Introduction
It is generally recognized that cancer
chemotherapy-induced nausea and vomiting (CINV) occurs more
commonly in women compared to men and also tends to occur more
often in younger compared to older patients (1). CINV compromises the quality of life
(QOL) of the patients and reduces treatment compliance, leading
even to treatment refusal or discontinuation (2). Therefore, it is crucial to establish
an antiemetic therapy capable of fully preventing, or at least
controlling, CINV.
A two-drug antiemetic regimen consisting of a
first-generation 5-hydroxytryptamine 3 receptor antagonist
(5-HT3RA), such as granisetron, together with dexamethasone, was
mainly prescribed in the 1990s. This combined drug regimen was
shown to be highly effective in the management of acute CINV (0–24
h following anticancer drug administration), but was not
sufficiently effective for the relief of delayed CINV (24–120 h
following anticancer drug administration) (3). Later, in the early 21th century, the
neurokinin 1 receptor antagonist (NK1RA) aprepitant and the
second-generation 5-HT3RA palonosetron became available for
clinical use. These antiemetics are also reportedly effective for
the relief of delayed CINV (4,
5). The regimen
paclitaxel/carboplatin (TC) is currently extensively administered
as chemotherapy for gynecological cancers; however, there are
currently no reports available on the effects of palonosetron on
delayed CINV. Therefore, we conducted a phase II clinical trial to
corroborate the efficacy and safety of palonosetron + dexamethasone
in patients receiving TC therapy.
Subjects and methods
Patients
The study population comprised 42 patients who had
been diagnosed with gynecological malignancies and treated with TC
at Iwate Medical University between January, 2011 and March, 2013.
For the assessment of therapeutic response, the following
definitions were employed to describe CINV outcomes: i) complete
response (CR), complete arrest/prevention of vomiting (no
vomiting-related events, no antiemetic treatment -irrespective of
the severity of nausea- and no rescue therapy); and ii) complete
control (CC), complete control of vomiting-related episodes (no
antiemetic treatment, only mild nausea, and no rescue therapy). The
efficacy and safety of palonosetron + dexamethasone medications
were evaluated by the self-completion method using the
Multinational Association of Supportive Care in Cancer (MASCC)
Antiemesis Tool (6) during an
observation period lasting from day 1 through day 8 of the initial
cycle of TC therapy. The severity of nausea was assessed using a
visual analog scale (VAS) and scored as follows: mild, 1–2;
moderate, 3–4; and severe, ≥ 5. The primary endpoint was the CR
rate for the delayed period (24–96 h after initiation of TC
therapy) and the secondary endpoints were as follows: i) CR rates
for the acute period (0–24 h after the initiation of TC therapy)
and for the overall period (0–96 h); ii) CC rates for the acute,
delayed and overall periods after the initiation of TC therapy;
iii) the severity of nausea during the acute, delayed and overall
periods after the initiation of TC therapy; and iv) adverse
reactions occurring during the observation period.
Subgroup analyses were performed to comparatively
assess the delayed period CR rate in the following strata: age (≥55
vs. <55 years), body surface area (≥1.47 vs. <1.47
m2), performance status (PS) (0 vs. 1–2) and
complications (present vs. absent).
Inclusion criteria
Patients meeting all the following criteria were
enrolled in this study: i) Aged ≥ 20 years at the time of
enrollment; ii) diagnosed with gynecological cancer; iii) naïve to
cancer chemotherapy or previous treatment with a single
antineoplastic agent less potent than ‘drugs with slight emetogenic
risk’ according to the NCCN Clinical Prectice Guidelines in
Oncology (Antiemesis Version 4, 2009) (7) (however, use of hormone preparations
for endocrinotherapy and use of antineoplastic agents for purposes
other than cancer treatment were not considered as chemotherapy);
iv) scheduled to receive the tri-weekly TC therapy regimen
(paclitaxel 175 mg/m2 and carboplatin area under the
curve 6 administered on day 1); v) patients whose bone marrow and
hepatic and renal functions were maintained and whose laboratory
test values within 8 days prior to enrollment, including the day of
enrollment, fulfilled the criteria of white blood cell count ≥
3,000/mm3, aspartate aminotransferase and alanine
aminotransferase < 100 IU/l [or grade ≤ 3 according to the
Common Terminology Criteria for Adverse Events (CTCAE) v4.0
(8), in the case of hepatic
metastasis evident on imaging] and creatinine clearance ≥ 60.0
ml/min; vi) Eastern Cooperative Oncology Group PS of 0 2; and vii)
patients provided written informed consent to participate in this
study.
