Introduction
Chronic renal failure (CRF) is chronic progressive
renal parenchyma damage caused by a variety of chronic kidney and
kidney systemic diseases. In CRF, the kidneys are unable to
discharge metabolic waste and regulate the water-salt and acid-base
balance, hormone secretion, hormone metabolism and other functions,
resulting in nitrogen qualitative hematic disease, metabolic
disorders and a series of clinical syndromes (1–4). A
previous study revealed that the number of potential CRF patients
is increasing worldwide (5). With
the exception of renal replacement therapy and kidney
transplantation, no other satisfactory treatments exist for CRF
(6–9). Alternative therapies, including
hemodialysis and peritoneal dialysis, are unable to improve the
pathological damage of kidney tissues. In addition, renal
transplantation is limited by the lack of kidney resources, the
expense and high risk of transplant rejection. Therefore, the
development of new medications that inhibit renal failure
progression and reduce the morbidity and mortality rates of CRF is
essential. Traditional Chinese medicine (TCM) is widely used to
treat various chronic diseases in China and Southeast Asia
(10), and the effective
prescription of TCM treatment for CRF has been increasingly
investigated.
Shenkang granules (SKGs) comprise a Chinese herbal
medicinal formula of rhubarb (Rheum palmatum L.), Salvia
miltiorrhiza, milkvetch root [Astragalus membranaceus
(Fisch.) Bunge] and safflower (Carthamus tinctorius L.). SKG
has the same components as Shenkang injection (SKI), which is a
commercially available treatment for CRF in China. Previous
pharmacological studies have revealed that SKI administration
alleviates the renal pathological lesions of 5/6 nephrectomized
(5/6 Nx) rats and delays CRF progression (11,12).
Clinical studies have also demonstrated that SKI reduces the levels
of microalbumin (mALB), total protein (U-TP), serum creatinine
(Scr) and blood urea nitrogen (BUN), and increases the nitric oxide
(NO) level, creatinine clearance rate (Ccr) and serum albumin (Alb)
level in CRF patients. In addition, SKI was shown to improve
clinical symptoms and delay the progress of CRF (13,14).
However, the injection is inconvenient and painful, and the
production process is complex and expensive. In addition, the
direct injection of SKI into the blood results in higher toxicity
and the risk of side-effects. Thus, oral medication is considered
to be more convenient and safe compared with the injections.
The beneficial role of angiotensin-converting enzyme
inhibitor (ACEI) drugs has been validated in human and experimental
models of renal failure (15,16). In
the present study, the effect of SKG on CRF was compared with that
of benazepril, which is a commonly used ACEI drug with a proven
efficacy (17). Therefore, the
inhibitory effect of SKG in 5/6 Nx rats suffering from CRF was
investigated using SKI and benazepril as the positive controls.
Materials and methods
Drugs
SKG and SKI were obtained from Xi'an Century
Shengkang Pharmaceutical Industry Co., Ltd. (Xi'an, China).
Benazepril was purchased from Beijing Novartis Pharma Co., Ltd.
(Beijing, China).
Animal model and experimental
groups
In total, 105 male Sprague-Dawley rats (weight,
180–240 g) were purchased from the Animal Center of Xi'an Jiaotong
University (Xi'an, China). The rats were housed at room
temperature, with a 12-h light/dark cycle and free access to tap
water and standard rat chow. The rats were cared for in accordance
with the principles of the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health). All the experiments were
approved by the Institutional Animal Investigation Committee of
Xi'an Jiaotong University. The rats were fed a normal diet one week
prior to the surgery in order to acclimatize to the laboratory
environment. CRF was induced in the rats by a 5/6 nephrectomy that
was performed under aseptic conditions. All surgical procedures
were carried out under 10% chloral hydrate anesthesia (3.5 ml/kg;
intraperitoneal). A left flank incision was made to expose the left
kidney. The renal artery was temporarily occluded, and the upper
and lower thirds of the kidney were ligated and resected. Bleeding
was controlled by compression. Thus, one-third of the left kidney
mass remained. The muscle and skin incisions were sutured with a
polypropylene suture. In total, 15 rats were randomly selected as
the sham-operated group that underwent the same procedure, but
without a nephrectomy. The animals were returned to the cages for
recovery and were injected daily with penicillin G (8,000,000
units; Harbin Pharmaceutical Group Co., Ltd., Harbin, China) for
five days. After two weeks, a right flank incision was made, the
renal vessels were tied, and the right kidney was excised. The
sham-operated group underwent the same surgery, without excision of
the kidney (15). Following suture
of the muscle and skin, the rats were returned to the cages and
were injected with penicillin daily for five days.
Four weeks after surgery, 2-ml blood samples were
collected from the eyeball venous plexus of the rats. The sera were
separated by 10-min centrifugation at 1,500 × g and 4°C. The levels
of BUN and Scr were detected using the Hitachi 7600 automated
biochemistry analyzer (Hitachi, Ltd., Tokyo, Japan) at the
Laboratory of the First Affiliated Hospital (School of Medicine,
Xi'an Jiaotong University). According to the levels of BUN and Scr,
the rats were randomly divided into seven groups (n=10 per group):
(i) 5/6 Nx (model group; intragastrically treated with SKG vehicle;
2.25 ml/kg/day normal saline); (ii) SKGL (low dose; 2 g crude
drug/kg/day SKG); (iii) SKGM (moderate dose; 4 g crude drug/kg/day
SKG); (iv) SKGH (high dose; 8 g crude drug/kg/day SKG); (v)
benazepril treatment group (5 mg/kg/day benazepril); (vi) SKI group
(13.3 ml/kg/day SKI); and (vii) sham-operated group (2.25 ml/kg/day
normal saline). Drugs and vehicle were administered by gastrogavage
once per day for 30 days, and the rats were weighed once per
week.
mALB and U-TP
Prior to and following the treatment period, the
rats were placed in metabolic cages (Shanghai Kangway Medical
Science and Technology Development Co., Ltd., Shanghai, China) for
24 h urine collection. The urine samples were centrifuged at 1,000
× g for 5 min and filtrated through a 2.2-µm membrane (Chekiang
Haining Hengtai Filter Equipment Factory, Chekiang, China). The
concentrations of mALB and U-TP in the urine samples were detected
at the Laboratory of the First Affiliated Hospital (School of
Medicine, Xi'an Jiaotong University).
