Introduction
The root cause of obesity is an imbalance between
energy intake and expenditure, a disturbance known to occur in
conditions, such as type 2 diabetes, dyslipidemia, hypertension and
other metabolic and cardiovascular diseases (1). At the cellular level, obesity is
characterized by an increase in the size (hypertrophy) and number
(hyperplasia) of adipoctyes. Hence, a complete understanding of the
mechanisms regulating adipogenesis is essential and of utmost
priority (2).
MicroRNAs (miRNAs or miRs) are small non-coding RNAs
that negatively regulate target gene expression at the
post-transcriptional level by binding to the 3′-untranslated region
(UTR) of mature mRNAs, affecting their stability (3). miRNAs have been shown to play
important regulatory roles in a variety of physiological processes,
including cell differentiation, apoptosis and proliferation
(4). Several miRNAs have been
reported to either accelerate or inhibit adipogenesis, through the
regulation of different signaling pathways (5). For example, miR-30 has been shown to
inhibit the differentiation of mesenchymal stem cells (MSCs) into
preadipocytes (6), and the
miR-17-92 cluster has been shown to accelerate the clonal expansion
of preadipocytes during early differentiation by targeting Rb2/p130
(7). Moreover, some miRNAs
(miR-130, miR-27a/b, miR-378 and miR-519d) can directly regulate
the levels of two critical transcription factors, peroxisome
proliferator-activated receptor γ (PPARγ) and
cytidine-cytidine-adenosine-adenosine-thymidine (CCAAT) enhancer
binding protein α (C/EBPα), thus affecting the dynamics of
adipogenesis (8–11). The signaling pathways mediated by
transforming growth factor (TGF)-β and Wnt have been shown to
negatively regulate adipogenesis. For example, the overexpression
of miR-21 has been shown to downregulate the expression of TGF-β
receptor 2 (TGFβR2), which in turn promotes adipogenesis, while the
inhibition of this function of miR-21 induces an increase in TGFβR2
expression accompanied by reduced adipogenic differentiation
(12). Three independent studies
have reported that some miRNAs affect adipogenesis by regulating
Wnt signaling. Specifically, miR-8 facilitates adipogenesis by
targeting Wntless and CG32767 (13), while miR-344 has been shown to
inhibit the adipocytic differentiation of 3T3-L1 cells by targeting
glycogen synthase kinase-3β (GSK3β) (14). Qin et al (15) used a microRNA array to identify
miRNas that regulate Wnt signaling either negatively (miR-210,
miR-148a, miR-194 and miR-322) or positively (miR-344, miR-27 and
miR-181) during adipogenesis in the 3T3-L1 cell line. In addition,
the authors demonstrated that miR-210 promotes adipogenesis by
targeting Tcf7l2, an important regulator of Wnt signaling (15).
The Wnt signaling pathway, due to its indispensable
role in development, is highly conserved during evolution. Both
canonical and non-canonical Wnt signaling have been shown to
negatively regulate adipogenesis. In canonical Wnt signaling
mediated by β-catenin, the ligands, Wnt10a and Wnt10b, bind to
frizzled 1 (FZD1) receptors and low-density lipoprotein
receptor-related protein (LRP)5/6 co-receptors leading to the
phosphorylation of dishevelled segment polarity protein (DVL) and
the degradation of Axin, followed by hypophosphorylation and the
nuclear translocation of β-catenin. This in turn leads to the
activation of downstream target genes, such as cyclin D1 (CCND1),
accompanied by the inhibition of PPARγ and C/EBPα, causing a
further decrease in adipogenesis (16).
Although miR-204 has been shown to promote the
adipogenesis and inhibit the osteogenesis of human MSCs through the
direct suppression of Runx2 (17), the effects of miR-204-5p on the
activity of Wnt/β-catenin signaling and, consequently, on
adipocytic differentiation remain unclear. In the present study, we
demonstrate that miR-204-5p promotes the differentiation of human
adipose-derived MSCs (hADSCs) into mature adipoctyes by suppressing
the expression of DVL3, a positive regulator of Wnt/β-catenin
signaling.
Materials and methods
Isolation and differentiation of
hADSCs
hADSCs were obtained through the liposuction of
subcutaneous adipose tissue, using previously described methods
(18). The donors were required
to provide signed informed consent prior to the commencement of the
study, which was approved by the Human Ethics Review Committee of
the Third Xiangya Hospital of the Central South University,
Changsha, China. Briefly, 10 g of freshly isolated adipose tissue
were washed with D-Hank’s buffer (Gibco/Life Technologies,
Shanghai, China) thrice, dissected into 1x1 mm sections and
digested with collagenase I (1 mg/ml; Sigma-Aldrich, St. Louis, MO,
USA) for 1 h at 37°C. The dissociated tissue was then filtered
through a 150-μm nylon mesh and centrifuged at 1,000 rpm for
5 min. The precipitate was lysed further with red blood cell lysis
buffer (Beyotime, Haimen, China) for 10 min, followed by
centrifugation at 1,000 rpm for 10 min. The fraction of cells thus
precipitated was washed with D-Hank’s buffer, resuspended in
DMEM-F12 (Gibco/Life Technologies) containing 10% fetal bovine
serum (FBS; Gibco/Life Technologies, Mulgrave, VIC, Australia) and
cultured at 37°C in 5% CO2. The medium was replaced
after 72 h; cells at passages 4–7 were used for further
experiments. To induce the differentiation of the hADSCs, confluent
hADSC cultures were grown in medium supplemented with 1
μmol/l dexamethasone, 10 μmol/l insulin, 0.5 mmol/l
isobutylmethyl-xanthine (IBMX) and 200 μmol/l indomethacin
(all purchased from Sigma-Aldrich). The induction medium was
replaced every 2 days.
