1. Introduction
Ca2+ is a ubiquitous cellular signal.
Changes in intracellular Ca2+ control various cellular
processes that are relevant to regulating normal function and to
developing diseases. Examples of such processes include blood
coagulation, nervous excitation, angiogenesis, cell apoptosis and
the development of cancer (1).
Ca2+ also plays an important role in the physiological
function and pathological processes of the digestive system. The
calcium-sensing receptor (CaSR) is a member of the G
protein-coupled receptor (GPCR) C superfamily and plays a key role
in maintaining systemic calcium homeostasis (2). It is expressed in diverse mammalian
tissues, including the parathyroid gland, the cardiovascular system
and the entire digestive system (3), and it mediates insulin and gastric
acid secretions as well as intestinal fluid transport (4). A mutation in the CaSR gene might be a
predisposing factor that leads to an increased susceptibility of
chronic and recurrent acute pancreatitis (5). Further, the CaSR gene is believed to
be an antitumor factor and is decreased or even absent in adults
with colorectal cancer (34).
However, the mechanism and function of CaSR in the gastrointestinal
tract have not yet been completely elucidated, particularly
relating to certain digestive diseases and tumors. To support
CaSR-based therapeutics of digestive diseases, we need to deepen
our understanding of CaSR structure and its biochemical features
and to determine the role of CaSR in the physiological and
pathophysiological processes of the gastrointestinal system.
2. The structural features of CaSR
CaSR, a member of the C family of G protein-coupled
receptors (GPCR), was first cloned from the bovine parathyroid
gland (6). The human CaSR gene is
located on the long arm of chromosome 3, and it encodes a
polypeptide composed of 1,078 amino acids. CaSR can be divided into
three parts: the extracellular domain (ECD), the transmembrane
domain (TMD) and the intracellular domain. The extracellular domain
(ECD) (N terminal) includes 612 amino acids, which contains the
binding site of Ca2+ or a CaSR agonist. The transition
from the activation to the deactivation of CaSR mostly occurs in
the ECD. Similar to other GPCRs, CaSR has a 250-amino-acid
transmembrane domain (TMD) that has 7 transmembrane helices
(7,8). A cysteine-rich domain exists between
the ECD and the first transmembrane of TMD, removing this domain
results in the loss of CaSR signaling (9). The final 216 amino acids form the
intracellular tail (C terminal) that contains protein kinase C, A
and D phosphorylation sites (7).
CaSR agonists include polyvalent metal cations (such as
Ca2+ and Gd3+) and organic polycations such
as polylysine, protamine and L-amino acids.
Because the concentration of Ca2+ in the
blood does not change obviously, the complex structure and many
binding sites of CaSR are required to monitor and regulate the
blood Ca2+ concentration. Hu and Spiegel (8) and Huang et al (10) reported that the molecular structure
of the ECD of CaSR is bilobed, where every two ECDs form a Venus
flytrap (VFT) that has a binding site for Ca2+ in the
crevice of the two lobes. The TMD also contains the Ca2+
binding site to regulate when the ECD is inactivated (11). Several transcription factors take
part in modulating the activation of CaSR, such as vitamin D
response elements (VDRE), Stat1/3, NFκB and Sp1/3, and their
binding sites are located in the CaSR promoters (12,13).
3. The distribution of CaSR and its function
in Ca2+ homeostasis and cellular processes
To date, studies have confirmed the important role
of CaSR in Ca2+ homeostasis (2,14),
indicating that CaSR is expressed in most organs that regulate the
Ca2+ balance, including the parathyroid glands, kidney,
thyroid C cells, stomach, osteoblasts and osteoclasts (15–17).
CaSR was also shown to be widely expressed in the nervous system,
esophagus, liver, pancreas, pituitary gland, peripheral blood,
breast and arterial smooth muscle cells (15,18–20).
It is well known that several factors affect the mechanism of
Ca2+ homeostasis, including Ca2+ sensors in
the cell membrane and Ca2+ regulating hormones. CaSR is
an important Ca2+ sensor that not only regulates the
release of Ca2+ from the endoplasmic reticulum (ER) but
also controls the switch of the Ca2+ ion channel in the
cellular membrane, which allows the transport of Ca2+
into or out of the extracellular fluid (21,22).
For example, CaSR activation can mediate Ca2+ entry into
human aortic smooth muscle cells via transient receptor potential 6
(TRPC6) (21). TRPC3, another
member of the TRPC family, was proved to participate in the CaSR
mediation of Ca2+ overload in the heart (23). In addition, hormones play an
important role in regulating Ca2+ balance. CaSR has been
proven to regulate the secretion and production of hormones,
including parathyroid hormone (PTH), calcitonin (CT), and
1,25-dihydroxyvitamin D 3 [1,25(OH)23], that maintain
Ca2+ homeostasis (24,25).
Taken together, in physiological conditions, CaSR is critical to
the Ca2+ balance in the human body.
The CaSR acts through at least two G proteins
(Gαi and Gαq/11) to regulate multiple
intracellular second messengers, including inositol trisphosphate
(IP3), cytoplasmic free Ca2+
([Ca2+]cyt) and cyclic adenosine
monophosphate (cAMP) (26). The
activated CaSR can stimulate the phospholipase C (PLC-IP3) signal
pathway to prompt the release of Ca2+ from endoplasmic
reticulum (ER) (27). The
intracellular signal pathways including mitogen activated protein
kinases (MAPKs), phosphatidylinositol 3 kinase/protein kinase B
(PI3K/AKT), and phospholipases A, C and D can also be activated by
the activation of CaSR (27,28),
they then regulate several cellular processes that are involved in
cellular secretion, proliferation, differentiation, chemotaxis,
apoptosis, gene expression, ion channel switch and aging (3,29,30).
Numerous transcription factors participate in the complex
regulation process that depends on CaSR. For example, activated
CaSR can regulate the cyclin D family genes, c-Myc and c-Fos to
affect cell proliferation (31,32),
and NFκB can also be stimulated by CaSR to promote the secretion of
proinflammatory factors and chemokines that cause the development
of obesity induced adipose tissue dysfunction disease (33). CaSR was reported to decrease
β-catenin-TCF-4 complex formation and thus suppress the development
of tumors in colon cancer (34).
In summary, the CaSR is considered to be a critical player in
cellular processes.
4. Role of CaSR in the esophagus
CaSR is known to be expressed in the basal cells of
the human esophagus and in the non-tumorigenic esophageal
epithelial cell line HET-1A (35,36).
In the study of the HET-1A cell line, activated CaSR increases
intracellular Ca2+ concentration mobilization and
activates ERK1/2 (MAPK) signal pathways to promote the
multifunctional cytokine IL-8 (CX-CL8) secretion (36). In oesophagitis, Mulder et al
proved that CaSR can be activated by eosinophil-released major
basic protein (MBP) to promote fibroblast growth factor 9 (FGF9)
secretion and that it then affects the downstream
proliferation-related genes BMP-2 and BMP-4 to induce the
proliferation of esophageal epithelial cells (37). CaSR may be implicated in the
proliferative response to injury and the pathogenesis of
oesophagitis. However, it is not yet known whether the CaSR
activation affects the proliferation or differentiation of cancer
cells in animals or human esophageal cancer.
