Introduction
Epstein-Barr virus (EBV) is associated with a number
of different types of aggressive non-Hodgkin's lymphoma (NHL). If B
cells infected with EBV express all 9 latent-cycle EBV antigens,
the cells become prone to developing a B-cell-derived proliferative
disease or tumor. EBV-associated NHLs always exhibit aggressive
characteristics, including rapid growth and necrosis. Particularly
in cases with EBV-related natural killer (NK)- or T-cell lymphoma,
there is a high risk of developing hemophagocytosis syndrome.
EBV-positive malignant diseases are associated with the virus
latent cycle and the EBV-infected lymphoblastoid cell lines usually
exhibit type 3 latency, which renders the cells more susceptible to
elimination by EBV-specific T cells (1). Cellular immunotherapy has been developed
over the last 20 years to overcome the poor responsiveness to
conventional chemotherapy in EBV-associated malignant diseases.
The most frequently used cells in cellular
immunotherapy include cytotoxic T lymphocytes (CTLs) and NK cells.
CTLs usually exert their effects through recognizing and binding to
the antigenic epitopes-MHC-I complex provided by the
antigen-presenting cells (2,3). In several types of viral infections,
including human immunodeficiency virus (4), EBV (5),
cytomegalovirus (6) and hepatitis B
and C viruses (7), CTLs play a
central role in the defense against viral invasion. In an immune
deficiency mouse model of human EBV-transformed B cells, it was
demonstrated that EBV-specific CTL treatment effectively prolonged
survival (8). According to reported
study results, the conventionally used chemotherapy drugs were able
to inhibit or promote CTL-mediated tumor cell elimination,
depending on the drug category (9).
Based on the abovementioned existing theories, we designed this
experiment with the aim to investigate the effect of EBV-specific
CTL treatment on EBV-related NHL and the potential underlying
mechanisms.
The tumor microenvironment has become a research
focus in the field of cancer research in recent years. The cells
and cytokines in the tumor microenvironment play a crucial role in
tumor initiation and progression. This effect is also consistent
with lymphoma occurrence. It was previously reported that the genes
associated with the tumor microenvironment, cell growth and/or
apoptosis and regulation of mitosis were associated with response
to treatment and the outcome of patients with Hodgkin's lymphoma
(10). In addition, the tissue
inhibitor of metalloproteinase 1 (TIMP-1) was found to promote
EBV-related lymphoma growth, and also inhibit tumor angiogenesis.
Therefore, TIMP-1 may be a crucial mediator in EBV-related lymphoma
(11). In a mouse model of
microenvironment-dependent human diffuse large B-cell lymphoma
(DLBCL), bevacizumab exerted a potent antitumor effect by
inhibiting tumor vascularization (12). In patients with follicular lymphoma,
tumor-associated macrophages were associated with poor prognosis
and definitely predicted the outcome (13). Therefore, in the present study, we
aimed to determine the post-treatment number of
lymphoma-infiltrating macrophages and determine whether adoptive
immunotherapy of lymphoma may be associated with tumor
microenvironment modulation.
Materials and methods
Cell lines and reagents
The Farage cell line, which is a human EBV-positive
lymphoma cell line, was purchased from American Type Culture
Collection (ATCC, Rockville, MD, USA). The cells were traditionally
cultured in RPMI-1640 (Sigma-Aldrich Shanghai Trading Co., Ltd.,
Shanghai, China) supplemented with 10% fetal bovine serum (Sigma
Chemical Co., St. Louis, MO, USA) under conditions of 5%
CO2 in an incubator at 37°C. Mitomycin C was purchased
from Genia Biology, Beijing, China. Antibodies for flow cytometry
were purchased from Becton-Dickinson, San Jose, CA, USA.
EBV-CTL culture and
characterization
Monocyte-depleted peripheral blood lymphocytes
(PBLs) from EBV-seropositive donors were stimulated with Farage
cells incubated overnight at 37°C. Prior to being added to the
PBLs, the Farage cells were treated with mitomycin C (10–80 µg/ml)
for 30 min. Following treatment, the Farage cells were added to the
PBLs to co-culture for 4 days, then half of the medium was
discarded. The PBLs were collected and re-added to 2×105
Farage cells for continuous stimulation. A total of 10 U/ml human
recombinant interleukin (rIL)-2 (Beijing Biodee Biotechnology Co.,
Ltd., Beijing, China) was added to the PBL medium on day 3; half of
the medium was changed every 3 days and the rIL-2 concentration was
maintained at 10 U/ml. The stimulation procedure was repeated
weekly. The T cells which were able to recognize EBV survived and
continued to proliferate, whereas the T cells which could not
recognize EBV gradually underwent apoptosis.mmune phenotype
characterization analysis of EBV-CTLs was performed using
fluorescein isothiocyanate (FITC)-conjugated antibodies against
human CD3 and CD8 (monoclonal rat anti-mouse anti-CD3 and anti-CD8
antibodies, cat. nos. 555275 and 553031, respectively;
Becton-Dickinson) by flow cytometry.