Transfection of hADSCs
Mimics, mimic-NC, agomir (cholesterol-conjugated
2′-O-methyl-modified mimics), agomiR-NC, antagomir and antagomiR-NC
of miR-204-5p, were synthe-sized by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The miR-204-5p target site was predicted using
online software, and found to be highly conserved among
vertebrates. pVAX1- DVL3 and pVAX1-NC were purchased from Biovector
Science Lab, Inc. (Beijing, China), and siRNA-DVL3 (si-DVL3) and
siRNA-NC (si-NC) were prepared by Invitrogen (Carlsbad, CA, USA).
The hADSCs were transfected with mimics, mimic-NC, agomir,
agomiR-NC (100 nM), antagomir, antagomiR-NC (200 nM), pVAX1-DVL3,
pVAX1-NC, siRNA-DVL3 or siRNA-NC using Lipofectamine 2000
(Invitrogen) according to the standard manufacturer-recommended
protocol. At 2 days following transfection, cell differentiation
was induced using the aforementioned protocol.
Bioinformatics analysis
The miRNA targets were predicted using PicTar
(http://pictar.org/), TargetScan (http://www.targetscan.org/vert_42/) and miRanda
(http://www.microrna.org/microrna/).
Luciferase reporter constructs and
assay
The 3′-UTR sequence of human DVL-3 containing the
seed target sequence of miR-204-5p was amplified by PCR and cloned
into the pmiR- RB-REPORT™ (Guangzhou RiboBio Co., Ltd.) dual
luciferase plasmid. The following primers were used: forward,
5′-CCGCTCGAGCCCAGTGAGTTCTTTGTGGATGTG-3′
and reverse, 5′-GAATGCGGCCGCTGTGTGCCAGGCACTGTGCTAG-3′.
The XhoI and NotI (all provided by Invitrogen)
restriction sites are underlined above. The mutation of this
sequence from AAAGGGA to ACCGGGA was performed using the
QuickChange Site-Directed Mutagenesis kit (Agilent Technologies,
Edinburgh, UK).
The pmiR-RB-REPORT vector (50 ng) containing either
wild-type or mutated human DVL3 3′-UTR was co-transfected into the
293T cells (obtained from Xiangya Cells Center of Central South
University, Changsha, China) together with miR-204-5p mimics (100
nM) using Lipofectamine 2000 (Invitrogen). A non-target control
mimic was used as a transfection control. Four replicates were
prepared for each transfection experiment. Firefly and
Renilla luciferase activities were measured using the
Dual-Glo Luciferase assay system (Promega, Madison, WI, USA).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using the TRIzol RNA
extraction kit (Life Technologies, Carlsbad, CA, USA). Reverse
transcription of miR-204 was carried out using a reverse
transcription kit (Life Technologies), according to a protocol with
specific instructions for miRNAs (Guangzhou RiboBio Co., Ltd.).
Briefly, the reverse transcription reaction was carried out in a
total volume of 11 μl, which included 4 μl (2
μg) total RNA, 2 μl specific reverse transcription
primer (500 nM) and 5 μl RNase-free water. This reaction
mixture was first incubated at 70°C for 10 min, followed by
incubation in ice for 2 min. We then centrifuged the tubes briefly
(5 sec) to collect the entire volume, and then added 5 μl 5X
reverse transcription Buffer, 2 μl dNTP mix (2.5 mM), 0.5
μl RNase inhibitor (40 U/μl), 0.5 μl reverse
transcriptase (200 U/μl) and 6 μl RNase-free water in
a total reaction volume of 25 μl. The reaction mixture was
then incubated at 42°C for 60 min, followed by 70°C for 10 min.
miR-204-5p primer was designed by Guangzhou RiboBio Co., Ltd. and
quantitative PCR (qPCR) was performed using the QuantiTect SYBR-
Green PCR Master Mix kit (Toyobo Co., Ltd., Osaka, Japan) and the
Mastercycler ep realplex RT-PCR cycler (Eppendorf, Hamburger,
Germany). The U6 small nuclear RNA was used as an endogenous
normalization control. The relative quantification difference for
gene expression between different groups was determined using the
2−ΔΔCT method, and the sequences of the primers are
listed in Table I.
 | Table ISequence information on the primers
used for RT-qPCR. |
Table I
Sequence information on the primers
used for RT-qPCR.