5. CaSR in the liver
Much evidence shows that CaSR is important for
normal hepatic physiological function and liver diseases. In 2001,
Canaff et al first identified expression in liver tissue and
primary cultured hepatocytes (20). Subsequently research demonstrated
the functional expression of CaSR in Buffalo rat liver cells and
that activated CaSR stimulated bile flow by the PLC-IP3 signal
transduction pathway (38). In the
hepatic ischemia/reperfusion (I/R) injury model, CaSR activation
induced cell apoptosis by promoting the p38 MAPK and ERK-1/2 signal
pathway phosphorylation and regulating the downstream Bcl-2, Cyt-c,
caspase-3 and Bax gene expression (39). More importantly, in the cirrhotic
animal model which was induced by carbon tetrachloride
(CCl4), CaSR has been reported to reduce the
intrahepatic resistance to portal flow (40). Thus, the regulation of CaSR might
serve as a new pharmacological target for the prevention and
treatment of drug- or alcohol-induced liver disease.
6. CaSR and the pancreas
In 2002, Rácz et al (19) were the first to report evidence for
the presence of CaSR in the normal human pancreas, pancreatic
cancer and chronic pancreatitis tissue. Immunohistochemistry
located the expression of CaSR in the endocrine and exocrine
pancreas, including β-islet cells, duct cell, acinar cells and
various non-exocrine cells such as intrapancreatic nerves and blood
vessels (19,41). These data suggest that CaSR has a
function in the human pancreas. However, the CaSR function varies
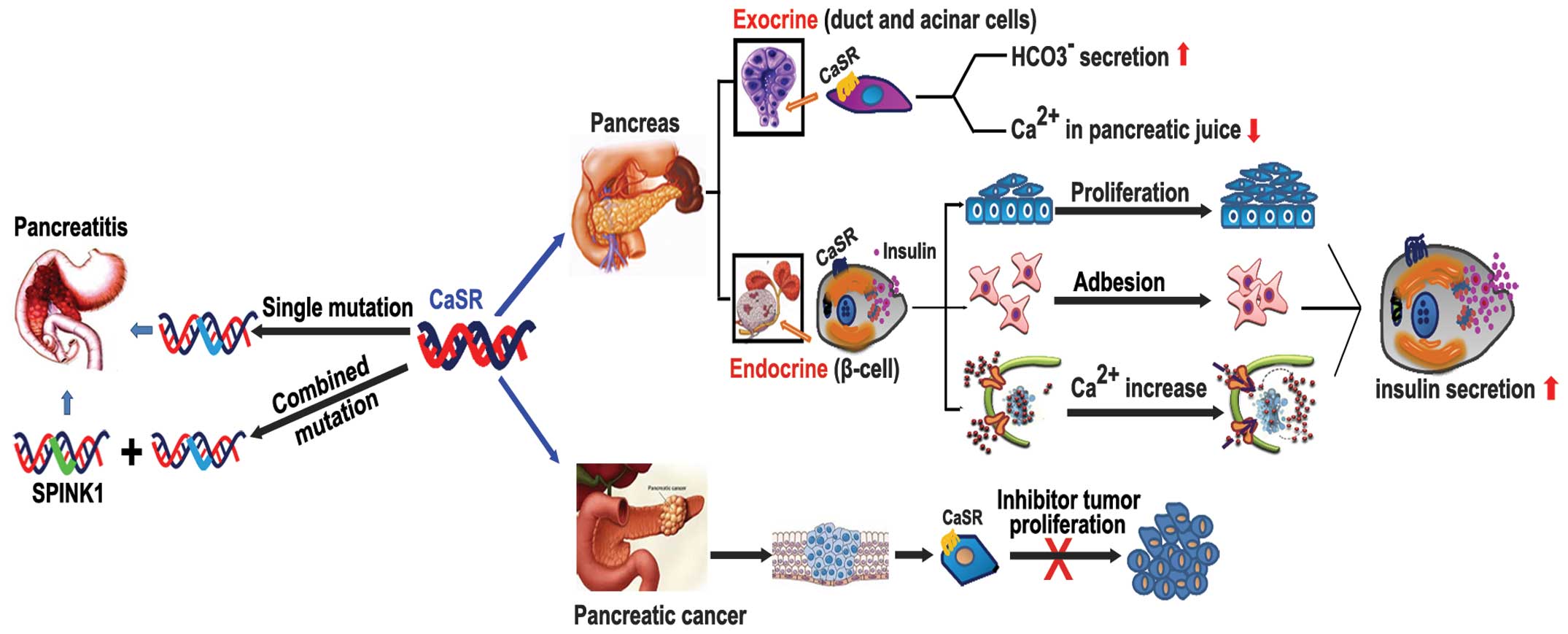
depending on where it is located in the pancreas (Fig. 1).
CaSR in exocrine and endocrine pancreas
physiology
The function of the exocrine pancreas mainly include
the secretion of various digestive enzymes and pancreatic juice.
CaSR was observed to be highly expressed in human pancreatic acinar
and ducts cells (19), suggesting
a potential role for CaSR in regulating pancreatic exocrine
function. In 1999, Bruce et al reported that the activation
of CaSR in the rat pancreas duct luminal membrane increased ductal
HCO3− secretion (41).
Moreover, CaSR could monitor and regulate the Ca2+
concentration in pancreatic juice by triggering ductal electrolyte
and fluid secretion, thereby reducing the precipitation of
Ca2+ salts in the duct lumen and decreasing both the
risk of carbonate stone formation and the progression to
pancreatitis (19). Although the
expression of CaSR could be detected in human acinar cells
(42), the function of the
receptor in this setting is not yet clear.
The function of CaSR in pancreatic endocrine tissue
is confirmed to participate in the regulation of glucose-induced
insulin secretion by β-cells (42–44).
It is known that change in plasma glucose concentration is the
major factor stimulating insulin release. The metabolism of glucose
could change the ATP/ADP ratio by closing ATP-dependent
K+ channels that then open the voltage-gated
Ca2+ channels (VGCC) to mediate intracellular
Ca2+ changes and induce insulin release (45). The extracellular Ca2+
levels were previously observed to induce transitory increases in
insulin secretion (46). The
experiments of Squires et al (45) and Gray et al (47) in human islets and insulin-secreting
cell lines (MIN6) also confirmed that low concentrations of
Ca2+ could induce a marked but transient insulin
secretion by activating the CaSR and the downstream MAPK signal
pathway. In addition, CaSR was shown to affect the β-cell
proliferation and cell-cell communication that cooperate in insulin
secretory responses (48). Hills
et al showed that CaSR was able to enhance the function of
the epithelial adhesion protein (E-cadherin) in β-cells and the
expression of L-type VDCC, which reinforces cell-cell adhesion and
β-cell function to promote the insulin secretion of neighboring
cells. However, the role of CaSR is dual because they have a
functional that varies between enhancing and inhibiting insulin
release. Studies have shown that CaSR in human β-cells have a
negative modulatory effect on insulin secretion (43). First, the CaSR is activated by
extracellular Ca2+ which is a promotive factor for
insulin secretion, but if there is a prolonged Ca2+
concentration-dependent activation, this is reversed and inhibition
of insulin secretion is affected. Moreover, the transduction
mechanism is confirmed to be unlikely through the cAMP or PLC-IP3
pathways. Squires et al (43) proposed that coupled receptor is the
possible mechanism for insulin secretion inhibition, but they have
not yet garnered enough evidence to prove this hypothesis. Although
it has been reported that the colocalization and the spatial
interactions between the L-type voltage-dependent calcium channels
(L-type VDCC) and CaSR enhanced the glucose-induced the secretion
of insulin (45), the latest
report found that the CaSR that is activated by L-histidine
depresses L-type VDCC activity and inhibits insulin secretion in
β-cells (49). Thus, the different
stimulation factors and space conformational changes of CaSR may be
a possible explanation for the role of CaSR in negatively
modulating insulin secretion.