Determination of Scr, BUN, total
cholesterol (CH), triglyceride (TG), low-density lipoprotein (LDL)
and glutathione peroxidase (GSH-PX) levels
At the end of the treatment period, the rats were
anesthetized by an injection of 10% chloral hydrate (3.5 ml/kg),
and blood samples were collected from the aorta ventralis. Sera
were obtained following 10-min centrifugation at 1,500 × g and 4°C,
and were frozen at −80°C until required for further analysis. Scr,
BUN, CH, TG and LDL levels were detected at the Laboratory of the
First Affiliated Hospital (School of Medicine, Xi'an Jiaotong
University). GSH-PX levels were detected using a GSH-PX biochemical
assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China).
Serum transforming growth
factor-β1 (TGF-β1) and angiotensin II (Ang
II)
A rat TGF-β1 ELISA kit (Shanghai Westang
Bio-Tech Co., Ltd., Shanghai, China) was used to determine the
TGF-β1 level in the rat serum. In a glass tissue
grinder, the rat kidneys were homogenized for 30 sec in prechilled
methanol. The homogenate was centrifuged at 4°C for 20 min at
13,000 × g, and the supernatant was obtained. Serum and kidney
levels of Ang II were detected using an Ang II radioimmunoassay kit
(Beijing Sino-UK Institute of Biological Technology, Beijing,
China).
Body weight, kidney weight/body weight
ratio (KW/BW) and heart weight index (HWI)
During the treatment period, the rats were weighed
once per week. At the end of the treatment period, the rats were
weighed and anesthetized using the aforementioned procedure.
Subsequently, the remnant left kidney and the heart were excised
and weighed, and the KW/BW and HWI were calculated.
Histology
To observe the renal lesions the
paraformaldehyde-fixed kidneys were embedded in paraffin and cut
into 4-µm sections, the sectioned kidney samples were then stained
with hematoxylin and eosin. In addition, the kidney sections were
stained with Masson's trichrome to measure the fibrotic areas. The
images were captured and viewed using an image analysis system
(Leica Q550CW; Leica Microsystems GmbH, Wetzlar, Germany).
Western blot analysis
For western blot analysis, the rat kidney samples
were lysed in radioimmunoprecipitation assay lysis buffer,
containing 0.1 M phenylmethylsulfonyl fluoride, and centrifuged at
13,000 × g for 20 min. The total protein concentrations of the
supernatants were determined using a BCA Protein Assay kit
(Applygen Technologies, Inc., Beijing, China). Extracts were boiled
at a 1:1 ratio with a loading buffer containing Tris (125 mmol/l;
pH 6.8), 4% w/v sodium dodecyl sulphate (SDS), 10% v/v glycerol, 4%
v/v 2-mercaptoethanol and 2 mg/ml bromophenol blue. Equal amounts
of protein were separated using 10% SDS-polyacrylamide gel
electrophoresis (18), and the
separated protein was transferred to a nitrocellulose membrane.
Subsequently, the membrane was blocked with 5% non-fat milk to
avoid non-specific protein binding (19). Next, the membrane was incubated at
4°C overnight with the following primary antibodies: Rabbit
anti-collagen I (1:100; cat. no. bs-7158R, Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China), rabbit anti-collagen III
(1:100; cat. no. bs-0948R, Beijing Biosynthesis Biotechnology Co.,
Ltd.), rabbit anti-matrix metalloproteinase (MMP)-2 (1:500; cat.
no. ab124294, Abcam, Hangzhou, China), rabbit anti-MMP-9 (1:500;
cat. no. ab7299, Abcam) and GAPDH (1:500; cat. no. ab181602, Abcam;
serving as an internal control). The membrane was incubated with a
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(1:20,000; cat. no. 111-035-00, Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 2 h at room
temperature, after which the membranes were visualized using a
Fujifilm LAS-1000 Luminescent Image Analyzer (Fujifilm Medical
Systems USA, Stamford, CT, USA) and the band intensity was
quantified by ImageJ software (http://rsb.info.nih.gov/ij/).
Statistical analysis
One-way analysis of variance, followed by Tukey's
multiple comparison test, were performed to detect the
statistically significant differences among three or more groups.
Statistical analyses were performed using SPSS version 17.0 (SPSS
Inc., Chicago, IL, USA). Results are presented as the mean ±
standard error of mean. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of SKG on body weight, KW/BW
and HWI
Sham-operated rats were found to have a higher body
weight compared with the 5/6 Nx animals prior to the treatment
period; however, no statistically significant difference was
observed (P>0.05; Fig. 1A). As
shown in Fig. 1A, the body weight of
the rats increased slowly during the treatment period, with the
exception of the sham-operated rats. However, no statistically
significantly differences were detected among the groups
(P>0.05). The left KW/BW of the sham-operated group was found to
be significantly lower compared with the model group and the other
treatment groups (P<0.01; Fig.
1B), indicating that the remnant kidney of the 5/6 Nx rats
exhibited hypertrophy. The HWI of the 5/6 Nx group was found to be
significantly higher compared with the sham-operated group
(P<0.05; Fig. 1C), whereas no
statistically significant differences were observed between the
sham-operated group and treatment groups (P>0.05). These results
indicated that SKG may prevent hypertrophy of the heart.