| Gene name | Sequences of
primers |
|---|
| DVL3 | F:
5′-ACAATGCCAAGCTACCATGCTTC-3′ |
| R:
5′-AGCTCCGATGGGTTATCAGCAC-3′ |
| C/EBPα | F:
5′-TGGACAAGAACAGCAACGAG-3′ |
| R:
5′-TTGTCACTGGTCAGCTCCAG-3′ |
| PPARγ | F:
5′-GAGAAGACTCAGCTCTAC-3′ |
| R:
5′-CAAGCATGAACTCCATAGTG-3′ |
| FABP4 | F:
5′-AGCACCATAACCTTAGATGGGG-3′ |
| R:
5′-CGTGGAAGTGACGCCTTTCA-3′ |
| GAPDH | F:
5′-GGCTGAGAACGGGAAGCTTGTCAT-3′ |
| R:
5′-CAGCCTTCTCCATGGTGGTGAAGA-3′ |
Western blot analysis
To extract total protein, the cells were first lysed
in ice-cold radio immunoprecipitation assay (RIPA) lysis buffer
(Beyotime). The total protein concentration in the lysates was
determined by BCA assay (Beyotime). A total of 50 μg protein
were separated by 10% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) gel and transferred onto a PVDF membrane
(Millipore, Boston, MA, USA). The membrane was blocked in blocking
buffer containing 0.1% Tween-20 and 5% skimmed milk for 1 h,
followed by incubation in primary antibody overnight at 4°C. The
following day, the membranes were washed in blocking buffer,
incubated with HRP-conjugated secondary antibodies (Proteintech,
Wuhan, China) for 1 h at room temperature, washed again and
developed using the ECL kit (Beyotime). The following primary
antibodies were used: DVL3 (ab76081; Abcam, Cambridge, UK),
β-catenin (51067-2-AP), C/EBPα (18311-1-AP), fatty acid binding
protein 4 (FABP4; 12802-1-AP) and PPARγ (16643-1-AP; all provided
by Proteintech), CCND1 (BM0771; Wuhan Boster Biological Technology,
Ltd., Wuhan, China).
Oil Red O staining
The cells were washed twice in PBS, fixed in 4%
paraformaldhyde (PFA; Beyotime) for 30 min, washed twice with PBS
and stained with freshly prepared Oil Red O (Beyotime) working
solution (60% Oil Red O stock solution, composed of 0.5% Oil Red O
dissolved in isopropanol and 40% H2O) for 20 min at room
temperature. The cells were then washed twice with water, and the
stained lipid droplets were observed and imaged under a microscope
(TE2000-E; Nikon, Tokyo, Japan).
Statistical analysis
All data are presented as the means ± SD. All
statistical analyses were performed using SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA). Statistical comparisons between 2 groups
were made using the unpaired Student’s t-test. Multiple groups were
analyzed and compared using one-way analysis of variance (ANOVA). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
miR-204-5p is upregulated and DVL3 is
downregulated in hADSCs during adipogenic differentiation
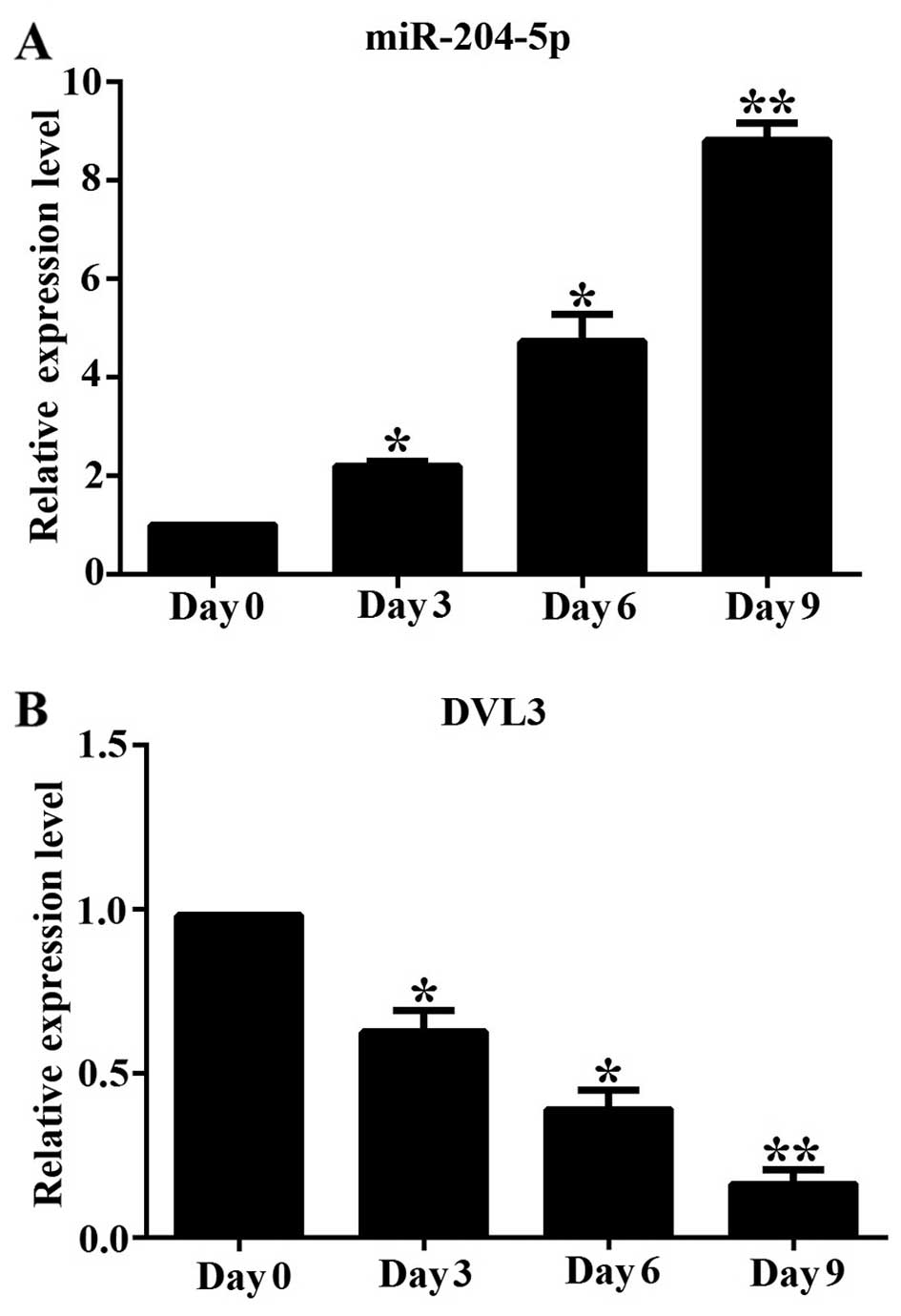
We first determined the changes in the mRNA