EBV-CTL cytotoxicity assay
Target cells (1×106) were co-cultured
with CTLs of varying concentrations [effector cells:target cells
(E:T ratio) = 2.5, 5.0, 10.0 and 20.0] for 4 h at 37°C.
Subsequently, MTT assays were performed to evaluate cell viability.
A total of 20 µl MTT solution (5 mg/ml) was added to maintain
incubation for 1–4 h. The supernatant was carefully discarded and
the plates were washed 3 times with phosphate-buffered saline
(PBS). Dimethylsulfoxide (150 µl) was added to each well and the
plates were placed on a shaker for 15–20 min to completely dissolve
the formazan crystal violet. Absorbance of Farage cells was tested
at 570 nm using an ELISA reader (Thermo Fisher Scientific Inc.,
Waltham, MA, USA). The cell death ratio was calculated as
follows:
Relative cell death ratio = (Ae-Ab) ×
100/(Ac-Ab),
where Ac, absorbance of control; Ae, absorbance of
experimental groups; and Ab, background absorbance.
Adoptive immunotherapy
A total of 20 NOD/scid nude female mice, aged 5–7
weeks and weighing 15–20 g were purchased from Beijing HFK
Bioscience Co., Ltd. (Beijing, China). The mice were housed in
sterile cages with unidirectional air flow and supplied with
sterile feed and sterile water. The mice were kept according to the
institutional guidelines approved by the pla General Hospital of
Chengdu Military Region (Sichuan, China) in line with the current
regulations and standards of the ministry of Health, Labor and
welfare. Logarithmic phase Farage cells (1×106) in 100
µl serum-free medium were subcutaneously inoculated into the backs
of the mice. The mice were randomly divided into four groups
(chemotherapy alone, immunotherapy alone, combined therapy and
control groups; n=5 per group) on the 5th day, when small tumors
were palpable. The applied dosage of cyclophosphamide, doxorubicin,
vincristine and prednisone (CHOP regimen) was consistent with the
existing literature (14). The drugs
were infused into the mice according to the study plan. The
immunotherapy alone group was administered 20×106 CTLs
per mouse per administration through intravenous injection for 5
consecutive days. The control group was injected with serum-free
RPMI-1640 medium. All the mice received 2×104 IU IL-2
through peritoneal injection daily, for a total of 10 days. The
tumor size was measured from the time of the first injection every
2 days. After the 7th measurement, the mice were sacrificed by
cervical dislocation and the tumors were excised and weighed.
Tumor-infiltrating immune cells
assay
Fresh tumor tissue was digested with 1 mg/ml
collagenase-1 (Gibco, Ltd, Grand Island, NY, USA) diluted in
serum-free RPMI-1640 for 1.5–2 h. The tissue homogenate was
centrifuged (377 × g) for 3 min, the supernatant was removed and
the pellet was washed 3 times with PBS. The pellet was resuspended
with 5–8 ml PBS (pH=7.4) and a cell suspension was formed following
filtration. Using a red blood cell counting instrument, single-cell
concentration was modulated to 1×105/100 µl.
Subsequently, 100 µl cell suspension was extracted to incubate with
antibodies for flow cytometry. The surface markers
CD3-phycoerythrin (PE) with CD8-FITC and CD11b-FITC with F4/80-PE
(BD Biosciences Pharmingen, San Jose, CA, USA) were incubated with
the cells for 30 min at 4°C. Isotype controls were set as the
negative controls. The specimens were rewashed with PBS to remove
the redundant antibodies. The cells were resuspended in 200 µl PBS
to be evaluated by flow cytometry (Becton-Dickinson). The total
cells to be harvested were set to 1×104 and the speed of
cell collection was controlled at 200–300 cells/sec. The data
analysis was completed by FlowJo software, version 7.6 (FlowJo,
Ashland, OR, USA).