 | Figure 1.Effect of SKG on the (A) body weight,
(B) KW/BW and (C) HWI. Values are presented as the mean ± standard
error of mean (n=10 per group). *P<0.05 and **P<0.01, vs. 5/6
Nx group. SKG, Shenkang granules; KW/BW, kidney weight/body weight
ratio; HWI, heart weight index; 5/6 Nx, 5/6 nephrectomized (model
group); SKI, Shenkang injection; SKGL, low-dose SKG; SKGM,
moderate-dose SKG; SKGH, high-dose SKG. |
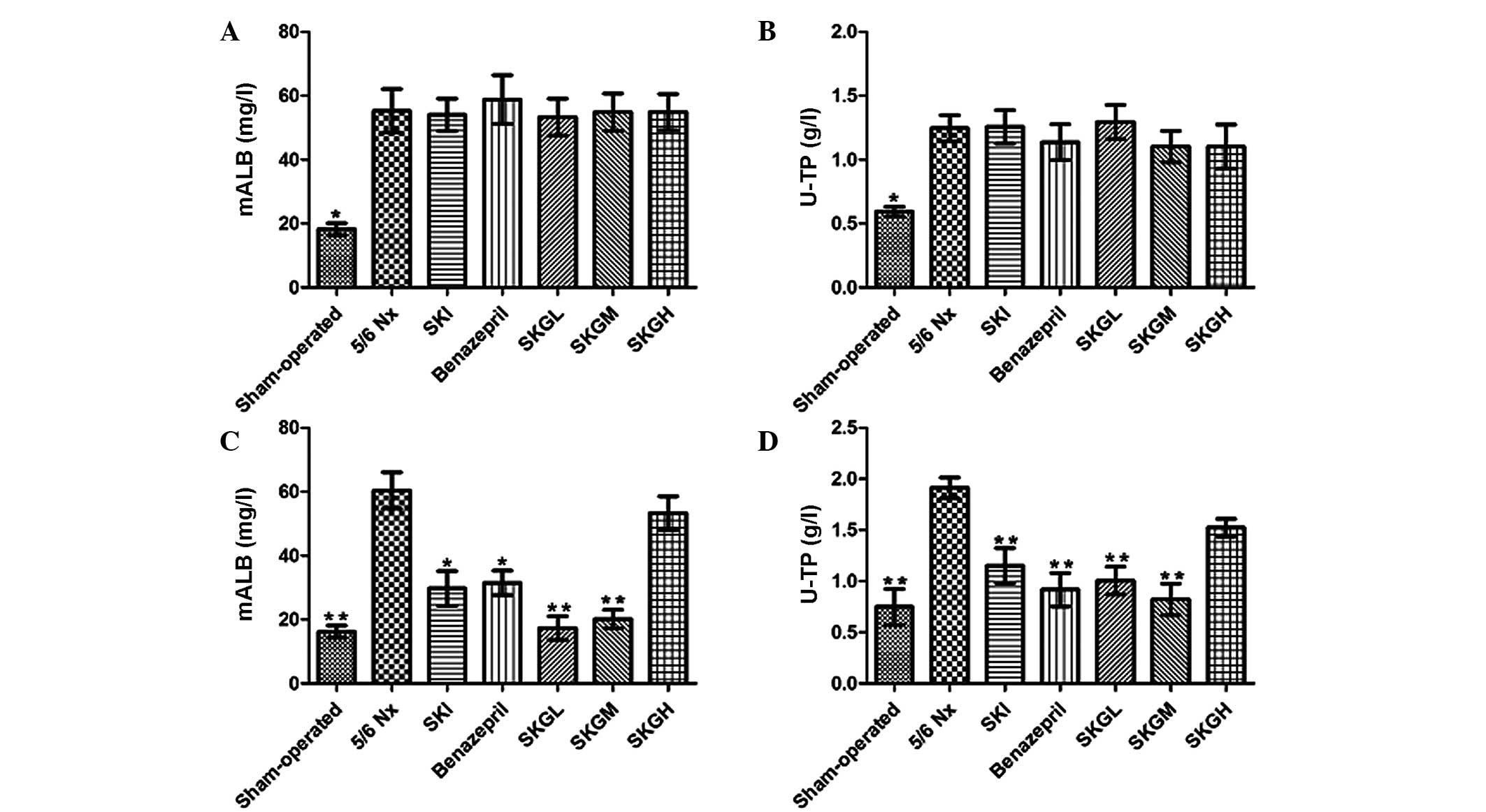
Effect of SKG on mALB and U-TP
As shown in Fig. 2A and
B, the mALB and U-TP concentrations in the 5/6 Nx rats were
higher compared with the sham-operated animals prior to treatment
(P<0.01), while no statistically significant differences were
observed among the treatment groups. At the end of the treatment
period, the levels of mALB and U-TP in the 5/6 Nx group (60.43±5.15
mg/l and 1.91±0.09 g/l, respectively) were significantly higher
compared with the sham-operated group (16.20±1.39 mg/l and
0.75±0.15 g/l, respectively; P<0.01). By contrast, treatment
with SKGL and SKGM decreased the level of mALB (17.35±3.15 and
20.17±2.46 mg/l, respectively; P<0.01, vs. 5/6 Nx group) and
U-TP (1.01±0.11 and 0.82±0.14 g/l, respectively; P<0.01, vs. 5/6
Nx group). However, treatment with SKGH did not reduce the levels
of mALB and U-TP (Fig. 2C and D). In
addition, SKI and benazepril were found to reduce the mALB and U-TP
levels significantly (P<0.05 and P<0.01, vs. 5/6 Nx group,
respectively).
Effect of SKG on renal function, serum
lipid levels and GSH-PX
Scr and BUN concentrations were found to be higher
in the 5/6 Nx group (73.03±3.31 µmol/l and 16.04±0.83 mmol/l,
respectively) when compared with the sham-operated group
(40.66±3.31 µmol/l and 6.87±0.52 mmol/l, respectively; P<0.01;
Fig. 3A and B). As shown in Fig. 3A and B, SKG, SKI and benazepril
lowered the increased Scr and BUN levels following 5/6 Nx surgery
(P<0.05 or P<0.01, vs. 5/6 Nx group); however, the levels did
not reach those of the sham-operated group. In addition, the levels
of CH, TG and LDL in the treatment groups decreased significantly
compared with the 5/6 Nx group (P<0.05 or P<0.01; Fig. 3C–E). The serum concentration of
GSH-PX was found to be significantly increased in the SKGL and SKGM
groups when compared with the 5/6 Nx group; however, the GSH-PX
concentrations remained lower than those in the sham-operated rats
(Fig. 3F; P<0.05, vs. 5/6 Nx
group).