expression of miR-204-5p and DVL3 during the adipogenic
differentiation of hADSCs in vitro at different time points
(0, 3, 6 and 9 days). We measured the mRNA expression level of
miR-204-5p and DVL3 by RT-qPCR, and found that miR-204-5p
expression gradually increased during adipogenesis, by
approximately 2.3-, 4.8- and 8.7-fold on days 3, 6 and 9,
respectively, compared to the undifferentiated cells on day 0
(Fig. 1A). By contrast, the mRNA
expression level of DVL3 decreased rapidly during adipogenesis, by
approximately 37, 53 and 82% on days 3, 6 and 9, when compared to
the undifferentiated cells on day 0 (Fig. 1B). This suggests that miR-204-5p
plays an important physiological role during the differentiation of
hADSCs into the adipocytic lineage, by regulating the levels of
DVL3, a critical regulator of the Wnt/β-catenin pathway.
miR-204-5p regulates the adipogenesis of
hADSCs
Although miR-204-5p is known to promote the
differentiation of MSCs into mature adipoctyes by suppressing the
expression of Runx2, to the very best of our knowledge, its effect
on the adipogenic differentiation of hADSCs has not been
demonstrated to date. To investigate this function of miR-204-5p,
we transfected the hADSCs with miR-204-5p agomir, agomiR-NC,
antagomir or antagomiR-NC, and then induced their differentiation
for 9 days; the cells were then stained with Oil Red O to observe
the lipid droplets in the differentiated cells. We found that the
overexpression of miR-204-5p enhanced adipogenesis, resulting in
increased numbers of stained cellular oil droplets in comparison to
the control (untransfected cells) or in the differentiated hADSCs
transfected with the negative control agomir (agomir-NC).
Conversely, the knockdown of miR-204-5p significantly decreased the
extent of adipogenesis (Fig. 2A).
Subsequently, we examined the expression of the adipogenic marker
genes, C/EBPα, PPARγ and FABP4, during normal adipogenesis, and
found a gradual increase in the transcript and protein levels.
Furthermore, the expression of all 3 genes markedly increased
following the overexpression of miR-204-5p, while the knockdown of
miR-204-5p led to a significant downregulation in the expression of
these genes (Fig. 2B–D). Our
results from RT-qPCR were consistent with those of western blot
results for the differentiation period of 9 days. The
overexpression of miR-204-5p in the hADSCs caused a significant
upregulation in the protein levels of C/EBPα, PPARγ and FABP4,
while its knockdown led to a significant decrease in the expression
of these genes (Fig. 2E and F).
Taken, our results suggest that miR-204-5p promotes the
adipogenesis of hADSCs.