The role of CaSR in pancreatic
disease
Recent studies have primarily focused on the acute
and chronic pancreatitis (CP) associated with CaSR receptor
mutation (6,50,51).
The mutations in the CaSR gene and the pancreatic secretory trypsin
inhibitor gene (SPINK1) were identified in patients with familial
hypocalciuric hypercalcemia (FHH), in 2010, genetic linkage and
candidate gene studies recognized that these two gene mutations
were associated with susceptibility to acute and/or chronic
pancreatitis (51,52). Felderbauer et al found that
mutations of the CaSR gene (R896H) were particularly associated
with SPINK1-related chronic pancreatitis (51). Clinical research has suggested that
pancreatitis patients usually have both the CaSR mutation and the
N34S SPINK1 gene mutation, whereas in healthy patients, only an
isolated CaSR or N34S SPINK1 gene mutation can be detected. This
finding suggests that the CaSR gene is an important co-factor in
SPINK1-related pancreatitis. In 2008, Murugaian et al found
that four new mutations of the CaSR gene in patients in India with
tropical chronic pancreatitis, which confirmed that CaSR variants
could lead to idiopathic CP with or without SPINK1 mutations
(53). Moreover, some literature
has elucidated the role of CaSR in pancreatic cancer. CaSR was
detected in human pancreatic cancer tissue and cell lines. Early
studies reported that CaSR is activated by elevated extracellular
Ca2+ or Gd3+ that can significantly reduce
cell proliferation in the well-differentiated human pancreatic
adenocarcinoma capan-1 cell line (19), but these results were obtained
in vitro. More clinical evidence is needed to determine the
role of CaSR in pancreatic tumors.
7. CaSR expression in the stomach
The regulatory function of
Ca2+ and CaSR in gastric acid secretion
The stomach is an important digestive organ. Gastric
acid plays an indispensable role in modulating digestion and
absorption, and an imbalance usually induces gastrointestinal
disease (54). Generally, the
classically modulated pathways of acid secretion are involved
including the paracrine, endocrine, and neuroendocrine systems
(55), in addition, the
H+-K+-ATPase activation was also shown to be
crucial to the formation of HCL and the regulation of gastric acid
secretion (56,57). Ca2+ is an important
second messenger and has a role in the above process. In previous
animal and human studies, experiments have proved that
Ca2+ and amino acids are useful in stimulating acid
secretion. High levels of intravenous or intestinal Ca2+
both promote the release of gastric acid (57,58).
Interestingly, we found that gastric acid increases that are
induced by hormonal or neuronal stimulation accompany an elevation
in intracellular Ca2+ levels that can be detected in the
gastric gland (59,60). Thus, there is a close connection
between Ca2+ and gastric acid secretion, and the CaSR is
confirmed to participate in the regulation of
Ca2+-induced gastric acid secretion.
The role of CaSR in regulating gastric acid
secretion has been proven in both in vivo and in
vitro experiments, and gastric cells of the basement membrane,
mucus cells, G cells and D cells all express CaSR (61). Ceglia et al provided the
first in vivo evidence that the activation of the CaSR
increases the serum gastrin levels and basal gastric acid secretion
in healthy older men and women (62). Activated CaSR increased the
intracellular Ca2+ concentration by leading to a release
of Ca2+ from the ER and causing an influx of
extracellular Ca2+. The elevated intracellular
Ca2+ level enhanced parietal cell
H+-K+-ATPase activity and subsequently
gastric acid secretion (62,63).
Further study showed that in isolated human gastric glands, the
signal pathway PLC-IP3 and ERK1/2 (MAPK) as well as
Ca2+-dependent and Ca2+-independent protein
kinase C (PKC) isoforms participate in CaSR activation and cause
acid secretion (64). The
regulation of gastrin release is another important pathway by which
CaSR moderates acid secretion. Research has shown that the G cells
expressing CaSR were sensitive to amino acids, pH and elevated
levels of Ca2+ and that these cells secreted gastrin in
the presence of these factors (4).
Double immunofluorescence studies validated the specific
colocalization of gastrin and CaSR in CaSR wild-type (WT) mice. The
lower extracellular Ca2+ concentrations activate CaSR to
produce a proliferative response in normal human gastric mucous
epithelial cells (65,66). Thus, a close connection may exist
between CaSR and gastrin. Indeed, many experiments showed that CaSR
was highly expressed on human gastrinoma cells and that activated
CaSR could increase the release of gastrin (67,68).
Taken together, these data support the hypothesis that the CaSR is
necessary for acid secretion, mucosal repair and the maintenance of
normal G-cell numbers.
Possible function of CaSR in gastric
cancer
In addition to the high level of CaSR expression in
the gastrinoma, few reports have described the role of CaSR in the
development of gastric diseases, particularly in the development of
gastric tumors (67,68). In 2007, Milne et al examined
CaSR expression in primary gastric carcinomas, corresponding
xenografts and two novel gastric carcinoma cell lines. The
immunohistochemistry data showed no significant loss of CaSR in
gastric cancers. A later analysis demonstrated that a gain in the
number of CaSR was observed in primary gastric tumor cells,
xenografts and cell lines (69).
The author suggested that CaSR does not appear to act as a tumor
suppressor gene in gastric cancer. Our laboratory data (not
published) support the above views. We found that CaSR expression
was significantly increased in gastric cancer patients and gastric
cancer cell lines MKN45 and 7901, and the high extracellular
Ca2+ can activate the CaSR to promote the proliferation
and migration of gastric cancer cells. Past studies have found that
high extracellular Ca2+ can mediate telomerase activity
in ovarian epithelial cells (70).
We speculated that CaSR might mediate this process because
extracellular Ca2+ is the agonist of CaSR. We
hypothesized that the same regulatory mechanism exists in the
development of gastric cancer. The activated CaSR could increase
human telomerase reverse transcriptase (hTERT) expression and
activity via the MAPK or the PI3K/AKT signal pathways to effect the
development of gastric cancer. However, we need to further test our
hypothesis. In conclusion, the above results suggest that CaSR
might act as a carcinogenic factor that participates in the gastric
tumor development process.
8. CaSR expression and function in the
intestines
Predominantly, research showed that CaSR is widely
expressed on the surface and basal region of the colonic crypt of
humans and rats (4), where it is involved in the
regulation of normal intestinal epithelial cell proliferation and
differentiation. In addition, rat colonic neurons comprising the
enteric nervous system have also shown to have CaSR expressed on
their surfaces (71,72). It is suggested that CaSR agonists
might act through neuronal pathways. However, the distribution of
CaSR in the colon crypt is controversial, and Chakrabarty et
al indicated an increase of CaSR expression along the crypt
axis, from the basal region to the top of the crypt (34,73).