 | Figure 3.Effect of SKG on the levels of (A)
Scr, (B) BUN, (C) CH, (D) TG, (E) LDL and (F) GSH-PX in 5/6 Nx rats
at day 30 following surgery. Values are presented as the mean ±
standard error of mean (n=10 per group). *P<0.05 and
**P<0.01, vs. 5/6 Nx group. SKG, Shenkang granules; Scr, serum
creatinine; BUN, blood urea nitrogen; CH, total cholesterol; TG,
triglyceride; LDL, low-density lipoprotein; GSH-PX, glutathione
peroxidase; 5/6 Nx, 5/6 nephrectomized (model group); SKI, Shenkang
injection; SKGL, low-dose SKG; SKGM, moderate-dose SKG; SKGH,
high-dose SKG. |
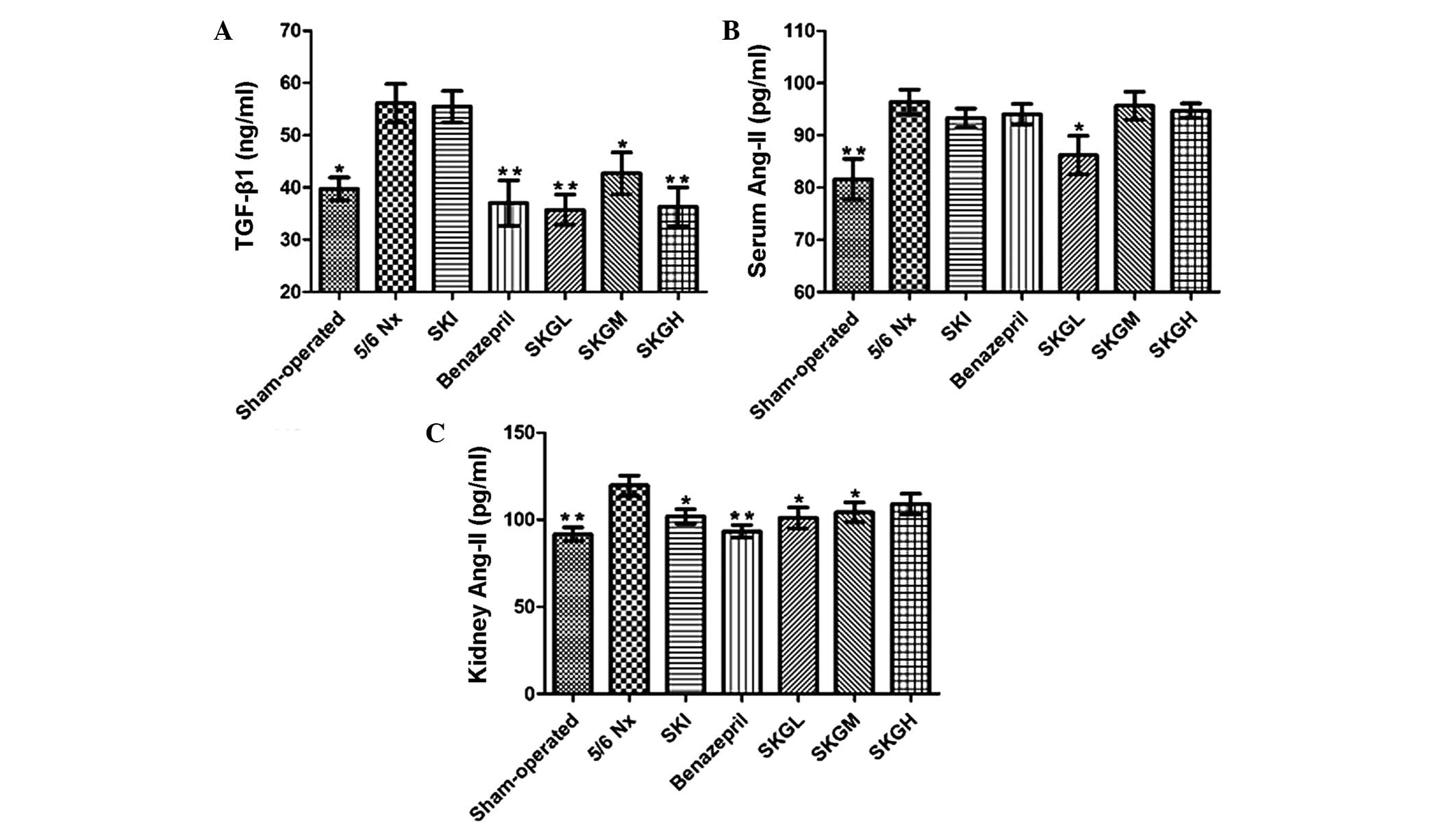
Effect of SKG on TGF-β1 and
Ang II
Serum TGF-β1 concentrations were
significantly higher in the 5/6 Nx group (56.14±3.14 ng/ml) when
compared with the sham-operated group (39.74±1.80 ng/ml; P<0.05;
Fig. 4A). Treatment with low-,
moderate- and high-dose SKG was found to reduce the serum
TGF-β1 level compared with the 5/6 Nx group (P<0.01,
P<0.05 and P<0.01, respectively; Fig. 4A). However, no statistically
significant difference was observed when comparing the
concentration in the SKI group and the 5/6 Nx group. To further
clarify the possible mechanisms of the aforementioned effects of
SKG on CRF, the serum and kidney levels of Ang II were measured. As
shown in Fig. 4B, the content of
serum Ang II was significantly increased in the 5/6 Nx group
compared with the sham-operated group (P<0.01). SKGL treatment
reduced the serum Ang II level (P<0.05, vs. 5/6 Nx group), while
the remaining treatment groups had no effect on the serum Ang II
level. By contrast, the kidney Ang II concentration was found to be
significantly decreased in the treatment groups (P<0.01 or
P<0.05, vs. 5/6 Nx group; Fig.
4C), with the exception of the SKGH group.
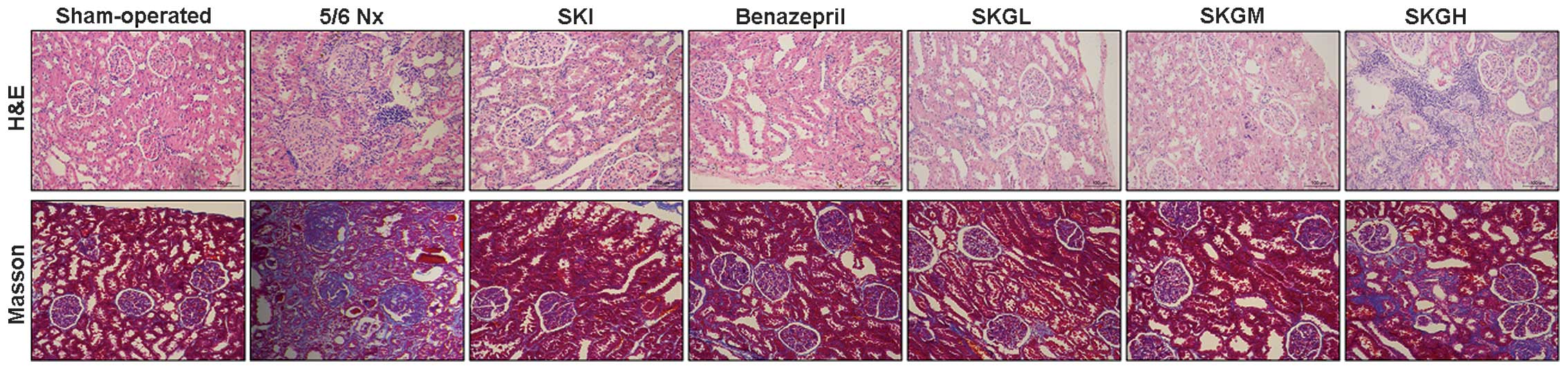
Histology
Sham-operated rats were found to have a normal
kidney structure (Fig. 5). By
contrast, the kidney histology of the 5/6 Nx group revealed
extensive fibrosis, various grades of sclerosis and fibrosis in the
glomeruli, mesangial cell hyperplasia and mesangial matrix
proliferation. In addition, atrophy and necrosis of renal tubules,
as well as detachment of epithelial cells, were observed.