DVL3 is a direct target of
miR-204-5p
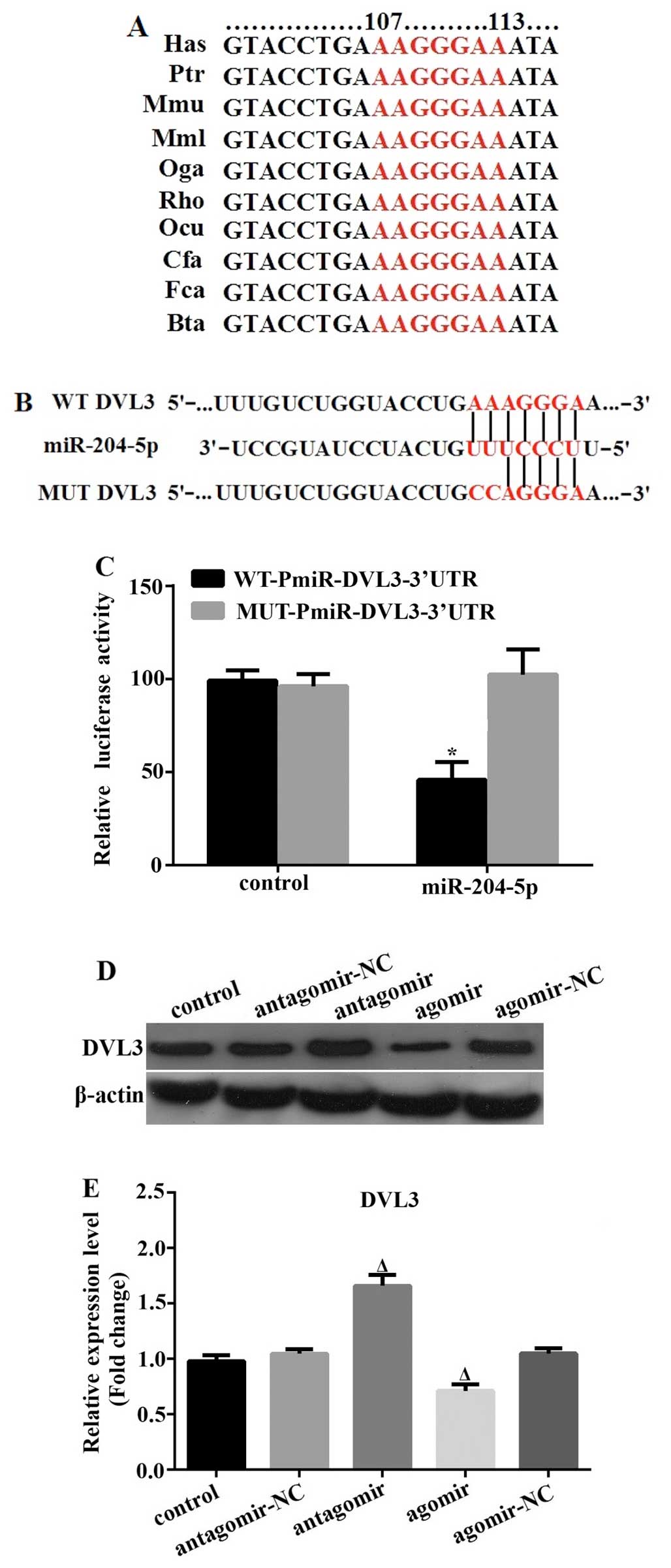
In order to further explore the molecular pathway
underlying the effects of miR-204-5p on the adipogenesis of hADSCs,
we first identified potential target genes using the online
prediction software, TargetScan, miRanda and PicTar. Specifically,
DVL3 was found to possess the miR-204-5p target site in its 3′-UTR,
and it was thus predicted, with a high score, to be a direct target
by all 3 programs. In addition, the target site was found to be
highly conserved among vertebrates (Fig. 3A). To confirm the predicted
molecular interaction between miR-204-5p and the 3′-UTR of DVL3, we
performed a standard dual-luciferase reporter assay using reporter
plasmids carrying the wild-type or mutated 3′-UTR (of DVL3) seed
target sequence (Fig. 3B). In
comparison with the control miR mimic-transfected 293T cells, we
found that co-transfection with the wild-type 3′-UTR luciferase
plasmid and miR-204-5p resulted in a significant suppression of
luciferase activity by 47%. By contrast, miR-204-5p had no effect
on the luciferase activity of the mutated 3′-UTR reporter (Fig. 3C). Finally, we examined the
protein expression level of DVL3 in the hADSCs following
transfection with miR-204-5p antagomir or agomir for 72 h. In
accordance with the above-mentioned findings, DVL3 expression was
significantly upregulated following transfection with the
miR-204-5p antagomir, and was markedly downregulated following
transfection with the miR-204-5p agomir (Fig. 3D and E). These results clearly
indicate that DVL3 is a direct target of miR-204-5p.
DVL3 regulates adipogenesis through
Wnt/β-catenin signaling
To determine whether DVL3 regulates adipogenesis, we
first explored its effect on the activity of Wnt/β-catenin
signaling by inducing its overexpression or knocking down its
expression in hADSCs for a period of 72 h. We found that both
β-catenin and CCND1 expression was upregulated in response to
transfection with pVAX1-DVL3, and downregulated following
transfection with siRNA-DVL3 (si-DVL3; Fig. 4A and B). Subsequently, we examined
the changes in the extent of adipogenesis using Oil Red O staining
in response to the knockdown of DVL3 in the hADSCs, following the
induction of adipogenic differen-tation for 9 days. Our results
indicated that transfection with si-DVL3 significantly promoted
adipogenesis when compared with the control (untransfected cells)
or the si-NC-transfected group. Furthermore, the expression of
several markers of mature cells of the adipogenic lineage (C/EBPα,
PPARγ and FABP4) was upregulated following the knockdown of DVL3,
as determined by RT-qPCR (Fig. 4C and
D). Our data suggest that DVL3 regulates the extent of
adipogenesis by modulating the activity of Wnt/β-catenin
signaling.