Exclusion criteria
The exclusion criteria were as follows: i) Severe
uncontrollable complications, apart from malignant tumors (e.g.,
intestinal paralysis, pulmonary fibrosis, diabetes mellitus, heart
failure, myocardial infarction, angina pectoris, renal failure,
hepatic failure, mental disorders, cerebrovascular disorder and
gastric/duodenal active ulcers); ii) symptomatic brain metastasis
or clinical suspicion of brain metastasis; iii) convulsive disorder
requiring treatment with anticonvulsants; iv) symptomatic ascites
or pleural effusions requiring therapeutic paracentesis; v) pyloric
stenosis or intestinal obstruction; vi) vomiting-related episodes
or CTCAE v4.0 grade ≥ 2 nausea; vii) history of hypersensitivity to
palonosetron or ingredients of any other 5-HT3RA preparation; viii)
history of hypersensitivity to ingredients of dexamethasone
preparations; ix) pregnancy, lactation, and/or refusal to practice
contraception during the study period; x) past history of
palonosetron use; xi) incapable or reluctant to cooperate with the
procedures necessary for this study; and xii) any patients who were
deemed inappropriate to be subjects of this study, for any reason,
by the investigator (or coinvestigator).
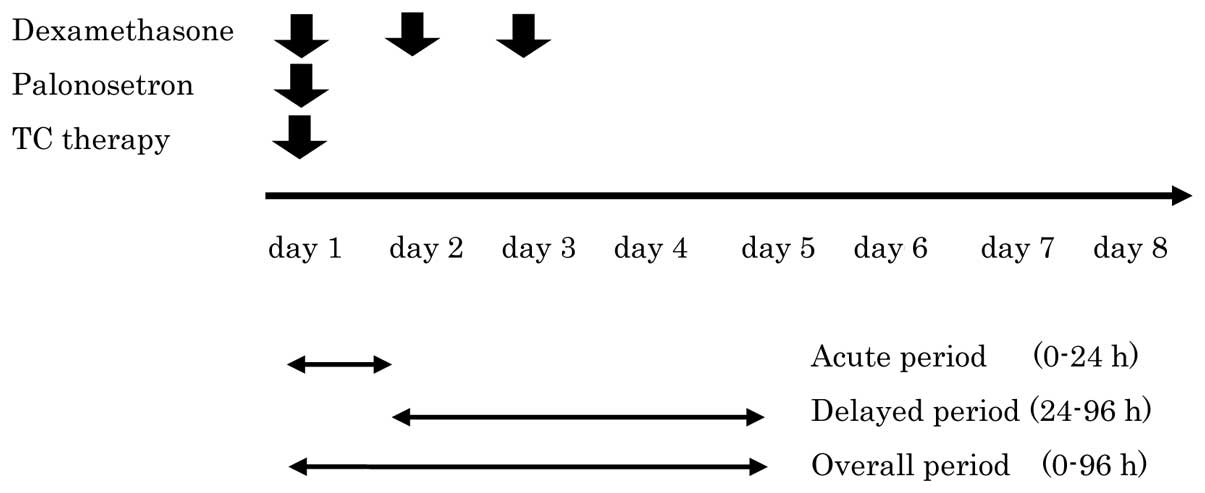
Medication administration
On day 1, 0.75 mg/body of palonosetron and 19.8
mg/body of dexamethasone were dissolved in 100 ml of physiological
saline and administered intravenously over 15 min immediately prior
to TC therapy. Dexamethasone in daily doses of 6.6 mg/body in 100
ml physiological saline was also administered intravenously on days
2 and 3 (Fig. 1).