Tubulointerstitial analysis revealed evident fibrosis and
inflammation of the infiltrate. Renal damage was significantly
improved in the treatment groups, particularly in the SKGL and SKGM
groups; however, the renal damage was not modulated effectively in
the SKGH group.
Effect of SKG on MMP-2 and MMP-9
Renal concentrations of MMP-2 and MMP-9 in the 5/6
Nx group were significantly higher compared with the sham-operated
group. As shown in Fig. 6, SKG
markedly decreased the MMP-2 and MMP-9 levels (P<0.01, vs. 5/6
Nx group). The level of MMP-2 in the SKGL and SKGM groups exhibited
no statistical difference with that of the sham-operated rats. In
addition, the MMP-9 level in the SKGL group was lower compared than
the level in the sham-operated rats, but the difference was not
statistically significant.
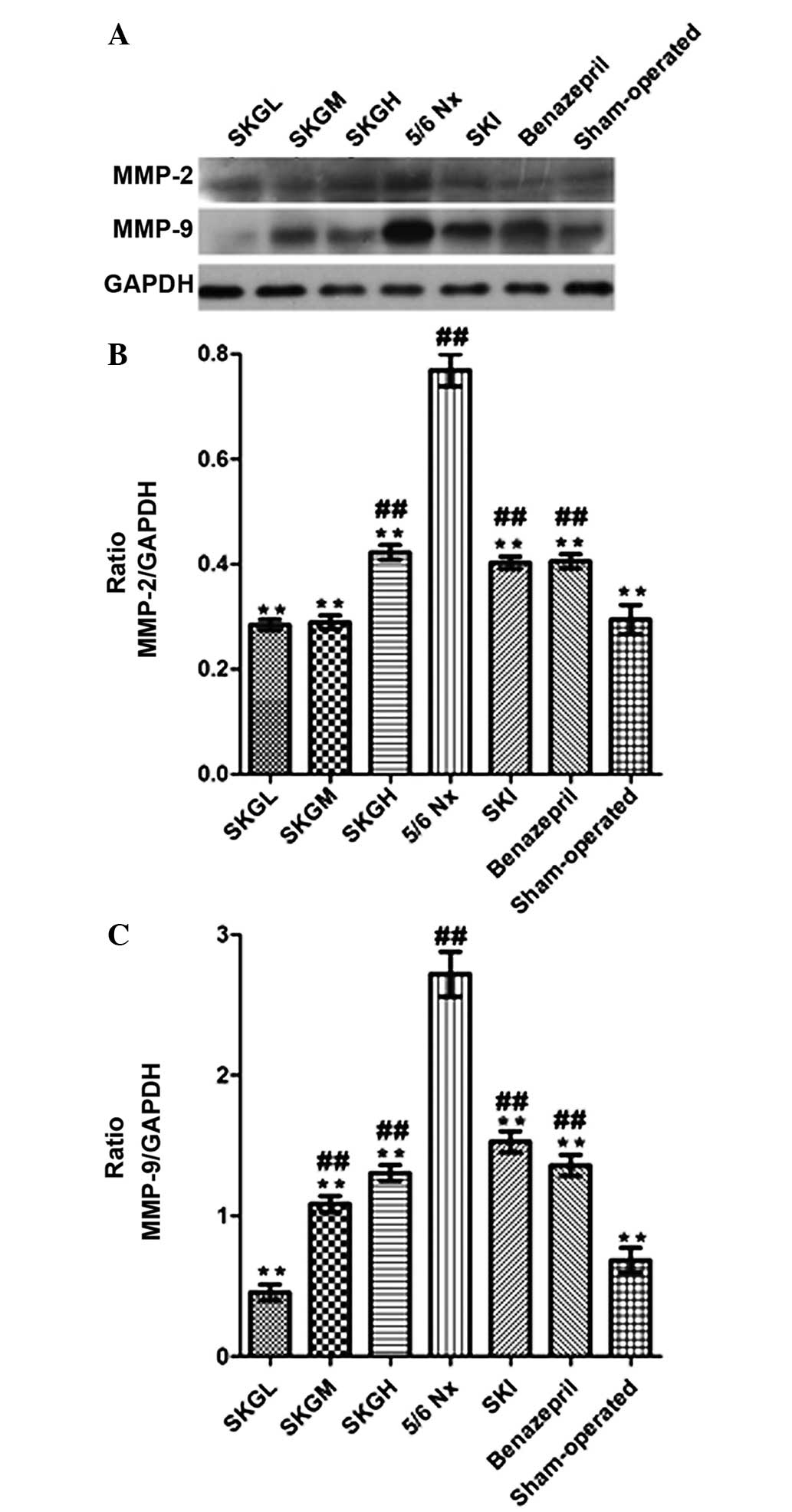 | Figure 6.(A) Western blot analyses showing
MMP-2 and MMP-9 expression in the renal tissue. Quantification of
the expression levels of (B) MMP-2 and (C) MMP-9 in the different
groups. Values are presented as the mean ± standard error of mean
(n=10 per group). **P<0.01, vs. 5/6 Nx group;
##P<0.01, vs. sham-operated group. MMP, matrix
metalloproteinase; SKG, Shenkang granule; SKGL, low-dose SKG; SKGM,
moderate-dose SKG; SKGH, high-dose SKG; 5/6 Nx, 5/6 nephrectomized
(model group); SKI, Shenkang injection; GAPDH, glyceraldehyde
3-phosphate dehydrogenase. |
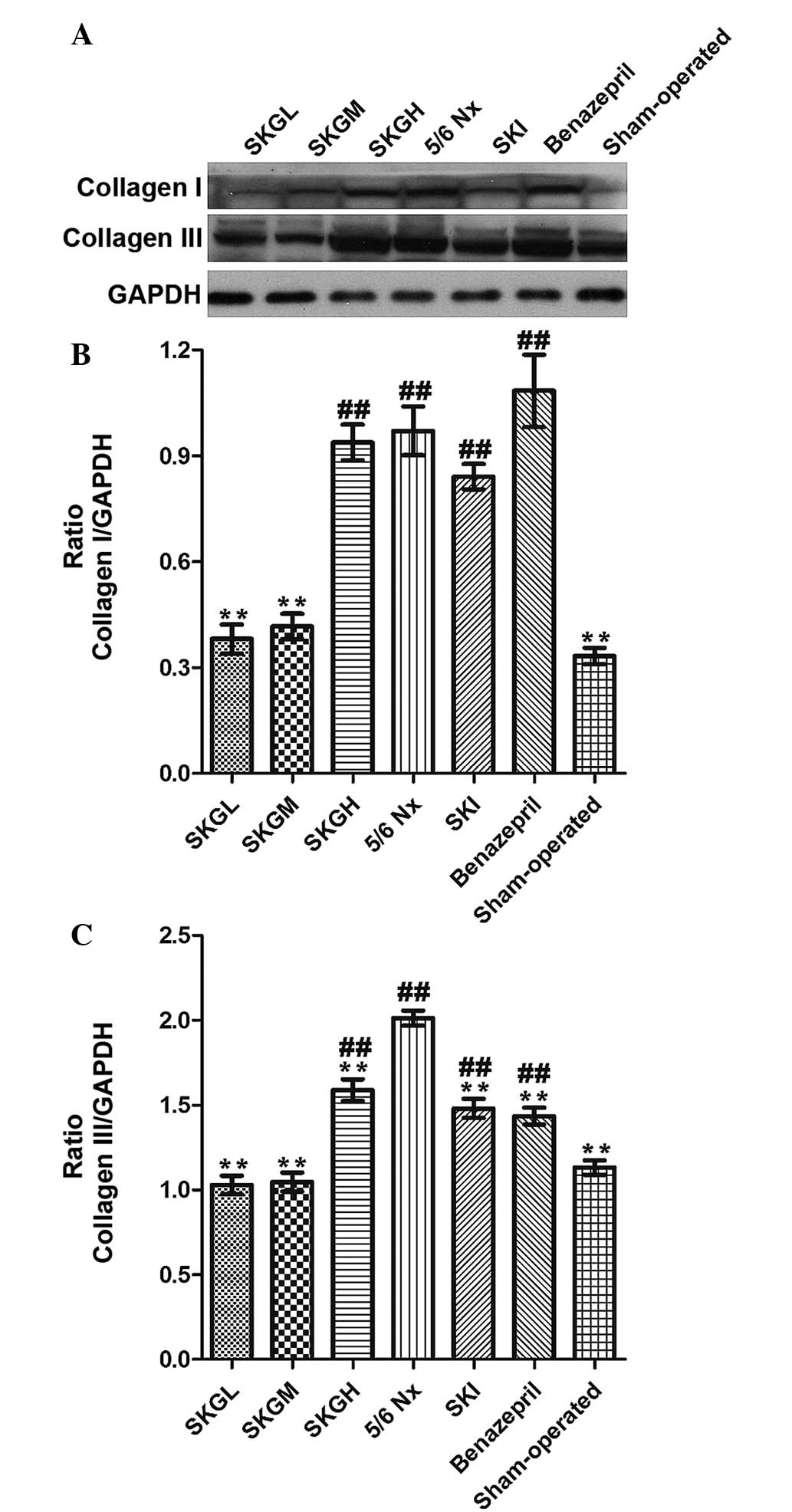
Effect of SKG on collagen I and
III
Western blot analysis was used to analyze the
extracellular matrix (ECM) protein expression, which was found to
be significantly increased in the 5/6 Nx group. By contrast, the
expression levels of collagen I and III were reduced in the
treatment groups. As shown in Fig.
7, the SKGL and SKGM groups exhibited significantly reduced
expression levels of collagen I, showing similar levels to the
sham-operated group. However, the SKI, benazepril and SKGH groups
had no effect on the collagen I expression levels. The content of
collagen III in all the treatment groups was evidently decreased
when compared with the 5/6 Nx group (P<0.01, Fig. 7B); however, the collagen III level in