miR-204-5p suppresses Wnt/β-catenin
signaling, thus promoting adipogenesis
DVL3 is a critical regulator of canonical
Wnt/β-catenin signaling. Based on the above- mentioned results
demonstrating that DVL3 is a direct target of miR-204-5p, we wished
to determine whether this miRNA regulates the activity of the
Wnt/β-catenin pathway. For this purpose, we transfected the hADSCs
with either miR-204-5p agomir, agomiR-NC, antagomir or antagomiR-NC
and 2 days later, adipogenic differentiation was induced for 9
days, and we then determined the relative activity of Wnt/β-catenin
signaling by measuring the expression level of DVL3. As shown in
Fig. 5A and B, the DVL3 protein
levels were markedly decreased in the cells transfected with the
miR-204-5p agomir, while transfection with the miR-204-5p antagomir
led to a significant increase in DVL3 expression. Of note, the
expression of β-catenin, the mediator of canonical Wnt signaling,
and that of CCND1, a downstream target of this pathway, showed the
same pattern of alteration as DVL3. Thnus, our results suggest that
miR-204-5p suppresses canonical Wnt/β-catenin signaling, possibly
by regulating DVL3 expression, which subsequently promotes
adipogenesis.
Discussion
The differentiation of MSCs into mature adipoctyes
involves two main steps: i) the differentiation of multipotent MSCs
into committed preadipocytes; and ii) the maturation of
preadipocytes into adipoctyes through a molecular mechanism
mediated by PPAR and C/EBP, two transcription factors known to
regulate adipogenesis (19).
miR-204 has been described as a ‘switch’ molecule that can control
the fate choice between osteogenesis and adipogenesis. The
overexpression of miR-204 has been shown to promote adipogenesis
(17). However, little is known
about its role in regulating Wnt/β-catenin signaling during
adipocytic differentiation.
The role of miR-204 in regulating adipocytic
pathways is unclear. A previous study demonstrated a downregulation
in the expression level of miR-204 in the mature adipose tissue of
C57BLJ6 mice fed a high-fat diet compared to those mice fed a
standard diet (20). Another
study demonstrated that the miR-204 levels were significantly
upregulated in rat stromal vascular fraction (SVF) cells following
adipocytic differentiation (21).
Similar findings were reported by two other groups that studied
C3H10T1/2 (22) and mesenchymal
progenitor cell lines (17).
In the present study, we first examined the dynamics
of miR-204-5p expression during adipogenesis in hADSCs, and found
that its expression is gradually upregulated during this process.
This result is consistent with the results of a previous study
using SVF and C3H10T1/2 cells (22). We then demonstrated that the
overexpression of miR-204-5p enhanced the adipogenesis of hADSCs,
as illustrated by the significant increase in the quantity of lipid
droplets and in the mRNA expression of PPARγ, C/EBPα and FABP4.
Conversely, the downregulation of miR-204-5p suppressed adipogenic
differentiation. Our data strongly suggest that miR-204-5p plays an
important physiological role in regulating the adipogenesis of
MSCs.
Wnt/β-catenin signaling has been shown to negatively
regulate adipogenic differentiation in both MSCs and preadipocytes.
The disruption of any component of this pathway significantly
affects the generation of adipocytes. For example, as previously
demonstrated in 3T3-L1 cells, the overexpression of Wnt1, Wnt10b or
a GSK3β-mediated phosphorylation-defective form of β-catenin,
inhibit adipogenesis (23). The
constitutive expression of Axin or T-cell transcription factor 4
(TCF4) has been shown to induce spontaneous adipogenic
differentiation (24). Similar
observations have been made using MSCs (16). DVL is a positive regulator of
Wnt/β-catenin signaling. Accordingly, the exposure of 3T3-L1 cells
to anti-adipogenic compounds, such as
5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR),
epigallocatechin gallate (EGCG), platycodin D or
1,25-dihydroxyvitaminD3 [1,25(OH)2D3] has
been shown to induce the upregulation of DVL (25–29). In the present study, we
demonstrated that DVL3 expression progressively decreased during
the differentiation of hADSCs, suggesting that the former may be a
negative regulator of adipogenesis. Similar findings were reported
in the studies by Lagathu et al (30) and Lee et al (25).
In order to further explore the mechanisms
underlying the effects of miR-204-5p on adipocytic differentiation,
we performed bioinformatics analysis and a dual luciferase reporter
assay and verified that DVL3 is direct target of this miRNA. We
then examined the direct role of DVL3 in regulating adipogenesis.
Lee et al (31) performed
a series of experiments through which they determined that
compounds that inhibit adipogenesis, do so by upregulating
components of Wnt/β-catenin signaling. For example, treatment with
200 μM baicalin, a natural flavonoid compound extracted from
Suctellaria baicalensis, was shown to significantly inhibit
adipogenesis by downregulating the expression of PPARγ, C/EBPα and
FABP4 in mature adipoctyes. In the control groups (0 μM
baicalin), the β-catenin and CCND1 mRNA levels were gradually
downregulated during adipogenesis, while in the cells exposed to
baicalin, these levels were markedly upregulated at different time
points during differentiation. These findings were further verified
using β-catenin-targeted siRNA (31). In addition, in this study, we
demonstrated that the overexpression of DVL3 led to an increase in
β-catenin and CCND1 expression, and conversely, the knockdown of
DVL3 led to a decrease in β-catenin and CCND1 expression. In
addition, the knockdown of DVL3 in hADSCs significantly promoted
adipogenesis following induction for 9 days. Our findings indicate
that DVL3 regulates the extent of adipogen-esis by modulating
Wnt/β-catenin signaling. These findings were further corroborated
by inducing the overexpression or knockdown of miR-204-5p, that led
to alterations in the protein level of DVL3. To demonstrate the
effect of miR-204-5p on the activity of Wnt/β-catenin signaling, we
analyzed the expression of downstream effectors and targets of this
pathway in response to the overexpression or knockdown of
miR-204-5p in the hADSCs. We demonstrated that DVL3, β-catenin and
CCND1 expression was downregulated and upregulated in mature
adipoctyes, in response to transfection with the miR-204-5p agomir
or antagomir, respectively.