However, Ahearn et al revealed the converse conclusion in
normal mucosa of colorectal adenoma patients (74). This difference in distribution may
be caused by the different genera or the functional changes in CaSR
expression during the development of colonic disease.
Ca2+ and CaSR regulate
intestinal secretion/absorption and fluid transport
The intestine is the main organ of nutrient
absorption and secretion that adjusts the flow of water and several
ions (Na+, Cl−, K+,
Ca2+) to maintain water and electrolyte homeostasis.
Human intestinal mucosal epithelium contains a Ca2+
sensing mechanism. In the early 1980s, changes in extracellular
Ca2+ as well as modulations in 1,25-dihydroxy vitamin D3
levels were observed to regulate the absorption and/or secretion of
Ca2+ in rat isolated colonic mucosa (75,76).
CaSR participated in the regulation of intestinal secretion and
absorption associated with Ca2+, organic nutrients and
amino acids. Mace et al found that CaSR was involved in the
L-amino acid stimulation of enteroendocrine (IEC) K-cell and L-cell
activity in the rat small intestine (77). The intestinal brush border
expresses CaSR, which helps to sense the presence of intraluminal
calcium and modifies calcium transcellular and paracellular
absorption by co-operating with the vitamin D system (78). The presence of CaSR provides a
fundamental mechanism that intestinal cells use to detect and
respond to Ca2+-related biologic behavior in intestinal
secretion and absorption.
In addition, the CaSR is known to play a central
role in intestinal fluid transport. It was reported that colonic
CaSR is activated by Ca2+/spermine and that this
activation could reverse the forskolin-stimulated net fluid
secretion in isolated rat colonic crypts (79,80).
The secretagogues such as forskolin or cholera toxin could induce
fluid secretion by three important mechanisms, including the second
message addition of cAMP and cGMP, the decrease in the activity of
Na+-dependent fluid absorption ion channel
sodium-hydrogen exchanger (NHE) (81,82),
and an increase in the phosphorylation activation of
Cl+-transport bumetanide-sensitive Na-K-2Cl
cotransporter (NKCC1) by CFTR (83). CaSR was shown to reverse the
generous fluid secretion by increasing the NHE activity, inhibiting
CFTR-NKCC1 pathway and enhancing the cyclic nucleotide destruction
to abrogate the addition of cAMP and cGMP (79,83,84).
Thus, the presence of CaSR may have important implications for the
prevention and treatment of certain diarrheal diseases. Moreover,
Ca2+ and CaSR were required for polyamine (spermine >
spermidine > putrescine) regulated fluid secretion. Cheng et
al (85) observed in rat
colonic crypts that spermine can not directly induce agonist
response in the absence of Ca2+ and that the interaction
of spermine with extracellular Ca2+ is able to enhance
the Ca2+ sensitivity of the CaSR and modulate luminal or
basolateral fluid.
The role of CaSR in colonic cancer and
drug resistance
The consensus viewpoint is that CaSR as a tumor
suppressor is down-regulated during colorectal tumorigenesis
(86,87). The activation of CaSR promotes
colonic cancer epithelial cell differentiation and decreases cell
growth. Thus, high Ca2+ dietary intake could reduce the
risk of colorectal cancer development (88). According to a previous study, the
mechanisms of CaSR that suppress colon cancer were relatively
clear: the cell-cell intercellular adhesion protein and
epithelial-mesenchymal transition important marker E-cadherin
played key roles in the CaSR-related tumor-suppressing effects in
colon cancer (34). On the one
hand, the increase in E-cadherin stimulated by CaSR can interact
with β-catenin, an important protooncogene, which is the member of
the Wnt pathway family that enhances the cell-cell and cell-matrix
adhesion via the actin-based cytoskeleton (89). This may contribute to reducing the
ability of cancer cells to move and invade surrounding tissues. On
the other hand, the activation of CaSR can also prevent the nuclear
translocation of β-catenin, thereby reducing β-catenin-TCF4 complex
formation and downregulating the c-myc and cyclin D1 expression.
This inhibits cell proliferation and the defective Wnt pathway
activation in colon cancer cell lines (34,90).
In addition, CaSR has been reported to mediate non-canonical Wnt
signaling (Wnt5a/Ror2) and to decrease the risk of
colitis-associated colon cancer by suppressing NFκB activity and
reducing inflammation TNFα secretion and TNFR1 expression (91). Thus, there is no doubt as to the
anti-oncogenic role of CaSR. Interestingly, in 2013, Singh et
al found that CaSR-null colon cancer cells regained CaSR
expression through the methylation and demethylation of the CaSR
gene, followed by the concurrent reversal of stem cell markers,
drug resistance and epithelial-mesenchymal transition (EMT) related
transcription factors (92). Taken
together, these data show that the inactivation of CaSR may serve
as a key role in colon carcinogenesis.
In recent years, CaSR research has focused on the
drug resistance of colonic cancer chemotherapy. Activated CaSR can
enhance the sensitivity of human colon carcinoma cells to mitomycin
C (MMC) and fluorouracil (5-FU). Cancer cells with a high level of
expression of thymidylate synthase (TS) and survivin are relatively
resistant to the 5-FU (93).
Nevertheless, NAD(P)H:quinine oxidoreductase 1 (NQO1) is a key
enzyme involved in the bioreductive activation of MMC, and its
reduced level is associated with MMC resistance in colon cancer
cells. The activated CaSR could up-regulate the expression of NQO1
and downregulate the expression of both TS and survivin to promote
cell apoptosis during chemotherapy. The conceivable mechanism is
that CaSR activation suppresses the transcriptional activation of
the survivin gene by inhibiting the β-catenin/Wnt signal pathway
and decreasing the formation of the T-cell factor/β-catenin dimer
(34). The dimer binding site is
the survivin gene promoter (94,95).
Moreover, CaSR can suppress c-Myc expression to negatively affect
the transcription of the TS gene (96,97).
In 2011, Liu et al found that CaSR could act synergistically
with the voltage-activated L-type Ca2+ channel (VGCC)
blocker nifedipine to increase the release of Ca2+ from
intracellular stores and enhance its sensitivity to 5-FU and 5-FU
metabolism (98). The human
multidrug resistance 1 (MDR1) gene and the expression of its
product gp170 might participate in this process (99–101). In summary, the G-protein-coupled
CaSR could act as a potential target for improving the
chemotherapeutic effect of colon cancer.
