Spandidos Publications style
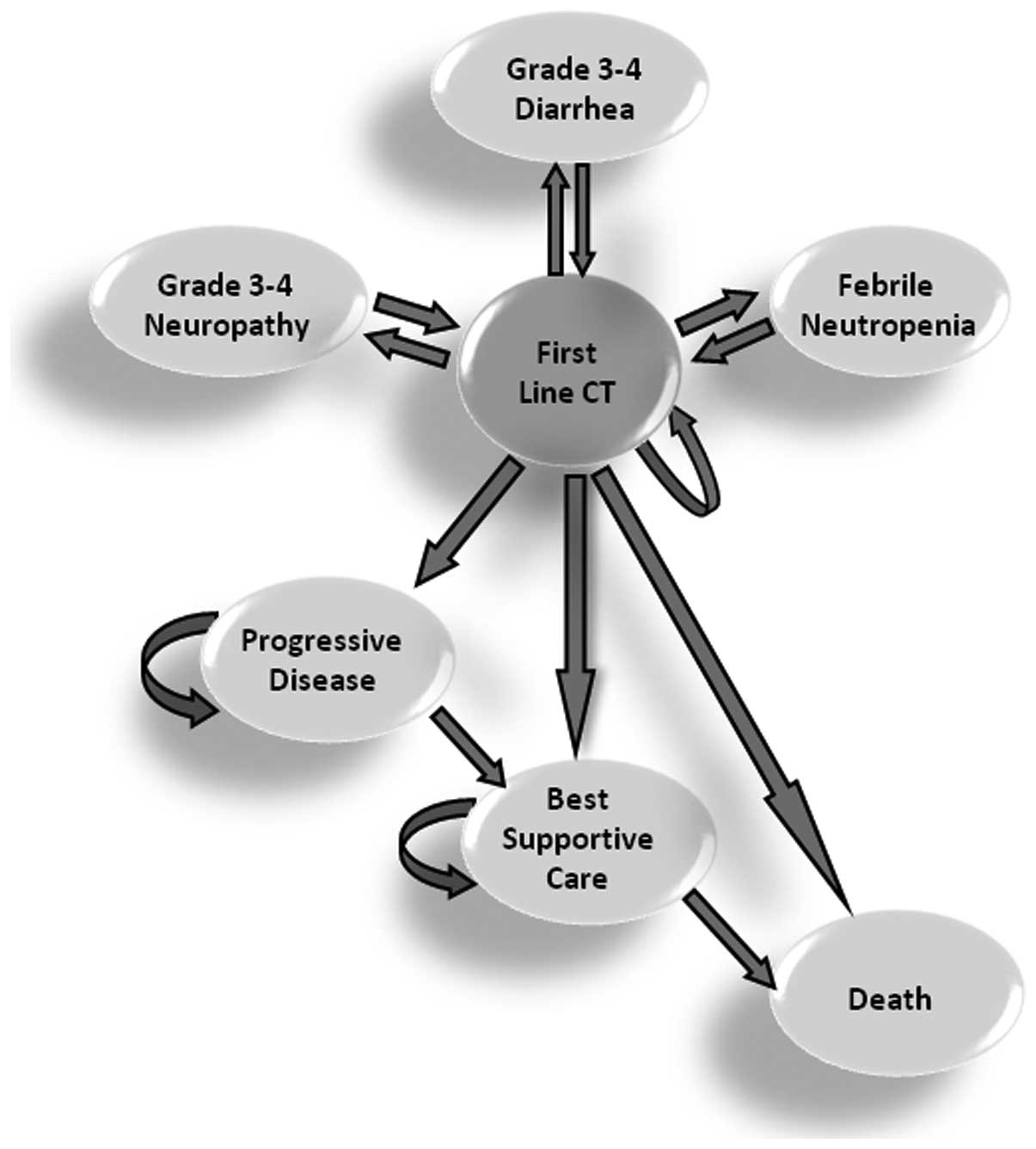
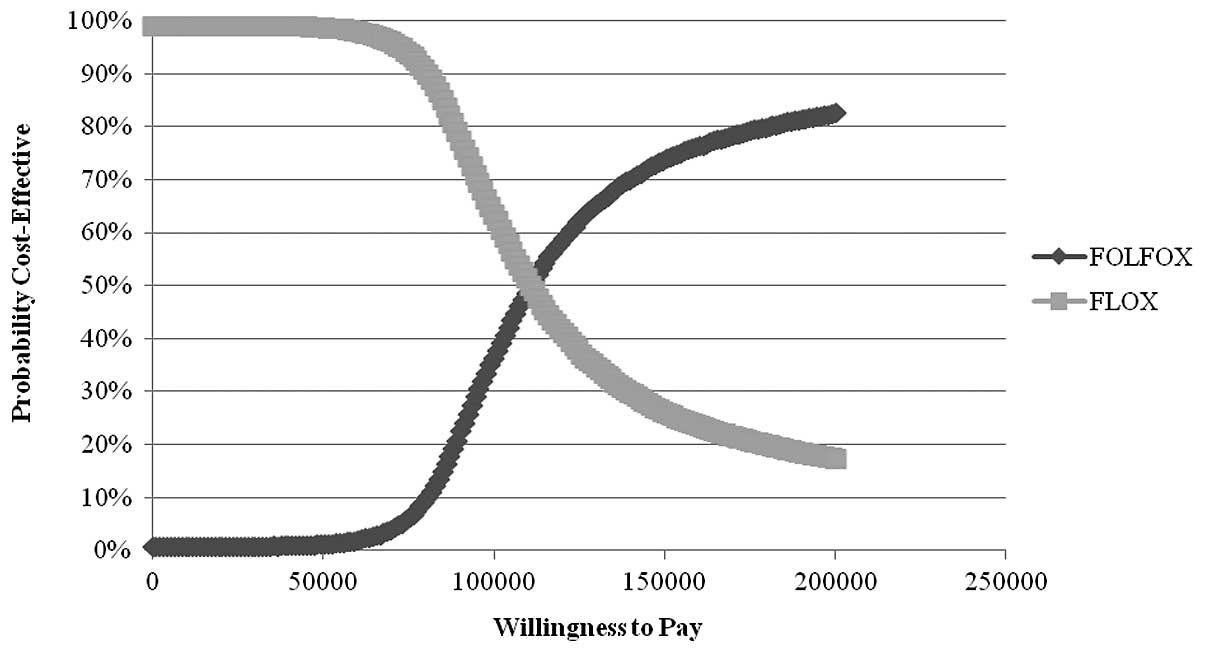
Nebuloni DR, Mak MP, Souza FH, Saragiotto DF, Júlio T, De Castro Jr G, Sabbaga J and Hoff PM: Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: Effectiveness and cost-utility analysis. Mol Clin Oncol 1: 175-179, 2013.
APA
Nebuloni, D.R., Mak, M.P., Souza, F.H., Saragiotto, D.F., Júlio, T., De Castro Jr, G. ... Hoff, P.M. (2013). Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: Effectiveness and cost-utility analysis. Molecular and Clinical Oncology, 1, 175-179. https://doi.org/10.3892/mco.2012.12
MLA
Nebuloni, D. R., Mak, M. P., Souza, F. H., Saragiotto, D. F., Júlio, T., De Castro Jr, G., Sabbaga, J., Hoff, P. M."Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: Effectiveness and cost-utility analysis". Molecular and Clinical Oncology 1.1 (2013): 175-179.
Chicago
Nebuloni, D. R., Mak, M. P., Souza, F. H., Saragiotto, D. F., Júlio, T., De Castro Jr, G., Sabbaga, J., Hoff, P. M."Modified FLOX as first-line chemotherapy for metastatic colorectal cancer patients in the public health system in Brazil: Effectiveness and cost-utility analysis". Molecular and Clinical Oncology 1, no. 1 (2013): 175-179. https://doi.org/10.3892/mco.2012.12