In conclusion, the present study provides direct
evidence that miR-204-5p induces the post-transcriptional silencing
of DVL3, an important negative regulator of adipogenesis. We also
demonstrate that miR-204-5p promotes adipogenesis by regulating the
activity of canonical Wnt/β-catenin signaling in hADSCs. These
findings suggest that DVL3 and miR-204-5p may serve as potential
therapeutic targets for the treatment and management of obesity and
other related metabolic disorders.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 81200599) and the Hunan
Province Nature Science Foundation of China (no. 13JJ4027). We
would like to thank Medjaden Bioscience Limited for their
assistance in the preparation of this manuscript.
References
|
1
|
Kanuri G and Bergheim I: In vitro and in
vivo models of non-alcoholic fatty liver disease (NAFLD). Int J Mol
Sci. 14:11963–11980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung YY, Yoon T, Yang WK, Moon BC and Kim
HK: Anti-obesity effects of Actinidia polygama extract in mice with
high-fat diet-induced obesity. Mol Med Rep. 7:396–400. 2013.
|
|
3
|
Ma J, Jiang Z, He S, Liu Y, Chen L, Long
K, Jin L, Jiang A, Zhu L, Wang J, Li M and Li X: Intrinsic features
in microRNA transcriptomes link porcine visceral rather than
subcutaneous adipose tissues tometabolic risk. PLoS One.
8:e800412013. View Article : Google Scholar
|
|
4
|
Song G, Xu G, Ji C, Shi C, Shen Y, Chen L,
Zhu L, Yang L, Zhao Y and Guo X: The role of microRNA-26b in human
adipocyte differentiation and proliferation. Gene. 10:481–487.
2014. View Article : Google Scholar
|
|
5
|
Chen L, Song J, Cui J, Hou J, Zheng X, Li
C and Liu L: microRNAs regulate adipocyte differentiation. Cell
Biol Int. 37:533–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karbiener M, Neuhold C, Oprriessnig P,
Prokesch A, Bogner- Strauss JG and Scheideler M: MicroRNA-30c
promotes human adipocyte differentiation and co-represses PAI-1 and
ALK2. RNA Biol. 8:850–860. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg
RJ and Li X: miR-17-92 cluster accelerates adipocyte
differentiation by negatively regulating tumor-suppressor Rb2/p130.
Proc Natl Acad Sci USA. 26:2889–2894. 2008. View Article : Google Scholar
|
|
8
|
Lee EK, Lee MJ, Abdelmohsen K, Kim W, Kim
MM, Srikantan S, Martindale JL, Hutchison ER, Kim HH, Marasa BS,
Selimyan R, Egan JM, Smith SR, Fried SK and Gorospe M: miR-130
suppresses adipogenesis by inhibiting peroxisome
proliferator-activated receptor gamma expression. Mol Cell Biol.
31:626–638. 2011. View Article : Google Scholar :
|
|
9
|
Kim SY, Kim AY, Lee HW, Son YH, Lee GY,
Lee JW, Lee YS and Kim JB: miR-27a is a negative regulator of
adipocyte differentiation via suppressing PPARgamma expression.
Biochem Biophys Res Commun. 392:323–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gerin I, Bommer GT, McCoin CS, Sousa KM,
Krishnan V and MacDougald OA: Roles for miRNA-378/378* in adipocyte
gene expression and lipogenesis. Am J Physiol Endocrinol Metab.
299:E198–E206. 2010.PubMed/NCBI
|
|
11
|
Martinelli R, Nardelli C, Pilone V,
Buonomo T, Liguori R, Castanò I, Buono P, Masone S, Persico G,
Forestieri P, Pastore L and Sacchetti L: miR-519d overexpression is
associated with human obesity. Obesity (Silver Spring).
18:2170–2176. 2010. View Article : Google Scholar
|
|
12
|
Kim YJ, Hwang SJ, Bae YC and Jung JS:
MiR-21 regulates adipogenic differentiation through the modulation
of TGF-beta signaling in mesenchymal stemcells derived from human
adipose tissue. Stem Cells. 27:3093–3102. 2009.PubMed/NCBI
|
|
13
|
Kennell JA, Gerin I, MacDougald OA and
Cadigan KM: The microRNA miR-8 is a conserved negative regulator of
Wnt signaling. Proc Natl Acad Sci USA. 105:15417–1522. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Wang S, Chen L, Chen Y, Wu M,
Zhang Y, Yu K, Huang Z, Qin L and Mo D: MicroRNA-344 inhibits
3T3-L1 cell differentiation via targeting GSK3β of Wnt/β-catenin
signaling pathway. FEBS Lett. 588:429–435. 2013. View Article : Google Scholar
|
|
15
|
Qin L, Chen Y, Niu Y, Chen W, Wang Q, Xiao
S, Li A, Xie Y, Li J, Zhao X, He Z and Mo D: A deep investigation
into the adipogenesis mechanism: profile of microRNAs regulating
adipogenesis by modulating the canonical Wnt/β-catenin signaling
pathway. BMC Genomics. 11:3202010. View Article : Google Scholar
|
|
16
|
Christodoulides C, Lagathu C, Sethi JK and
Vidal-Puig A: Adipogenesis and WNT signaling. Trends Endocrinol
Metab. 20:16–24. 2009. View Article : Google Scholar
|
|
17
|
Huang J, Zhao L, Xing L and Chen D:
MicroRNA-204 regulates Runx2 protein expression and mesenchymal
progenitor cell differentiation. Stem Cells. 28:357–364. 2010.