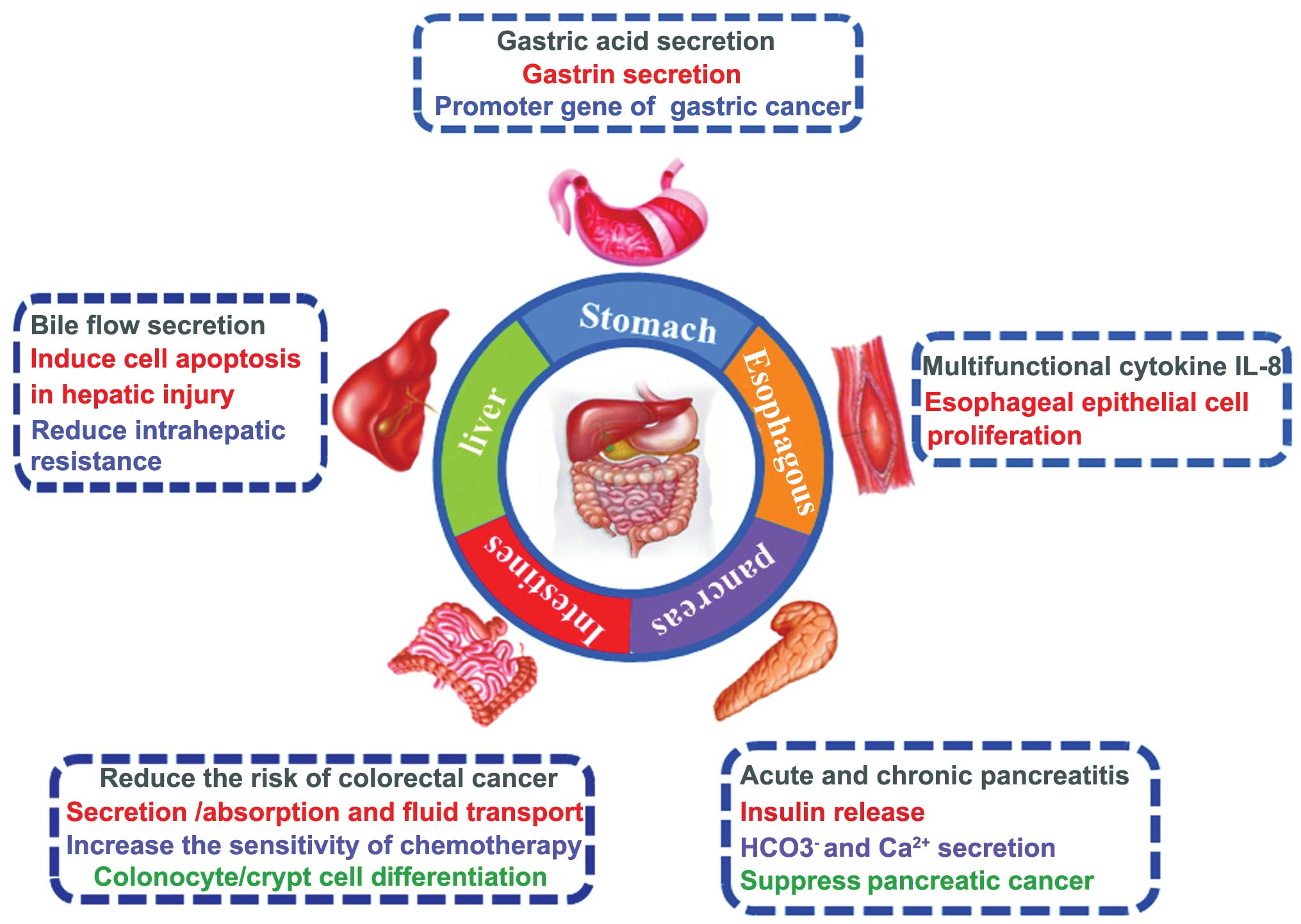
9. Conclusions
Presently evidence demonstrates that the CaSR
directly or indirectly regulates a variety of aspects of
gastrointestinal physiological function and disease occurrence
(Fig. 2). Recently, the function
of CaSR in inflammation-associated digestive disease (such as
esophagitis and colitis-associated colon cancer in humans) have
been a direction of new research. CaSR as a new therapeutic target
for treating digestive diseases has extensive clinical
significance, further research should pay more attention to the
application of scientific research for clinical applications.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81301731). We also thank Professor Hui
Dong (Department of Gastroenterology, Xinqiao Hospital, Third
Military Medical University) for highly professional services.
Abbreviations:
|
CaSR
|
calcium sensing receptor
|
|
ECD
|
extracellular domain
|
|
TMD
|
transmembrane domain
|
|
VDRE
|
vitamin D response elements
|
|
TRPC
|
transient receptor potential
|
|
ER
|
endoplasmic reticulum
|
|
MAPKs
|
mitogen activated protein kinases
|
|
PI3K/AKT
|
phosphatidylinositol 3 kinase/protein
kinase B
|
|
CX-CL8
|
multifunctional cytokine IL-8
|
|
FGF9
|
fibroblast growth factor 9
|
|
E-cadherin
|
epithelial adhesion protein
|
|
VGCC
|
voltage-gated Ca2+
channels
|
|
VDCC
|
voltage-dependent Ca2+
channel
|
|
SPNIK1
|
pancreatic secretory trypsin
inhibitor gene
|
|
PKC
|
protein kinase C
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
MMC
|
mitomycin C
|
|
5-FU
|
5-fluorouracil
|
References
|
1
|
Monteith GR: Calcium and cancer: targeting
Ca2+ transport. Nat Rev Cancer. 7:519–530. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown EM: Role of the calcium-sensing
receptor in extracellular calcium homeostasis. Best Pract Res Clin
Endocrinol Metab. 27:333–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brennan SC, Thiem U, Roth S, Aggarwal A,
Fetahu ISh, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F,
Mentaverri R, Riccardi D and Kallay E: Calcium sensing receptor
signalling in physiology and cancer. Biochim Biophys Acta.
1833:1732–1744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Geibel JP and Hebert SC: The functions and
roles of the extracellular Ca2+-sensing receptor along
the gastrointestinal tract. Annu Rev Physiol. 71:205–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felderbauer P, Hoffmann P, Einwächter H,
Bulut K, Ansorge N, Schmitz F and Schmidt WE: A novel mutation of
the calcium sensing receptor gene is associated with chronic
pancreatitis in a family with heterozygous SPINK1 mutations. BMC
Gastroenterol. 3:342003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brown EM, Gamba G, Riccardi D, Lombardi M,
Butters R, Kifor O, Sun A, Hediger MA, Lytton J and Hebert SC:
Cloning and characterization of an extracellular Ca(2+)-sensing
receptor from bovine parathyroid. Nature. 366:575–580. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garrett JE, Capuano IV, Hammerland LG,
Hung BC, Brown EM, Hebert SC, Nemeth EF and Fuller F: Molecular
cloning and functional expression of human parathyroid calcium
receptor cDNAs. J Biol Chem. 270:12919–12925. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu J and Spiegel AM: Structure and
function of the human calcium-sensing receptor: insights from
natural and engineered mutations and allosteric modulators. J Cell
Mol Med. 11:908–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu J, Hauache O and Spiegel AM: Human
Ca2+ receptor cysteine-rich domain. Analysis of function
of mutant and chimeric receptors. J Biol Chem. 275:16382–16389.
2000.
|
|
10
|
Huang Y, Zhou Y, Yang W, Butters R, Lee
HW, Li S, Castiblanco A, Brown EM and Yang JJ: Identification and
dissection of Ca(2+)- binding sites in the extracellular domain of
Ca(2+)-sensing receptor. J Biol Chem. 282:19000–19010. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nearing J, Betka M, Quinn S, Hentschel H,
Elger M, Baum M, Bai M, Chattopadyhay N, Brown EM, Hebert SC and
Harris HW: Polyvalent cation receptor proteins (CaRs) are salinity
sensors in fish. Proc Natl Acad Sci USA. 99:9231–9236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Canaff L and Hendy GN: Human
calcium-sensing receptor gene. Vitamin D response elements in
promoters P1 and P2 confer transcriptional responsiveness to
1,25-dihydroxyvitamin D. J Biol Chem. 277:30337–30350. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Canaff L and Hendy GN: Calcium-sensing
receptor gene transcription is up-regulated by the proinflammatory
cytokine, interleukin-1beta. Role of the NF-kappaB pathway and
kappaB elements. J Biol Chem. 280:14177–14188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brown EM: The extracellular
Ca2+-sensing receptor: central mediator of systemic
calcium homeostasis. Annu Rev Nutr. 20:507–533. 2000.
|
|
15
|
Ward BK, Magno AL, Walsh JP and Ratajczak
T: The role of the calcium-sensing receptor in human disease. Clin
Biochem. 45:943–953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dvorak MM and Riccardi D: Ca2+
as an extracellular signal in bone. Cell Calcium. 35:249–255.