Statistical analysis
The data are presented as means ± standard
deviation. The statistical analysis was performed with one-way
analysis of variance employing SPSS 16.0 software (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
Cytotoxicity of EBV-CTLs
As CTLs have been recognized as an important tool in
cancer immunotherapy, we attempted to generate a significant number
of CTLs in vitro by immune stimulation and we cloned out
numerous EBV-specific CTLs, which were able to recognize tumor
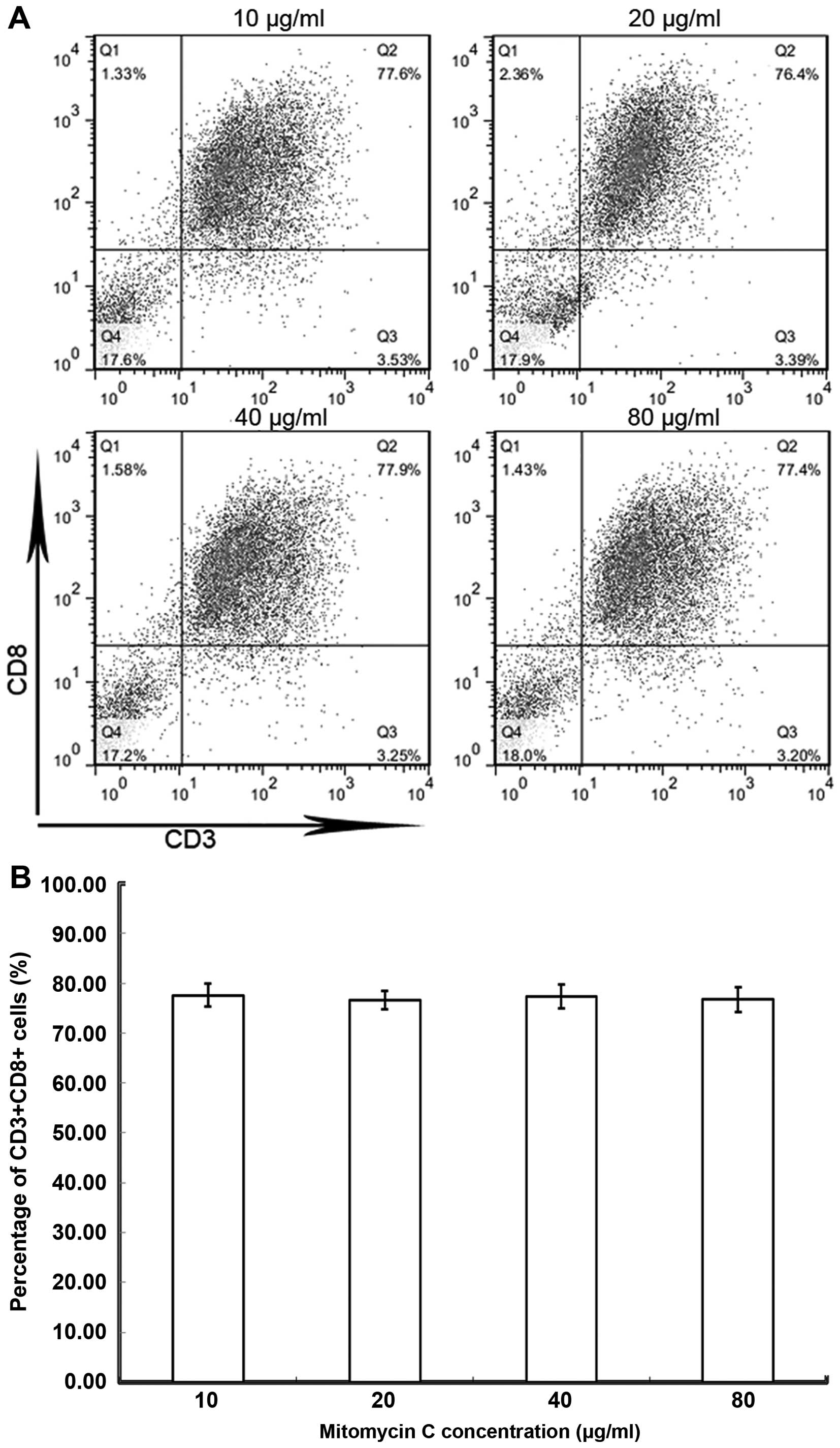
cells containing EBV. Tumor cells treated by mitomycin C at varying
concentrations (10–80 µg/ml) did not result in PBLs proliferating
into significantly different numbers of CD8+ T cells
(Fig. 1). However, all these
CD8+ T cells displayed specific recognition and
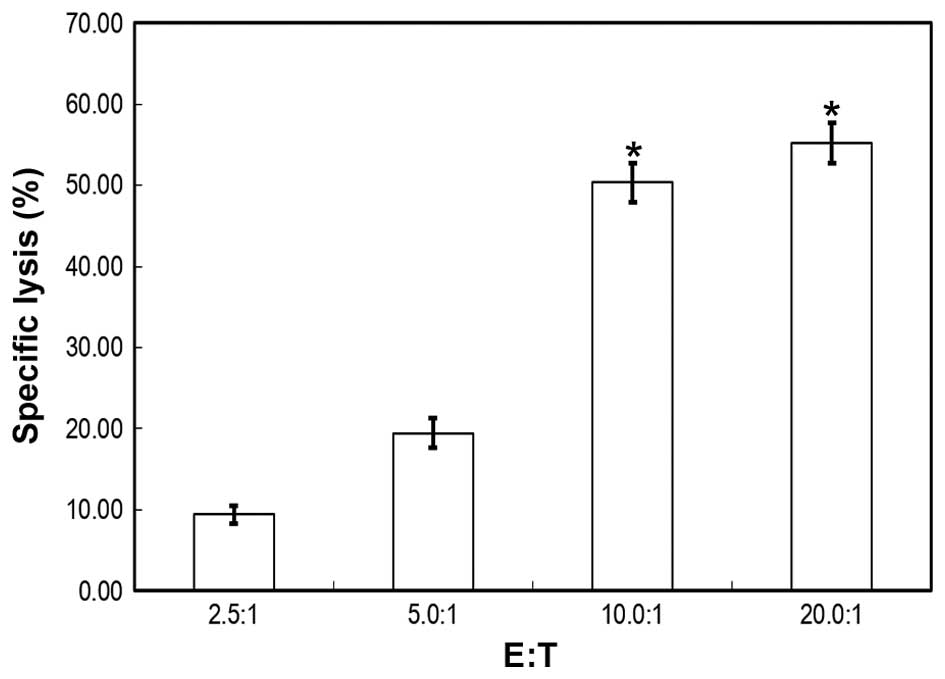
cytotoxic abilities. On MTT assays, the specific lysis ratio
differed along with the E:T ratio. The results demonstrated that
the mean lysis ratio was 9.41, 19.45, 50.34 and 55.26% for an E:T
