7 days
post-surgery | 3.2±1.5 | 5.5±1.1 | −2.30 (−2.80 to
−1.80) | <0.00001 |
| Saline volume of
irrigation (ml) | 3880±1205 | 4650±909 | −770.00 (−1082.82 to
−457.18) | <0.00001 |
| Length of hospital
stay (days) | 15±4.1 | 22.5±2.3 | −7.50 (−8.74 to
−6.26) | <0.00001 |
The mean wound healing time was also significantly
shorter (MD, −9.40; 95% CI, −10.99 to −7.81; P<0.00001) in group
A (10.0±2.5 days) than in group B (19.4±5.2 days). The mean VAS
score 7 days after surgery in group A was significantly lower than
that of group B (MD, −2.30; 95% CI, −2.80 to −1.80; P<0.00001).
The length of the hospital stay in group A was significantly
shorter than that in group B (MD, −7.50; 95% CI, −8.74 to −6.26;
P<0.00001).
All the patients enrolled were followed up for at
least 4 weeks following surgery, and there were no clinical
complications in either group during this period.
Discussion
Since skin is normally colonized by a range of
microorganisms that may cause infection, defining an SSI requires
evidence of clinical signs and symptoms of infection rather than
microbiological evidence alone. Frequently, SSIs only affect
superficial tissues, but certain more serious infections affect the
deeper tissues or other parts of the body or organs manipulated
during the procedure. The majority of SSIs become apparent within
30 days of an operative procedure, and most often between the fifth
and tenth day. However, where a prosthetic implant is employed,
SSIs affecting the deeper organs or tissues may occur several
months or more after surgery (8,9).
Under this context, our study included SSI patients who were more
than 30 days after the primary surgery and excluded patients with
any prosthetic implant.
Surveillance of SSI provides data that does not
inform but influences clinical practice to decrease the risk of
SSI, as well as communicating the risks of infection to patients
more clearly. Since certain SSIs may take several days or longer to
develop, signs of infection may not become apparent until after the
patient has been discharged from hospital (10). Surveillance focused on detecting
SSI during the inpatient stay is thus likely to underestimate the
true rate of SSI. This issue is exacerbated by the increasing trend
towards shorter lengths of postoperative hospital stay (11).
For various types of surgery, it is known that the
risk of SSI is affected by the specific site of the surgery; for
example, laminectomy at the cervical vertebrae is associated with a
lower risk of SSI than laminectomy performed at other levels (OR,
6.7; 95% CI, 1.4 to 33.3) (12).
The complexity and duration of the procedure are also indicated as
risk factors of SSI. Studies of general and vascular surgery
estimated that there was a two- to three-fold increased risk of SSI
with increasing surgical complexity, measured as work relative
value units (13–15). These studies noted that particular
attention should be paid to operative patients with high risk
factors of SSI. Once SSI is recognized, it should be treated
promptly and effectively. Conventional treatment of SSI includes
changing dressings, administration of antibiotics, removal of
sutures, drainage of pus and irrigation of the wound with saline.
Minor infections may respond sensitively to these treatments;
however, serious cases of SSI may become exacerbated.
In serious SSI cases, the commonly used strategy is
re-operation to open and clean the wound with sterile saline, but
this is not always effective. Experts and researchers are making
good progress in exploring novel and effective approaches for the
treatment of SSI. In the present trial, an endoscopy procedure was
employed for the treatment of SSI. The endoscopy procedure is in
widespread use in the field of surgery and is a procedure favored
by many surgeons. The procedure has advantages including minimal
invasion, low risk and high cost-effectiveness. However, there are
few studies in the literature reporting this minimally invasive
procedure as a treatment for SSI.
The purpose of this randomized trial was to examine
the clinical effectiveness of using endoscopy for the treatment of
SSI in wound healing. Unlike in conventional surgery for SSI, we
were able to completely remove the necrotic tissue and infected
suture in visualization under the endoscopy procedure. For small
sinuses that could not be reached by endoscopy, the necrosis was
removed by biopsy clip (16,17).
The results demonstrated that endoscopy decreased the wound healing
time, the rate of skin transplantation, the blood loss and the
length of hospital stay. Subsequently, the cost of treatment for
the endoscopy group was significantly lower than that for the
conventional surgery group. In addition, the patients in group A
experienced less pain than those in group B. During the follow-up
period, there were no clinical complications in either group.