2004.
|
|
17
|
Quarles LD: Extracellular calcium-sensing
receptors in the parathyroid gland, kidney, and other tissues. Curr
Opin Nephrol Hypertens. 12:349–355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wonneberger K, Scofield MA and Wangemann
P: Evidence for a calcium-sensing receptor in the vascular smooth
muscle cells of the spiral modiolar artery. J Membr Biol.
175:203–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rácz GZ, Kittel A, Riccardi D, Case RM,
Elliott AC and Varga G: Extracellular calcium sensing receptor in
human pancreatic cells. Gut. 51:705–711. 2002.PubMed/NCBI
|
|
20
|
Canaff L, Petit JL, Kisiel M, Watson PH,
Gascon-Barré M and Hendy GN: Extracellular calcium-sensing receptor
is expressed in rat hepatocytes coupling to intracellular calcium
mobilization and stimulation of bile flow. J Biol Chem.
276:4070–4079. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chow JY, Estrema C, Orneles T, Dong X,
Barrett KE and Dong H: Calcium-sensing receptor modulates
extracellular Ca(2+) entry via TRPC-encoded receptor-operated
channels in human aortic smooth muscle cells. Am J Physiol Cell
Physiol. 301:C461–C468. 2011. View Article : Google Scholar
|
|
22
|
Breitwieser GE: Calcium sensing receptors
and calcium oscillations: calcium as a first messenger. Curr Top
Dev Biol. 73:85–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng SL, Sun MR, Li TT, Yin X, Xu CQ and
Sun YH: Activation of calcium-sensing receptor increases TRPC3
expression in rat cardiomyocytes. Biochem Biophys Res Commun.
406:278–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown EM: Clinical lessons from the
calcium-sensing receptor. Nat Clin Pract Endocrinol Metab.
3:122–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeon US: Kidney and calcium homeostasis.
Electrolyte Blood Press. 6:68–76. 2008. View Article : Google Scholar
|
|
26
|
Brown EM and MacLeod RJ: Extracellular
calcium sensing and extracellular calcium signaling. Physiol Rev.
81:239–297. 2001.PubMed/NCBI
|
|
27
|
Brennan SC and Conigrave AD: Regulation of
cellular signal transduction pathways by the extracellular
calcium-sensing receptor. Curr Pharm Biotechnol. 10:270–281. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smrcka AV: G protein betagamma subunits:
central mediators of G protein-coupled receptor signaling. Cell Mol
Life Sci. 65:2191–2214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin KI, Chattopadhyay N, Bai M, Alvarez R,
Dang CV, Baraban JM, Brown EM and Ratan RR: Elevated extracellular
calcium can prevent apoptosis via the calcium-sensing receptor.
Biochem Biophys Res Commun. 249:325–331. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh N, Promkan M, Liu G, Varani J and
Chakrabarty S: Role of calcium sensing receptor (CaSR) in
tumorigenesis. Best Pract Res Clin Endocrinol Metab. 27:455–463.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chattopadhyay N, Yano S, Tfelt-Hansen J,
Rooney P, Kanuparthi D, Bandyopadhyay S, Ren X, Terwilliger E and
Brown EM: Mitogenic action of calcium-sensing receptor on rat
calvarial osteoblasts. Endocrinology. 145:3451–3462. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kállay E, Kifor O, Chattopadhyay N, Brown
EM, Bischof MG, Peterlik M and Cross HS: Calcium-dependent c-myc
protooncogene expression and proliferation of Caco-2 cells: a role
for a luminal extracellular calcium-sensing receptor. Biochem
Biophys Res Commun. 232:80–83. 1997.
|
|
33
|
Cifuentes M, Fuentes C, Tobar N, Acevedo
I, Villalobos E, Hugo E, Ben-Jonathan N and Reyes M: Calcium
sensing receptor activation elevates proinflammatory factor
expression in human adipose cells and adipose tissue. Mol Cell
Endocrinol. 361:24–30. 2012. View Article : Google Scholar
|
|
34
|
Chakrabarty S, Radjendirane V, Appelman H
and Varani J: Extracellular calcium and calcium sensing receptor
function in human colon carcinomas: promotion of E-cadherin
expression and suppression of beta-catenin/TCF activation. Cancer
Res. 63:67–71. 2003.
|
|
35
|
Stoner GD, Kaighn ME, Reddel RR, Resau JH,
Bowman D, Naito Z, Matsukura N, You M, Galati AJ and Harris CC:
Establishment and characterization of SV40 T-antigen immortalized
human esophageal epithelial cells. Cancer Res. 51:365–371.
1991.PubMed/NCBI
|
|
36
|
Justinich CJ, Mak N, Pacheco I, Mulder D,
Wells RW, Blennerhassett MG and MacLeod RJ: The extracellular
calcium-sensing receptor (CaSR) on human esophagus and evidence of
expression of the CaSR on the esophageal epithelial cell line
(HET-1A). Am J Physiol Gastrointest Liver Physiol. 294:G120–G129.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mulder DJ, Pacheco I, Hurlbut DJ, Mak N,
Furuta GT, MacLeod RJ and Justinich CJ: FGF9-induced proliferative
response to eosinophilic inflammation in oesophagitis. Gut.
58:166–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xing W, Li G, Xi Y, Guo J, Li H, Li H,
Zhang W, Zhang L, Wu L, Wang R and Xu C: The functional expression
of calcium-sensing receptors in BRL cells and related signal
transduction pathway responsible for intracellular calcium
elevation. Mol Cell Biochem. 343:13–19. 2010. View Article : Google Scholar
|
|
39
|
Xing WJ, Kong FJ, Li GW, Qiao K, Zhang WH,
Zhang L, Bai SZ, Xi YH, Li HX, Tian Y, Ren H, Wu LY, Wang R and Xu
CQ: Calcium-sensing receptors induce apoptosis during simulated
ischaemia-reperfusion in Buffalo rat liver cells. Clin Exp
Pharmacol Physiol. 38:605–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sansoè G, Aragno M, Mastrocola R,
Paternostro C and Parola M: Calcium receptors located in fibrotic
septa: a new target to reduce portal pressure in liver cirrhosis.
Clin Sci (Lond). 125:67–75. 2013.PubMed/NCBI
|
|
41
|
Bruce JI, Yang X, Ferguson CJ, Elliott AC,
Steward MC, Case RM and Riccardi D: Molecular and functional
identification of a Ca2+ (polyvalent cation)-sensing
receptor in rat pancreas. J Biol Chem. 274:20561–20568. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jones PM, Kitsou-Mylona I, Gray E, Squires
PE and Persaud SJ: Expression and function of the extracellular
calcium-sensing receptor in pancreatic beta-cells. Arch Physiol
Biochem. 113:98–103. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Squires PE, Harris TE, Persaud SJ, Curtis
SB, Buchan AM and Jones PM: The extracellular calcium-sensing
receptor on human beta-cells negatively modulates insulin
secretion. Diabetes. 49:409–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Parkash J: Glucose-mediated spatial
interactions of voltage dependent calcium channels and calcium
sensing receptor in insulin producing beta-cells. Life Sci.