Concomitant and contraindicated
drugs
Dexamethasone preparations were contraindicated,
unless otherwise specified, from 24 h prior to the administration
of palonosetron until completion of the observation period. From 24
h prior to the administration of palonosetron until day 5,
concomitant use of any of the following drugs with an antiemetic
effect was prohibited, except for use as post-TC antiemetic
treatment (rescue therapy): i) NK1 receptor antagonist
antiemetics; ii) 5-HT3RA antiemetics; iii) all adrenocortical
steroids except dexamethasone; iv) antidopaminergic drugs, such as
metoclopramide and domperidone; v) phenothiazine antipsychotics,
such as prochlorperazine and perphenazine; vi) antihistamines; vii)
all benzodiazepines, except for the use of triazolam for insomnia
as needed; and viii) other drugs, including haloperidol,
droperidol, scopolamine and olanzapine. Concomitant use of
serotonin-specific reuptake inhibitors and serotonin-norepinephrine
reuptake inhibitors was also prohibited from 24 h prior to the
administration of palonosetron through day 5.
Statistical analysis
Logistic regression analysis was used for comparison
of background variables. Inter-group or inter-subgroup differences
were considered to be statistically significant when P<0.05.
Results
Background variables
The data for background variables are presented in
Table I. The median age was 60.5
years (range, 32–83 years) and the median body surface area was
1.44 m2 (range, 1.19-1.87 m2). The PS score
was 0 in 34, 1 in 4 and 2 in 4 patients. The diagnosis was uterine
cervical cancer in 2 patients, endometrial cancer in 18, ovarian
cancer in 21 and triple cancer in 1 patient. Of the 42 patients, 29
received TC therapy as adjuvant chemotherapy and 13 as systemic
chemotherapy. A total of 10 patients developed complications
(diabetes mellitus in 4; hypertension in 3; thrombosis in 2; and
arrhythmia, hyperlipidemia, insomnia and osteoarthritis of the hip
in 1 patient each), whereas 32 patients had no concurrent
disorders.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Patient no.(%)
(n=42) |
|---|
| Age (years) |
|
| Median
(range) | 60.5
(32–83) |
| Body surface area
(m2) |
|
| Median
(range) | 1.44
(1.19-1.87) |
| ECOG PS |
|
| 0 | 34
(81.0) |
| 1 | 4
(9.5) |
| 2 | 4
(9.5) |
| Type of cancer | |
|
Cervical | 2 (4.8) |
|
Endometrial | 18 (42. 8) |
|
Ovarian | 21 (50.0) |
|
Others | 1 (2.4) |
| Chemotherapy |
|
|
Adjuvant | 29 (69.0) |
|
Systemic | 13 (31.0) |
| Complications |
|
|
Present | 10 (23.8) |
|
Absent | 32 (76.2) |
Response to palonosetron +
dexamethasone therapy
For the acute, delayed and overall periods, the CR
rates were 95.2, 90.5 and 85.7%, respectively, whereas the CC rates
were 90.5, 85.7 and 78.6%, respectively (Table II). The severity grades of nausea
during the acute, delayed and overall periods were 0.45±1.38,
1.13±2.40, and 0.79±1.99, respectively. Episodes of nausea occurred
in 4 patients during the acute phase, 7 during the delayed period
and 9 during the overall period. The mean severity grades of nausea
for the patients with this adverse event during the periods
investigated in this study were 3.17±2.19, 3.96±2.99, and
3.69±2.77, respectively (data not shown).
 | Table II.Complete response (CR) and complete
control (CC) rate (n=42) . |
Table II.
Complete response (CR) and complete
control (CC) rate (n=42) .
| Period | CR rate, no.(%) | CC rate, no. (%) |
|---|
| Acute | 40 (95.2) | 38 (90.5) |
| Delayed | 38 (90.5) | 36 (85.7) |
| Overall | 36 (85.7) | 33 (78.6) |
Rescue therapy
Rescue therapy was administered to 2, 4, and 6
patients during the acute, delayed and overall periods,
respectively. Granisetron and dexamethasone were prescribed for
this purpose in the 2 patients with nausea during the acute period.
Granisetron and dexamethasone were used for this purpose during the
delayed period in all 4 affected patients, whereas 1 patient who
responded poorly also received an additional ramosetron regimen
(Table III).
 | Table III.Rescue therapy for complete response
(CR) and complete control (CC) cases. |
Table III.