In conclusion, the present study demonstrated that
the endoscopy procedure for the treatment of SSI reduces the wound
healing time compared with traditional surgery, without increasing
the risk of any clinical events. Endoscopy was not only effective
but also safe in the therapy of serious SSI. However, a further
randomized control trial is necessary to determine the
effectiveness and safety for the treatment of serious SSI.
References
|
1
|
Smyth ET, McIlvenny G, Enstone JE,
Emmerson AM, Humphreys H, Fitzpatrick F, et al: Four country
healthcare associated infection prevalence survey 2006: overview of
the results. J Hosp Infect. 69:230–248. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruce J, Russell EM, Millinson J and
Krukowksi ZH: The measurement and monitoring of surgical adverse
events. Health Tech Assess. 5:1–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coello R, Charlett A, Wilson J, Ward V,
Pearson A and Borriello P: Adverse impact of surgical site
infections in English hospitals. J Hosp Infect. 60:93–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanner J, Khan D, Aplin C, Ball J, Thomas
M and Bankart J: Post-discharge surveillance to identify colorectal
surgical site infection rates and related costs. J Hosp Infect.
72:243–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Astagneau P, Rioux C, Golliot F and
Brücker G; INCISO Network Study Group. Morbidity and mortality
associated with surgical site infections: results from the
1997–1999 INCISO surveillance. J Hosp Infect. 48:267–274. 2001.
|
|
6
|
Wilson AP, Gibbons C, Reeves BC, et al:
Surgical wound infection as a performance indicator: agreement of
common definitions of wound infection in 4773 patients. BMJ.
329:7202004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bayat A, McGrouther DA and Ferguson MW:
Skin scarring. Br Med J. 326:88–92. 2003. View Article : Google Scholar
|
|
8
|
Barie PS and Eachempati SR: Surgical site
infections. Surg Clin North Am. 85:1115–1135. 2005. View Article : Google Scholar
|
|
9
|
Chang WK, Srinivasa S, MacCormick AD and
Hill AG: Gentamicin-collagen implants to reduce surgical site
infection: systematic review and meta-analysis of randomized
trials. Ann Surg. 258:59–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dieter AA, Amundsen CL, Edenfield AL,
Kawasaki A, Levin PJ, Visco AG and Siddiqui NY: Oral antibiotics to
prevent postoperative urinary tract infection: a randomized
controlled trial. Obstet Gynecol. 123:96–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mannien J, Wille JC, Snoeren RL and van
der Hof S: Impact of postdischarge surveillance on surgical site
infection rates for several surgical procedures: results from the
nosocomial surveillance network in The Netherlands. Infect Control
Hosp Epidemiol. 27:809–816. 2006. View
Article : Google Scholar
|
|
12
|
Friedman ND, Sexton DJ, Connelly SM and
Kaye KS: Risk factors for surgical site infection complicating
laminectomy. Infect Control Hosp Epidemiol. 28:1060–1065. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neumayer L, Hosokawa P, Itani K, et al:
Multivariable predictors of postoperative surgical site infection
after general and vascular surgery: results from the patient safety
in surgery study. J Am Coll Surg. 204:1178–1187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaye KS, Schmit K, Pieper C, et al: The
effect of increasing age on the risk of surgical site infection. J
Infect Dis. 191:1056–1062. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Russo PL and Spelman DW: A new
surgical-site infection risk index using risk factors identified by
multivariate analysis for patients undergoing coronary artery
bypass graft surgery. Infect Control Hosp Epidemiol. 23:372–376.
2002. View
Article : Google Scholar
|
|
16
|
Ridgeway S, Wilson J, Charlet A, et al:
Infection of the surgical site after arthroplasty of the hip. J
Bone Joint Surg Br. 87:844–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rashid A, Nazir S, Kakroo SM, Chalkoo MA,
Razvi SA and Wani AA: Laparoscopic interval appendectomy versus
open interval appendectomy: a prospective randomized controlled
trial. Surg Laparosc Endosc Percutan Tech. 23:93–96. 2013.
View Article : Google Scholar : PubMed/NCBI
|