the SKI, benazepril and SKGH groups remained higher compared with
the sham-operated group.
Discussion
The aim of the present study was to investigate the
beneficial effect of SKG during the progressive phase of renal
injury in 5/6 Nx rats. A previous study demonstrated that in a 5/6
Nx rat model of CRF, proteinuria and renal dysfunction associated
with glomerular sclerosis and tubulointerstitial fibrosis were
observed (20). In the present
study, SKG was shown to significantly reduce the levels of mALB,
U-TP, Scr, BUN, CH, TG, LDL, TGF-β1 and kidney Ang II.
In addition, SKG increased the level of GSH-PX and attenuated the
progression of pathological renal damage in the CRF model; thus,
SKG was demonstrated to improve renal function.
Lipid metabolism disorder is a common complication
of CRF that induces an increase in CH and TG levels and upregulates
the LDL level (8,21). Hyperlipidemia may lead to biological
metabolic disorders, and alter the blood- and hemodynamics of
kidney tissues, aggravating proteinuria and renal pathological
changes (7,22). Abnormal lipoprotein levels can result
in the formation of foam cells in the kidney and promote glomerular
sclerosis (23). In the current
study, SKG was found to significantly reduce the levels of CH, TG
and LDL, thus inhibiting abnormal lipid deposition and alleviating
the lipid-induced damage of the renal tissues. Therefore, SKG may
improve renal damage by reducing the serum lipid concentration.
Furthermore, CRF leads to a decreased glomerular filtration rate
and the accumulation of Scr, BUN, proteinuria and other
metabolites, resulting in an increase in the total oxygen free
radical content (24,25). GSH-PX is an important enzyme that
eliminates free radicals (26,27). In
the present study, the concentration of GSH-PX in the 5/6 Nx group
was found to be significantly lower compared with the sham-operated
group, whereas SKG was found to significantly increase the level of
GSH-PX. The results indicated that SKG may increase the removal of
toxicity products, remove oxygen free radicals and reduce the free
radical-induced damage of kidney tissues, subsequently improving
renal function.