Rescue therapy for complete response
(CR) and complete control (CC) cases.
| Cases | Acute period
(VAS) | Rescue therapy | Delayed period
(VAS) | Rescue therapy |
|---|
| 1 | 5 | Granisetron,
dexamethasone | 1 | None |
| 2 | 2 | Granisetron,
dexamethasone | 0 | N/A |
| 3 | 0 | N/A | 10 | Granisetron,
dexamethasone |
| 4 | 0 | N/A | 8 | Granisetron,
dexamethasone |
| 5 | 7 | None | 7 | Granisetron,
dexamethasone, ramosetron |
| 6 | 0 | N/A | 4 | Granisetron,
dexamethasone |
| 7 | 3 | None | 0 | N/A |
| 8 | 0 | N/A | 3 | None |
| 9 | 0 | N/A | 6 | None |
Subgroup analysis
The subgroup analysis results are summarized in
Table IV. Higher delayed period
CR rates were recorded for the following subgroups: age <55
years, body surface area ≥ 1.47 m2, PS score 0 and
absence of complications.
 | Table IV.Subgroup analysis. |
Table IV.
Subgroup analysis.
| Variables | CR rate (%) | Odds ratio | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
| ≥ 55 (n
=29) | 86.1 | 4.76 | 0.24-95.3 | 0.005 |
| <55 (n
=13) | 100.0 |
|
|
|
| BSA,
m2 |
|
|
|
|
| ≥ 1.42 (n
=26) | 92.3 | 0.59 | 0.09-3.84 | 0.91 |
|
<1.42(n =16) | 87.5 |
|
|
|
| ECOG PS |
|
|
|
|
| 0
(n=34) | 94.1 | 0.02 | 0.03-1.40 | 0.36 |
| 1–2
(n=8) | 75.0 |
|
|
|
| Complications |
|
|
|
|
| Present
(n =10) | 80.0 | 3.59 | 0.53-24.3 | 0.56 |
| Absent
(n=32) | 93.8 |
|
|
|
Adverse events
Adverse event data are presented in Table V. As regards non-hematotoxic
adverse events, grade ≥ 2 constipation and diarrhea occurred in 1
patient (2.4%) each. No grade ≥ 3 hematotoxicities were identified
when the blood biochemical test parameters were measured.
 | Table V.Adverse events (n =42). |
Table V.
Adverse events (n =42).
| Toxicities | Grade1, no. (%) | Grade ≥ 2, no.
(%) |
|---|
|
Non-hematological |
|
|
|
Headache | 3 (7.1) | 0 |
|
Vertigo | 1 (2.4) | 0 |
|
Constipation | 14 (33.3) | 1 (2.4) |
| Abdominal
pain | 0 | 0 |
|
Hiccup | 0 | 0 |
|
Allergy | 0 | 0 |
|
Vascular pain | 0 | 0 |
|
Diarrhea | 0 | 1 (2.4%) |
| Hematological | 0 | 0 |
Discussion
It is generally recognized that, among the adverse
reactions to treatment with antineoplastic drugs, nausea and
vomiting are the most disagreeable symptoms suffered by patients
(9). Persistent nausea and
vomiting may lead to dehydration, electrolyte disturbances and
malnutrition, whereas patient willingness to undergo cancer
treatment diminishes in the presence of severe CINV. Therefore,
control of nausea and vomiting is considered to be crucial for the
continuation of cancer treatment by maintaining the patient's
general condition and QOL. Previously, antiemetic therapy for CINV
consisted solely of corticosteroids, with a rate of successful
acute CINV control of ∼30% (10).
The CINV control rate increased to ∼70% with the advent of
first-generation 5-HT3RA medications (11). The first-generation 5-HT3RAs proved
to be effective in the control of acute nausea and vomiting;
however, a proportion of patients suffer delayed nausea and
vomiting, which constitutes a major problem in cancer chemotherapy.
In 2010, palonosetron, a second-generation 5-HT3RA, was approved in
Japan. The efficacy of palonosetron in controlling delayed as well
as acute nausea and vomiting is attributed to its long plasma
elimination half-life (40 h) and strong affinity for 5-HT3
receptors.
There have been several reports demonstrating an
antiemetic effect of palonosetron on patients receiving highly
emetogenic chemotherapeutic agents (HEC) (12, 13). As regards moderate emetogenic
chemotherapeutic agents (MEC), however, the majority of the studies
have focused on antiemetic therapy regimens including an
anthracycline classified under HEC in the National Comprehensive
Cancer Network guidelines combined with cyclophosphamide (14, 15). However, the number of studies
assessing the efficacy of palonosetron in a combined antiemetic
regimen in patients treated with MEC alone is currently limited.