ratio of 2.5:1, 5.0:1, 10.0:1 and 20.0:1, respectively. Compared
with the 2.5:1 and 5.0:1 groups, the 10.0:1 and 20.0:1 groups
exhibited significant differences in terms of lysis percentage.
There were no significant lysis ability differences for the 2.5:1
vs. 5.0:1 and the 10.0:1 vs. 20.0:1 groups (Fig. 2).
Antitumor effect of EBV-CTL
To investigate the antitumor effect of EBV-CTLs, we
mimicked the human NHL model in BALB/c nude mice and applied immune
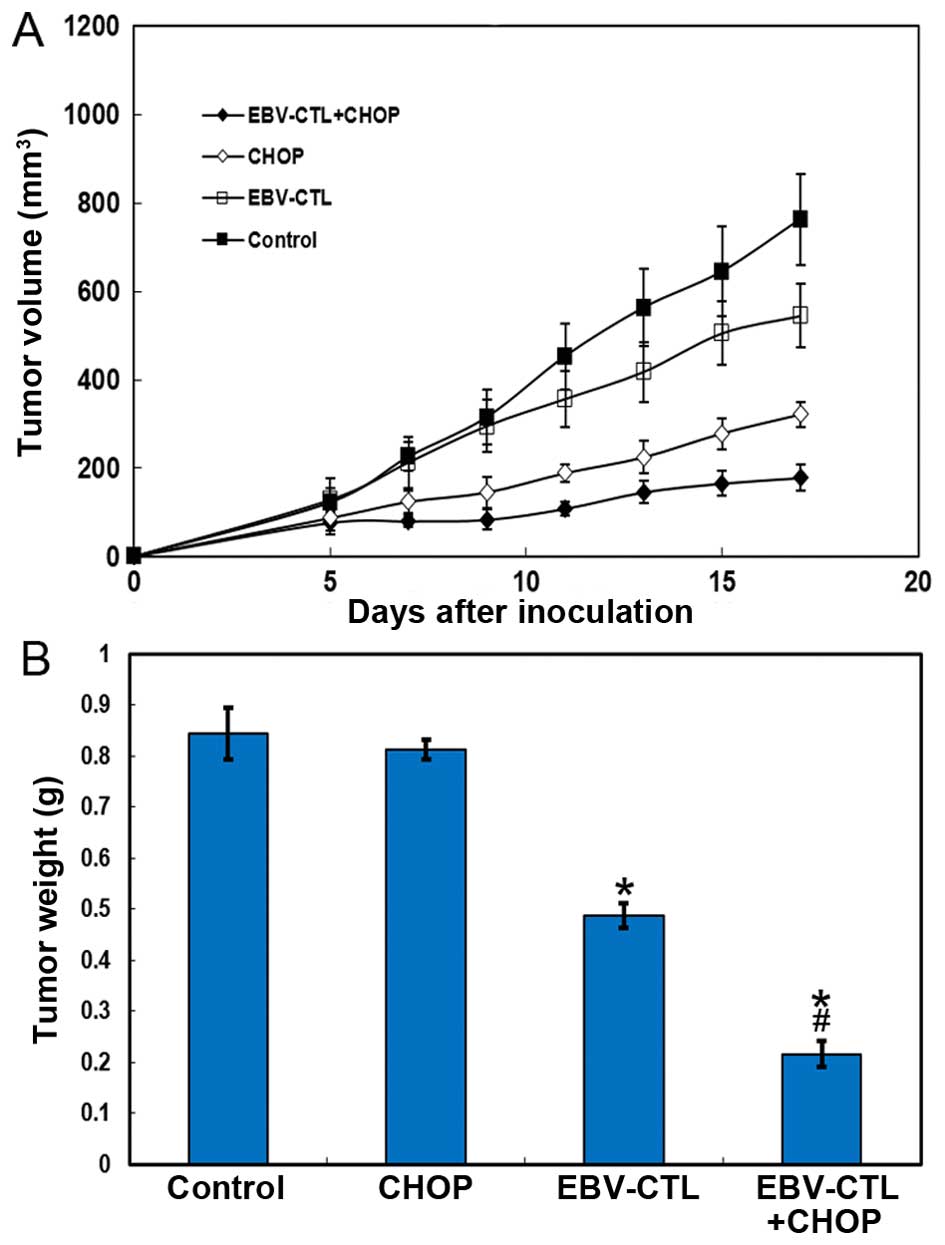
therapy on the mouse model. The results demonstrated that the
immunotherapy alone group was not superior compared with the
chemotherapy alone group. However, the antitumor effect was clearly
superior when the mice were treated with combination therapy. From
the 3rd day of cell immunotherapy onwards, compared with
chemotherapy, statistically significant retardation of tumor growth
was observed with combination therapy (Fig. 3A). This retardation effect was
enhanced by prolongation of the treatment time. In addition, the