88:257–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Squires PE: Non-Ca2+
homeostatic functions of the extracellular Ca2+-sensing
receptor (CaR) in endocrine tissues. J Endocrinol. 165:173–177.
2000.
|
|
46
|
Devis G, Somers G and Malaisse WJ:
Stimulation of insulin release by calcium. Biochem Biophys Res
Commun. 67:525–529. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gray E, Muller D, Squires PE, Asare-Anane
H, Huang GC, Amiel S, Persaud SJ and Jones PM: Activation of the
extracellular calcium-sensing receptor initiates insulin secretion
from human islets of Langerhans: involvement of protein kinases. J
Endocrinol. 190:703–710. 2006. View Article : Google Scholar
|
|
48
|
Hills CE, Younis MY, Bennett J,
Siamantouras E, Liu KK and Squires PE: Calcium-sensing receptor
activation increases cell-cell adhesion and beta-cell function.
Cell Physiol Biochem. 30:575–586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Parkash J and Asotra K: L-histidine
sensing by calcium sensing receptor inhibits voltage-dependent
calcium channel activity and insulin secretion in beta-cells. Life
Sci. 88:440–446. 2011. View Article : Google Scholar
|
|
50
|
Whitcomb DC: Genetic aspects of
pancreatitis. Annu Rev Med. 61:413–424. 2010. View Article : Google Scholar
|
|
51
|
Felderbauer P, Klein W, Bulut K, Ansorge
N, Dekomien G, Werner I, Epplen JT, Schmitz F and Schmidt WE:
Mutations in the calcium-sensing receptor: a new genetic risk
factor for chronic pancreatitis? Scand J Gastroenterol. 41:343–348.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Muddana V, Lamb J, Greer JB, Elinoff B,
Hawes RH, Cotton PB, Anderson MA, Brand RE, Slivka A and Whitcomb
DC: Association between calcium sensing receptor gene polymorphisms
and chronic pancreatitis in a US population: role of serine
protease inhibitor Kazal 1 type and alcohol. World J Gastroenterol.
14:4486–4491. 2008. View Article : Google Scholar
|
|
53
|
Murugaian EE, Premkumar RM, Radhakrishnan
L and Vallath B: Novel mutations in the calcium sensing receptor
gene in tropical chronic pancreatitis in India. Scand J
Gastroenterol. 43:117–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aditi A, Graham DY and Vitamin C:
Gastritis, and gastric disease: a historical review and update. Dig
Dis Sci. 57:2504–2015. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Schubert ML: Gastric secretion. Curr Opin
Gastroenterol. 27:536–542. 2011. View Article : Google Scholar
|
|
56
|
Prinz C, Kajimura M, Scott D, Helander H,
Shin J, Besancon M, Bamberg K, Hersey S and Sachs G: Acid secretion
and the H,K ATPase of stomach. Yale J Biol Med. 65:577–596.
1992.PubMed/NCBI
|
|
57
|
Reeder DD, Conlee JL and Thompson JC:
Calcium carbonate antacid and serum gastrin concentration in
duodenal ulcer. Surg Forum. 22:308–310. 1971.PubMed/NCBI
|
|
58
|
Levant JA, Walsh JH and Isenberg JI:
Stimulation of gastric secretion and gastrin release by single oral
doses of calcium carbonate in man. N Engl J Med. 289:555–558. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hirschowitz BI, Keeling D, Lewin M, Okabe
S, Parsons M, Sewing K, Wallmark B and Sachs G: Pharmacological
aspects of acid secretion. Dig Dis Sci. 40:3S–23S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sachs G, Shin JM, Briving C, Wallmark B
and Hersey S: The pharmacology of the gastric acid pump: the
H+,K+ ATPase. Annu Rev Pharmacol Toxicol.
35:277–305. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cheng I, Qureshi I, Chattopadhyay N,
Qureshi A, Butters RR, Hall AE, Cima RR, Rogers KV, Hebert SC,
Geibel JP, Brown EM and Soybel DI: Expression of an extracellular
calcium-sensing receptor in rat stomach. Gastroenterology.
116:118–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ceglia L, Harris SS, Rasmussen HM and
Dawson-Hughes B: Activation of the calcium sensing receptor
stimulates gastrin and gastric acid secretion in healthy
participants. Osteoporos Int. 20:71–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Geibel JP, Wagner CA, Caroppo R, Qureshi
I, Gloeckner J, Manuelidis L, Kirchhoff P and Radebold K: The
stomach divalent ion-sensing receptor scar is a modulator of
gastric acid secretion. J Biol Chem. 276:39549–39552. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Remy C, Kirchhoff P, Hafner P, Busque SM,
Müeller MK, Geibel JP and Wagner CA: Stimulatory pathways of the
calcium-sensing receptor on acid secretion in freshly isolated
human gastric glands. Cell Physiol Biochem. 19:33–42. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rutten MJ, Bacon KD, Marlink KL, Stoney M,
Meichsner CL, Lee FP, Hobson SA, Rodland KD, Sheppard BC, Trunkey
DD, Deveney KE and Deveney CW: Identification of a functional
Ca2+-sensing receptor in normal human gastric mucous
epithelial cells. Am J Physiol. 277:G662–G670. 1999.PubMed/NCBI
|
|
66
|
Feng J, Petersen CD, Coy DH, Jiang JK,
Thomas CJ, Pollak MR and Wank SA: Calcium-sensing receptor is a
physiologic multimodal chemosensor regulating gastric G-cell growth
and gastrin secretion. Proc Natl Acad Sci USA. 107:17791–17796.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Itami A, Kato M, Komoto I, Doi R, Hosotani
R, Shimada Y and Imamura M: Human gastrinoma cells express
calcium-sensing receptor. Life Sci. 70:119–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Goebel SU, Peghini PL, Goldsmith PK,
Spiegel AM, Gibril F, Raffeld M, Jensen RT and Serrano J:
Expression of the calcium-sensing receptor in gastrinomas. J Clin
Endocrinol Metab. 85:4131–4137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Milne AN, Sitarz R, Carvalho R, Polak MM,
Ligtenberg M, Pauwels P, Offerhaus GJ and Weterman MA: Molecular
analysis of primary gastric cancer, corresponding xenografts, and 2
novel gastric carcinoma cell lines reveals novel alterations in
gastric carcinogenesis. Hum Pathol. 38:903–913. 2007. View Article : Google Scholar
|
|
70
|
Alfonso-De Matte MY, Moses-Soto H and Kruk
PA: Calcium-mediated telomerase activity in ovarian epithelial
cells. Arch Biochem Biophys. 399:239–244. 2002.PubMed/NCBI
|
|
71
|
Sheinin Y, Kállay E, Wrba F, Kriwanek S,
Peterlik M and Cross HS: Immunocytochemical localization of the
extracellular calcium-sensing receptor in normal and malignant
human large intestinal mucosa. J Histochem Cytochem. 48:595–602.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cheng SX: Calcium-sensing receptor
inhibits secretagogue-induced electrolyte secretion by intestine
via the enteric nervous system. Am J Physiol Gastrointest Liver
Physiol. 30:G60–G70. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chakrabarty S, Wang H, Canaff L, Hendy GN,
Appelman H and Varani J: Calcium sensing receptor in human colon
carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3).