Furthermore, no studies have yet assessed the antiemetic effect of
palonosetron on delayed-onset nausea and vomiting in gynecological
cancer patients treated with MEC. Thus, we conducted this phase II
clinical trial to assess the efficacy and safety of palonosetron +
dexamethasone in patients receiving TC therapy.
In the PROTECT study, comparing a combined regimen
of the first-generation 5-HT3RA granisetron and dexamethasone with
a combined regimen of the second-generation 5-HT3RA palonosetron
and dexamethasone, there was no improvement with respect to acute
nausea induced by HECs, such as cisplatin or
doxorubicin/cyclophosphamide, frequently used in breast cancer
cases; however, the results confirmed a significantly higher
efficacy of palonosetron compared to granisetron in controlling
delayed nausea and vomiting (13).
We evaluated nausea and vomiting using the MASCC
Antiemesis Tool in the present clinical trial, utilizing VAS for
nausea and in terms of the frequency of vomiting episodes. The
majority of the previous clinical studies evaluated the severity of
nausea indirectly, according to whether or not any rescue therapy
was undertaken, rather than directly. The use of VAS allowed the
objective assessment of the severity of nausea in this study, as
the severity of nausea depends on the patient's (subjective)
viewpoint.
TC therapy is classified into the MEC category. The
present data demonstrated high CR and CC rates for delayed vomiting
in patients receiving TC therapy with concomitant palonosetron +
dexamethasone regimen.
In patients who failed to attain CR and CC,
reduction of nausea was achieved with granisetron, dexamethasone or
ramosetron, which are agents with different mechanisms of action. A
phase III clinical trial designed to compare palonosetron +
dexamethosone vs. granisetron, or palonosetron + dexamethasone vs.
aprepitant, is required to determine whether palonosetron +
dexamethasone exerts a prophylactic effect against nausea in
patients receiving TC therapy. Such a clinical trial is currently
being planned by the Japanese Gynecologic Oncology Group.
Young patients are reportedly at high risk of CINV
(16, 17). The present subgroup analysis
revealed a significantly higher CR rate in women aged <55 years
compared to that in women aged ≥ 55 years, in terms of treatment of
delayed-onset CINV. Previous studies have focused on the use of
first-generation 5-HT3RA, while the present data suggest the
potential efficacy of the second-generation 5-HT3RA palonosetron in
combination with dexamethasone for the control of delayed-onset
vomiting, which reportedly occurs more frequently among younger
patients. Furthermore, the present analysis revealed marginally
higher CR rates for the following subgroups: Body surface area
≥1.47 m2, PS score 0, adjuvant chemotherapy and absence
of complications, although the differences did not reach
statistical significance.
Constipation occurred as an adverse event in 16
patients (38.1%), but was rated as ≥grade 2 in only 1 patient
(2.4%). It is a general rule at our facility that all the patients
receive oral laxative medications following gynecological cancer
surgery; thus, none of the patients in the present series
experienced serious constipation. Headache occurred in 3 patients
(7.1%), vertigo in 1 (2.4%) and diarrhea in 2 patients (4.8%); the
causes for these adverse events remain unclear. No grade ≥ 3
hematotoxicity was observed in the blood biochemical tests. Thus,
the adverse reactions experienced were considered to be within
acceptable limits and were thus considered to have been effectively
controlled.
The present phase II clinical trial demonstrated the
efficacy of palonosetron + dexamethasone in preventing
delayed-onset nausea in gynecological cancer patients receiving TC
therapy. This combined antiemetic regimen was associated with only
mild adverse reactions and may serve as supportive therapy,
allowing cancer chemotherapy to be continued while maintaining the
patients' QOL.
References
|
1
|
Sekine I, Segawa Y, Kubota K and Saeki T:
Risk factors of chemotherapy-induced nausea and vomiting: index for
personalized antiemetic prophylaxis. Cancer Sci. 104:711–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richardson JL, Marks G and Levine A: The
influence of symptoms of disease and side effects of treatment on
compliance with cancer therapy. J Clin Oncol. 6:1746–1752.