Previous studies have demonstrated that
TGF-β1 is a key profibrotic growth factor involved in
renal fibrogenesis and an important factor among cytokines, with
TGF-β1 expression upregulated in CRF (28–31). In
the present study, SKG was shown to markedly reduce the serum
concentration of TGF-β1. Ang II is an inflammatory
molecule that activates TGF-β1 (32), and Ang II and TGF-β1 are
critical mediators of renal fibrosis (33). Previous studies have indicated that
Ang II is expressed in renal interstitial cells in a Nx model,
paralleling the severity of renal injury (34,35). In
the current study, Ang II levels increased in the serum and kidney
tissues of 5/6 Nx rats, while SKG was found to markedly decrease
the kidney Ang II level. However, the various treatments had no
statistically significant effect on the serum Ang II level, with
the exception of the low-dose SKG treatment. In addition, the level
of Ang II was in accordance with the level of TGF-β1,
indicating that SKG may prevent CRF by regulating Ang II and
TGF-β1.
The progression of CRF is characterized
histologically by progressive glomerulosclerosis and
tubulointerstitial fibrosis (9,36). A
significant observation of the present study was the marked
improvement effect of SKG on renal pathological damage, through
inhibition of the relative area of renal glomerulosclerosis and
tubulointerstitial fibrosis, particularly in the SKGL and SKGM
groups. Since the magnitude of fibrosis has been shown to indicate
the degree and progression of renal failure (37), the antifibrotic effect of SKG may be
relevant to the attenuation of renal disease progression. The
effect of SKG in ameliorating renal pathological damage may be
directly associated with a reduction in the synthesis of major ECM
components (38,39), as demonstrated by the diminished
production of collagen I and III in the kidney tissues of the
SKG-treated 5/6 Nx rats. As revealed by western blot analysis, SKGL
and SKGM may significantly reduce the content of collagen I and
III, with no statistically significant difference observed with the
sham-operated group.
Matrix metalloproteinases (MMPs) are a large family
of zinc-dependent proteases that regulate tissue remodeling, cell
proliferation and angiogenesis through affecting ECM accumulation
(40). MMPs are known to play an
important role in the pathophysiology of renal diseases (41). In addition, increasing evidence
indicates that MMP abnormalities are involved in the vascular
changes associated with kidney failure (42). MMP-2 and MMP-9 are important factors
participating in the progression of atherosclerosis in CRF patients
(42). Deposition of ECM also
results in an increase in MMP-2 and MMP-9 levels (43). The present study investigated the
MMP-2 and MMP-9 levels in kidney tissues using western blot
analysis. SKG was found to significantly reduce the levels of MMP-2
and MMP-9, as compared with the 5/6 Nx group.
In conclusion, the current study demonstrated that
SKG ameliorated renal injury in a 5/6 Nx rat model of CRF, through
the prevention of albuminuria, glomerulosclerosis and interstitial
fibrosis, and the reduction of Scr, BUN and serum lipid levels.
These effects may be associated with the impact of SKG on ECM
deposition and the inhibition of TGF-β1 and kidney Ang
II. The observations demonstrate that SKG may have a good
therapeutic effect on chronic renal failure. However, high-dose SKG
did not have a marked renoprotective effect in 5/6 Nx rats; thus,
further study is required.
References
|
1
|
Norman JT and Fine LG: Progressive renal
disease: fibroblasts, extracellular matrix, and integrins. Exp
Nephrol. 7:167–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossert J, Fouqueray B and Boffa JJ:
Anemia management and the delay of chronic renal failure
progression. J Am Soc Nephrol. 14:(7 Suppl 2). S173–S177. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki S, Tohda C, Kim M and Yokozawa T:
Gamma-aminobutyric acid specifically inhibits progression of
tubular fibrosis and atrophy in nephrectomized rats. Biol Pharm
Bull. 30:687–691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsujimura T, Nagamine J, Sugaru E, et al:
Combination therapy with SMP-534 and an angiotensin-converting
enzyme inhibitor provides additional renoprotection in 5/6
nephrectomized rats. Biol Pharm Bull. 32:1991–1996. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
El Nahas Meguid A and Bello AK: Chronic
kidney disease: the global challenge. Lancet. 365:331–340. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dellê H, Rocha JR, Cavaglieri RC, Vieira
JM Jr, Malheiros DM and Noronha IL: Antifibrotic effect of
tamoxifen in a model of progressive renal disease. J Am Soc
Nephrol. 23:37–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jian DY, Chao YW, Ting CH, et al: Losartan
ameliorates renal injury, hypertension, and adipocytokine imbalance
in 5/6 nephrectomized rats. Eur J Pharmacol. 709:85–92. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cho KH, Kim HJ, Kamanna VS and Vaziri ND:
Niacin improves renal lipid metabolism and slows progression in
chronic kidney disease. Biochim Biophys Acta. 1800:6–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyoda M, Shibata T, Wada Y, et al: Long-
and short-term treatment with imatinib attenuates the development
of chronic kidney disease in experimental anti-glomerular basement
membrane nephritis. Nephrol Dial Transplant. 28:576–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Duan H and He L: Inhibitory effect
of atractylenolide I on angiogenesis in chronic inflammation in
vivo and in vitro. Eur J Pharmacol. 612:143–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du J, Chen H and Wang XB: Effect of
shenkang injection on hypertrophy and expressions of p21 and p27 in
glomerular mesangial cells of rats cultured in high glucose.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 26:(Suppl). 68–71. 2006.[(In
Chinese)]. PubMed/NCBI
|
|
12
|
Xiao W, Wei LB, Ma Y, Long HB and Chen GB:
Renal protective effect of Shenkang pill on diabetic rats. Zhongguo
Zhong Yao Za Zhi. 31:1006–1009. 2006.[(In Chinese)]. PubMed/NCBI
|
|
13
|
Guo L, Liu Y and Mao W: Contrast study on
effect of shenkang injection and benazepril on human glomerular
mesangial extracellular matrix. Zhongguo Zhong Xi Yi Jie He Za Zhi.