Cancer Res. 65:493–498. 2005.PubMed/NCBI
|
|
74
|
Ahearn TU, McCullough ML, Flanders WD,
Long Q, Sidelnikov E, Fedirko V, Daniel CR, Rutherford RE, Shaukat
A and Bostick RM: A randomized clinical trial of the effects of
supplemental calcium and vitamin D3 on markers of their metabolism
in normal mucosa of colorectal adenoma patients. Cancer Res.
71:413–423. 2011. View Article : Google Scholar
|
|
75
|
Favus MJ, Kathpalia SC and Coe FL: Kinetic
characteristics of calcium absorption and secretion by rat colon.
Am J Physiol. 240:G350–G354. 1981.PubMed/NCBI
|
|
76
|
Favus MJ, Kathpalia SC, Coe FL and Mond
AE: Effects of diet calcium and 1,25-dihydroxyvitamin D3 on colon
calcium active transport. Am J Physiol. 238:G75–G78.
1980.PubMed/NCBI
|
|
77
|
Mace OJ, Schindler M and Patel S: The
regulation of K- and L-cell activity by GLUT2 and the
calcium-sensing receptor CasR in rat small intestine. J Physiol.
90:2917–2936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Garg MK, Kalra S and Mahalle N: The
intestinal calcistat: Determinant of clinical vitamin D deficiency.
Indian J Endocrinol Metab. 17:780–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Geibel J, Sritharan K, Geibel R, Geibel P,
Persing JS, Seeger A, Roepke TK, Deichstetter M, Prinz C, Cheng SX,
Martin D and Hebert SC: Calcium-sensing receptor abrogates
secretagogue-induced increases in intestinal net fluid secretion by
enhancing cyclic nucleotide destruction. Proc Natl Acad Sci USA.
103:9390–9397. 2006. View Article : Google Scholar
|
|
80
|
Cheng SX, Okuda M, Hall AE, Geibel JP and
Hebert SC: Expression of calcium-sensing receptor in rat colonic
epithelium: evidence for modulation of fluid secretion. Am J
Physiol Gastrointest Liver Physiol. 283:G240–G250. 2002.PubMed/NCBI
|
|
81
|
Kunzelmann K and Mall M: Electrolyte
transport in the mammalian colon: mechanisms and implications for
disease. Physiol Rev. 82:245–289. 2002.PubMed/NCBI
|
|
82
|
Donowitz M and Welsh MJ: Ca2+
and cyclic AMP in regulation of intestinal Na, K, and Cl transport.
Annu Rev Physiol. 48:135–150. 1986.
|
|
83
|
Haas M and Forbush B III: The Na-K-Cl
cotransporter of secretory epithelia. Annu Rev Physiol. 62:515–534.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Beavo JA: Cyclic nucleotide
phosphodiesterases: functional implications of multiple isoforms.
Physiol Rev. 75:725–748. 1995.PubMed/NCBI
|
|
85
|
Cheng SX, Geibel JP and Hebert SC:
Extracellular polyamines regulate fluid secretion in rat colonic
crypts via the extracellular calcium-sensing receptor.
Gastroenterology. 126:148–158. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Rogers AC, Hanly AM, Collins D, Baird AW
and Winter DC: Review article: loss of the calcium-sensing receptor
in colonic epithelium is a key event in the pathogenesis of colon
cancer. Clin Colorectal Cancer. 11:24–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Bresalier RS: Calcium, chemoprevention,
and cancer: a small step forward (a long way to go).
Gastroenterology. 116:1261–1263. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lamprecht SA and Lipkin M: Cellular
mechanisms of calcium and vitamin D in the inhibition of colorectal
carcinogenesis. Ann NY Acad Sci. 952:73–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Brembeck FH, Rosario M and Birchmeier W:
Balancing cell adhesion and Wnt signaling, the key role of
beta-catenin. Curr Opin Genet Dev. 16:51–59. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
MacLeod RJ, Hayes M and Pacheco I: Wnt5a
secretion stimulated by the extracellular calcium-sensing receptor
inhibits defective Wnt signaling in colon cancer cells. Am J
Physiol Gastrointest Liver Physiol. 293:G403–G411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kelly JC, Lungchukiet P and Macleod RJ:
Extracellular calcium-sensing receptor inhibition of intestinal
epithelial TNF signaling requires CaSR-mediated Wnt5a/Ror2
interaction. Front Physiol. 2:172011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Singh N and Chakrabarty S: Induction of
CaSR expression circumvents the molecular features of malignant
CaSR null colon cancer cells. Int J Cancer. 133:2307–2314. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Liu G, Hu X, Varani J and Chakrabarty S:
Calcium and calcium sensing receptor modulates the expression of
thymidylate synthase, NAD(P)H:quinone oxidoreductase 1 and survivin
in human colon carcinoma cells: promotion of cytotoxic response to
mitomycin C and fluorouracil. Mol Carcinog. 48:202–211. 2009.
View Article : Google Scholar
|
|
94
|
Kim PJ, Plescia J, Clevers H, Fearon ER
and Altieri DC: Survivin and molecular pathogenesis of colorectal
cancer. Lancet. 362:205–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Van de Wetering M, Oosterwegel M, Dooijes
D and Clevers H: Identification and cloning of TCF-1, a T
lymphocyte-specific transcription factor containing a
sequence-specific HMG box. EMBO J. 10:123–132. 1991.PubMed/NCBI
|
|
96
|
Lincz LF, Scorgie FE, Garg MB and Ackland
SP: Identification of a novel single nucleotide polymorphism in the
first tandem repeat sequence of the thymidylate synthase 2R allele.
Int J Cancer. 120:1930–1934. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Akopov SB, Chernov IP, Wahlström T,
Kostina MB, Klein G, Henriksson M and Nikolaev LG: Identification
of recognition sites for myc/max/mxd network proteins by a whole
human chromosome 19 selection strategy. Biochemistry (Mosc).
73:1260–1268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu G, Hu X, Premkumar L and Chakrabarty
S: Nifedipine synergizes with calcium in activating the calcium
sensing receptor, suppressing the expression of thymidylate
synthase and survivin and promoting sensitivity to fluorouracil in
human colon carcinoma cells. Mol Carcinog. 50:922–930. 2011.
View Article : Google Scholar
|
|
99
|
Hunter J, Hirst BH and Simmons NL:
Epithelial secretion of vinblastine by human intestinal
adenocarcinoma cell (HCT-8 and T84) layers expressing
P-glycoprotein. Br J Cancer. 64:437–444. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Gottesman MM, Pastan I and Ambudkar SV:
P-glycoprotein and multidrug resistance. Curr Opin Genet Dev.
6:610–617. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rimet O, Mirrione A and Barra Y:
Multidrug-resistant phenotype influences the differentiation of a
human colon carcinoma cell line. Biochem Biophys Res Commun.
259:43–49. 1999. View Article : Google Scholar : PubMed/NCBI
|