|
|
18
|
Yang X, Shang H, Katz A and Li X: A
modified aggregate culture for chondrogenesis of human
adipose-derived stem cells genetically modified with growth and
differentiation factor 5. Biores Open Access. 2:258–265. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hausman GJ, Dodson MV, Ajuwon K, Azain M,
Barnes KM, Guan LL, Jiang Z, Poulors SP, Srainz RD, Smith S,
Spurlock M, Novakofski J, Fernyhough ME and Bergen WG:
Board-invited review: the biology and regulation of preadipocytes
and adipocytes in meat animals. J Anim Sci. 87:1218–1246. 2009.
View Article : Google Scholar
|
|
20
|
Chartoumpekis DV, Zaravinos A, Ziros PG,
Iskrenova RP, Psyrogiannis AI, Kyriazopoulou VE and Habeos IG:
Differential expression of microRNAs in adipose tissue after
long-term high-fat diet-induced obesity in mice. PLoS One.
7:e348722012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fei J, Tamski H, Cook C and Santanam N:
MicroRNA regulation of adipose derived stem cells in aging rats.
PLoS One. 8:e592382013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Xie RL, Gordon J, LeBlanc K,
Stein JL, Lian JB, van Wijnen AJ and Stein GS: Control of
mesenchymal lineage progression by microRNAs targeting skeletal
gene regulators Trps1 and Runx2. J Biol Chem. 22:21926–21935. 2012.
View Article : Google Scholar
|
|
23
|
Liu J and Farmer SR: Regulating the
balance between peroxi-some proliferator-activated receptor gamma
andbeta-catenin signaling during adipogenesis. A glycogen synthase
kinase 3beta phosphorylation-defective mutant of beta-catenin
inhibits expression of a subset of adipogenic genes. J Biol Chem.
22:45020–45027. 2004. View Article : Google Scholar
|
|
24
|
Ross SE, Hemati N, Longo KA, Bennett CN,
Lucas PC, Erickson RL and MacDougald OA: Inhibition of adipogenesis
by Wnt signaling. Science. 11:950–953. 2000. View Article : Google Scholar
|
|
25
|
Lee H, Kang R, Bae S and Yoon Y: AICAR, an
activator of AMPK, inhibits adipogenesis via the WNT/β-catenin
pathway in 3T3-L1 adipocytes. Int J Mol Med. 28:65–71.
2011.PubMed/NCBI
|
|
26
|
Chan CY, Wei L, Castro-Muñozledo F and Koo
WL: (−)-Epigallocatechin-3-gallate blocks 3T3-L1 adipose conversion
by inhibition of cell proliferation and suppression of adipose
phenotype expression. Life Sci. 21:779–785. 2011. View Article : Google Scholar
|
|
27
|
Lee H, Bae S and Yoon Y: The
anti-adipogenic effects of (−)epigallocatechin gallate are
dependent on the WNT/β-catenin pathway. J Nutr Biochem.
24:1232–1240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee H, Bae S, Kim YS and Yoon Y:
WNT/β-catenin pathway mediates the anti-adipogenic effect of
platycodin D, a natural compound found in Platycodon grandiflorum.
Life Sci. 12:388–394. 2011. View Article : Google Scholar
|
|
29
|
Lee H, Bae S and Yoon Y: Anti-adipogenic
effects of 1,25-dihy-droxyvitamin D3 are mediated by the
maintenance of the wingless-type MMTV integration site/β-catenin
pathway. Int J Mol Med. 30:1219–1224. 2012.PubMed/NCBI
|
|
30
|
Lagathu C, Christodoulides C, Virtue S,
Cawthorn WP, Franzin C, Kimber WA, Nora ED, Campbell M, Medina-
Gomez G, Cheyette BN, Vidal-Puig AJ and Sethi JK: Dact1, a
nutritionally regulated preadipocyte gene, controls adipogen-esis
by coordinating the Wnt/beta-catenin signaling network. Diabetes.
58:609–619. 2009. View Article : Google Scholar :
|
|
31
|
Lee H, Kang R, Hahn Y, Yang Y, Kim SS, Cho
SH, Chung SI and Yoon Y: Anti obesity effect of baicalin involves
the modulations of proadipogenic and antiadipogenic regulators of
the adipogenesis pathway. Phytother Res. 23:1615–1623. 2009.
View Article : Google Scholar : PubMed/NCBI
|