20:50–52. 2000.[(In Chinese)]. PubMed/NCBI
|
|
14
|
Zhao Z, Li H and Zhang X: Effect of
shenkang injection on transforming growth factor-beta messenger
ribonucleic acid of LLC-PK1 renal tubular epithelial cells.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 20:931–933. 2000.[(In
Chinese)]. PubMed/NCBI
|
|
15
|
Anderson S, Rennke HG and Brenner BM:
Therapeutic advantage of converting enzyme inhibitors in arresting
progressive renal disease associated with systemic hypertension in
the rat. J Clin Invest. 77:1993–2000. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maschio G, Alberti D, Janin G, et al:
Effect of the angiotensin-converting-enzyme inhibitor benazepril on
the progression of chronic renal insufficiency. The
Angiotensin-Converting-Enzyme Inhibition in Progressive Renal
Insufficiency Study Group. N Engl J Med. 334:939–945. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghosh SS, Massey HD, Krieg R, et al:
Curcumin ameliorates renal failure in 5/6 nephrectomized rats: role
of inflammation. Am J Physiol Renal Physiol. 296:F1146–F1157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L, Xu CB, Zhang Y, Cao YX and
Edvinsson L: Secondhand smoke exposure induces
Raf/ERK/MAPK-mediated upregulation of cerebrovascular endothelin
ETA receptors. BMC Neurosci. 12:1092011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cao L, Zhang Y, Cao YX, Edvinsson L and Xu
CB: Cigarette smoke upregulates rat coronary artery endothelin
receptors in vivo. PLoS One. 7:e330082012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baraka A and El Ghotny S: Cardioprotective
effect of renalase in 5/6 nephrectomized rats. J Cardiovasc
Pharmacol Ther. 17:412–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai F, Makino T, Ono T and Mizukami H:
Anti-hypertensive effects of shichimotsukokato in 5/6
nephrectomized Wistar rats mediated by the DDAH-ADMA-NO pathway. J
Nat Med. 66:583–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma RX, Zhao N and Zhang W: The effects and
mechanism of Tripterygium wilfordii Hook F combination with
irbesartan on urinary podocyte excretion in diabetic nephropathy
patients. Zhonghua Nei Ke Za Zhi. 52:469–473. 2013.[(In Chinese)].
PubMed/NCBI
|
|
23
|
Cozzolino M, Staniforth ME, Liapis H, et
al: Sevelamer hydrochloride attenuates kidney and cardiovascular
calcifications in long-term experimental uremia. Kidney Int.
64:1653–1661. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tapia E, Zatarain-Barrón ZL,
Hernández-Pando R, et al: Curcumin reverses glomerular hemodynamic
alterations and oxidant stress in 5/6 nephrectomized rats.
Phytomedicine. 20:359–366. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao G, Zhao H, Tu L, et al: Effects and
mechanism of irbesartan on tubulointerstitial fibrosis in 5/6
nephrectomized rats. J Huazhong Univ Sci Technolog Med Sci.
30:48–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monostori P, Kocsis GF, Ökrös Z, et al:
Different administration schedules of darbepoetin alfa affect
oxidized and reduced glutathione levels to a similar extent in 5/6
nephrectomized rats. Clin Exp Nephrol. 17:569–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konda T, Enomoto A, Takahara A and
Yamamoto H: Effects of L/N-type calcium channel antagonist,
cilnidipine on progressive renal injuries in Dahl salt-sensitive
rats. Biol Pharm Bull. 29:933–937. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noda M, Matsuo T, Fukuda R, et al: Effect
of candesartan cilexetil (TCV-116) in rats with chronic renal
failure. Kidney Int. 56:898–909. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Romero F, Rodríguez-Iturbe B, Parra G,
González L, Herrera-Acosta J and Tapia E: Mycophenolate mofetil
prevents the progressive renal failure induced by 5/6 renal
ablation in rats. Kidney Int. 55:945–955. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mori Y and Hirano T: Ezetimibe alone or in
combination with pitavastatin prevents kidney dysfunction in 5/6
nephrectomized rats fed high-cholesterol. Metabolism. 61:379–388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zager RA, Johnson AC and Becker K: Acute
unilateral ischemic renal injury induces progressive renal
inflammation, lipid accumulation, histone modification, and
‘end-stage’ kidney disease. Am J Physiol Renal Physiol.
301:F1334–F1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Houlihan CA, Akdeniz A, Tsalamandris C,
Cooper ME, Jerums G and Gilbert RE: Urinary transforming growth
factor-beta excretion in patients with hypertension, type 2
diabetes, and elevated albumin excretion rate: effects of
angiotensin receptor blockade and sodium restriction. Diabetes
Care. 25:1072–1077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gaedeke J, Peters H, Noble NA and Border
WA: Angiotensin II, TGF-beta and renal fibrosis. Contrib Nephrol.
135:153–160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noronha IL, Fujihara CK and Zatz R: The
inflammatory component in progressive renal disease - are
interventions possible? Nephrol Dial Transplant. 17:363–368. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He L, Shen P, Fu Q, et al:
Nephro-protective effect of Kangqianling decoction on chronic renal
failure rats. J Ethnopharmacol. 122:367–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim SK, Jung KH and Lee BC: Protective
effect of Tanshinone IIA on the early stage of experimental
diabetic nephropathy. Biol Pharm Bull. 32:220–224. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Demosthenous P, Voskarides K, Stylianou K,
et al: Hellenic Nephrogenetics Research Consortium: X-linked Alport
syndrome in Hellenic families: phenotypic heterogeneity and
mutations near interruptions of the collagen domain in COL4A5. Clin
Genet. 81:240–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soylemezoglu O, Wild G, Dalley AJ, et al:
Urinary and serum type III collagen: markers of renal fibrosis.
Nephrol Dial Transplant. 12:1883–1889. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding J, Yang J, Liu J and Yu L:
Immunofluorescence study of type IV collagen alpha chains in
epidermal basement membrane: application in diagnosis of X-linked
Alport syndrome. Chin Med J (Engl). 110:584–586. 1997.PubMed/NCBI
|
|
40
|
Duong Van Huyen JP, Viltard M, Nehiri T,
et al: Expression of matrix metalloproteinases MMP-2 and MMP-9 is
altered during nephrogenesis in fetuses from diabetic rats. Lab
Invest. 87:680–689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marson BP, Lacchini R, Belo V, et al:
Matrix metalloproteinase (MMP)-2 genetic variants modify the
circulating MMP-2 levels in end-stage kidney disease. Am J Nephrol.
35:209–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee ES, Shen Q, Pitts RL, et al: Serum
metalloproteinases MMP-2, MMP-9, and metalloproteinase tissue
inhibitors in patients are associated with arteriovenous fistula
maturation. J Vasc Surg. 54:454–460. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pawlak K, Mysliwiec M and Pawlak D:
Peripheral blood level alterations of MMP-2 and MMP-9 in patients
with chronic kidney disease on conservative treatment and on
hemodialysis. Clin Biochem. 44:838–843. 2011. View Article : Google Scholar : PubMed/NCBI
|