1988.PubMed/NCBI
|
|
3
|
Kaizer L, Warr D, Hoskins P, Latreille J,
Lofters W, Yau J, Palmer M, Zee B, Levy M and Pater J: Effect of
schedule and maintenance on the antiemetic efficacy of ondansetron
combined with dexamethasone in acute and delayed nausea and emesis
in patients receiving moderately emetogenic chemotherapy: a phase
III trial by the National Cancer Institute of Canada Clinical
Trials Group. J Clin Oncol. 12:1050–1057. 1994.PubMed/NCBI
|
|
4
|
Yang LP and Scott LJ: Palonosetron: in the
prevention of nausea and vomiting. Drugs. 69:2257–2278. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curran MP and Robinson DM: Aprepitant: a
review of its use in the prevention of nausea and vomiting. Drugs.
69:1853–1878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MASCC antiemesis tool (MAT), . http://www.mascc.org/matAccessed. March
3–2010
|
|
7
|
NCCN Clinical Practice Guidelines in
Oncology-Antiemesis- ver.4 2009. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportiveAccessed.
March 3–2010
|
|
8
|
National Cancer Institute, . (2009) Common
Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS.
Available from. https://wiki.nci.nih.gov/display/VKC/Common+Terminology+Criteria+for+Adverse+Events+FAQ#CommonTerminology
Criteria for Adverse Events FAQ - What is the official reference
citation for CTCAE v40. Accessed. May 29–2009
|
|
9
|
Aapro MS: Palonosetron as an anti-emetic
and anti-nausea agent in oncology. Ther Clin Risk Manag.
3:1009–1020. 2007.
|
|
10
|
Ioannidis JP, Hesketh PJ and Lau J:
Contribution of dexamethasone to control of chemotherapy-induced
nausea and vomiting: a meta-analysis of randomized evidence. J Clin
Oncol. 18:3409–3422. 2000.PubMed/NCBI
|
|
11
|
Olver I, Paska W, Depierre A, Seitz JF,
Stewart DJ, Goedhals L, McQuade B, McRae J and Wilkinson JR: A
multicentre, double-blind study comparing placebo, ondansetron and
ondansetron plus dexamethasone for the control of cisplatin-induced
delayed emesis. Ondansetron Delayed Emesis Study Group. Ann Oncol.
7:945–952. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aapro MS, Grunberg SM, Manikhas GM,
Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B,
Lordick F and Macciocchi A: A phase III, double-blind, randomized
trial of palonosetron compared with ondansetron in preventing
chemotherapy-induced nausea and vomiting following highly
emetogenic chemotherapy. Ann Oncol. 17:1441–1449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito M, Aogi K, Sekine I, Yoshizawa H,
Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T and Mitsuhashi S:
Palonosetron plus dexamethasone versus granisetron plus
dexamethasone for prevention of nausea and vomiting during
chemotherapy: a double-blind, double-dummy, randomized, comparative
phase III trial. Lancet Oncol. 10:115–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenberg P, Figueroa-Vadillo J, Zamora R,
Charu V, Hajdenberg J, Cartmell A, Macciocchi A and Grunberg S99-04
Palonosetron Study Group: Improved prevention of moderately
emetogenic chemotherapy-induced nausea and vomiting with
palonosetron, a pharmacologically novel 5-HT3 receptor antagonist:
results of a phase III, single-dose trial versus dolasetron.
Cancer. 98:2473–2482. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gralla R, Lichinitser M, Van der Vegt S,
Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A
and Aapro M: Palonosetron improves prevention of
chemotherapy-induced nausea and vomiting following moderately
emetogenic chemotherapy: results of a double-blind randomized phase
III trial comparing single doses of palonosetron with ondansetron.
Ann Oncol. 14:1570–1577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hesketh PJ: Chemotherapy-induced nausea
and vomiting. N Engl J Med. 358:2482–2494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hesketh PJ, Aapro M, Street JC and Carides
AD: Evaluation of risk factors predictive of nausea and vomiting
with current standard-of-care antiemetic treatment: analysis of two
phase III trials of aprepitant in patients receiving
cisplatin-based chemotherapy. Support Care Cancer. 18:1171–1177.
2010. View Article : Google Scholar : PubMed/NCBI
|