tumor weight decreased after the completion of the combined
treatment (Fig. 3B).
Immune cell alterations in the tumor
microenvironment
Targeting the tumor microenvironment has been proven
to be crucial for cancer therapy; however, the effect of
immunotherapy on the microenvironment has not been fully
elucidated. We hypothesized that a CTL infusion may affect the
lymphoma microenvironment and modulate its homeostasis. We observed
alterations in the immune cells of the tumor microenvironment with
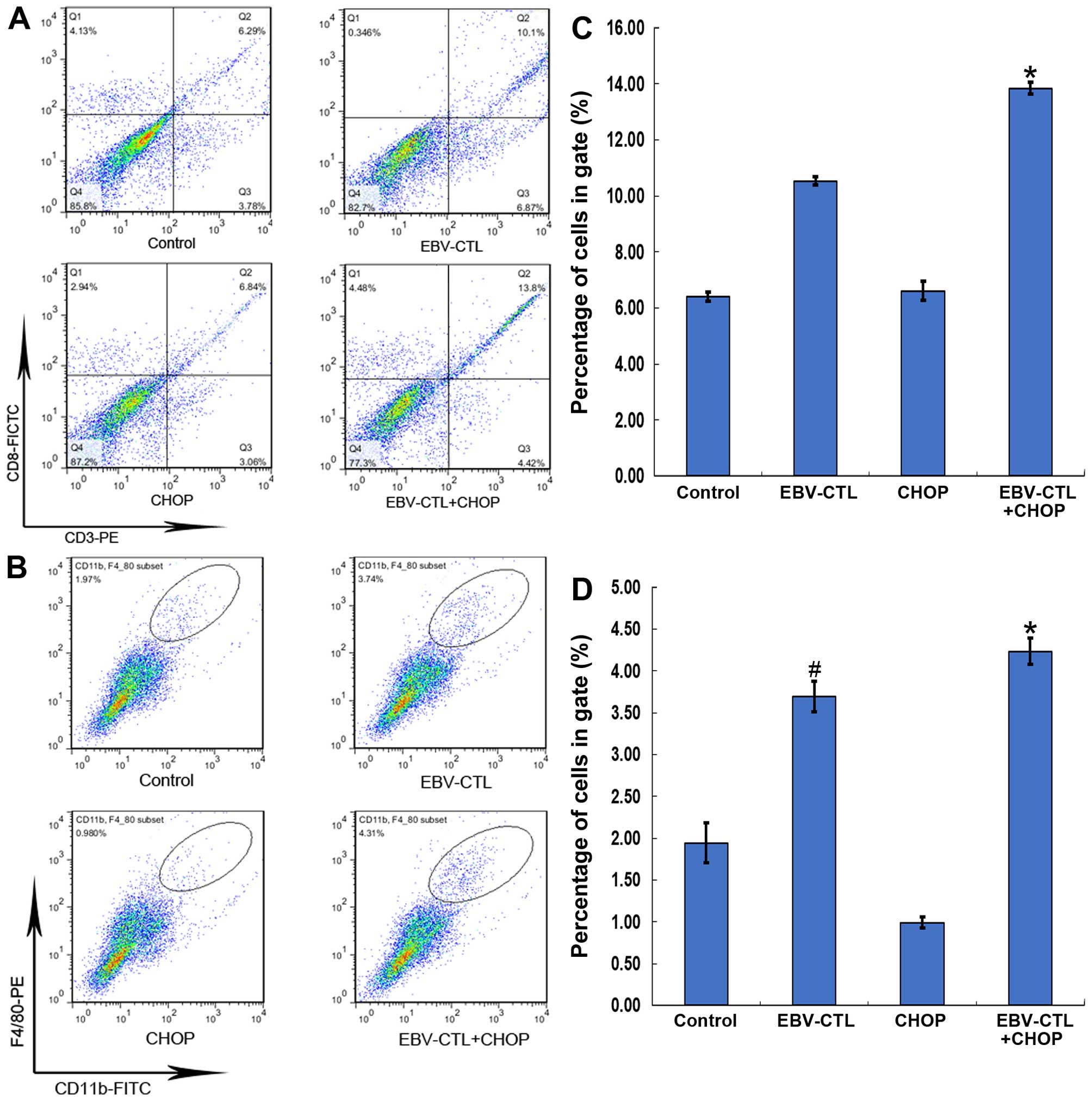
flow cytometry. It was observed that the numbers of
tumor-infiltrating CTLs and tumor-infiltrating macrophages (TIMs)
varied with the conditions under which the same total cell amounts
were collected. The results demonstrated that the mean percentage
of CD11b+F4/80+ cells increased following
immunotherapy (1.96, 3.82, 0.95 and 4.33% in the control, EBV-CTL,
CHOP and EBV-CTL+CHOP groups, respectively) (Fig. 4B). Furthermore, the mean percentage of
CD3+ CD8+ cells in the treatment groups also
increased to varying degrees (6.32, 10.24, 6.83 and 13.65% in the
control, EBV-CTL, CHOP and EBV-CTL+CHOP groups, respectively)
(Fig. 4A). The statistical results
are presented in Fig. 4C and D.
Discussion
The majority of EBV-associated tumors respond poorly
to conventional or intensive chemotherapy regimens, or exhibit a
high relapse rate. The presence of the EBV genome within these
tumors promotes the possibility of developing strategies directed
against viral targets. Potential intervention strategies include
adoptive immunotherapy approaches, interferon and small-molecule
compounds targeting different aspects of viral biology (15). To date, the strategy of adoptive
transfer of EBV-specific CTLs into lymphoma patients has been
investigated universally. It has been proven that EBV-specific CTL
lines may be generated from patients with confirmed EBV-positive
Hodgkin's disease (16), and such CTL
lines may contain clones with specificity for latent membrane
protein (LMP) 1 and LMP2 (17). In
transplant recipients, EBV-specific T-cell infusions may
significantly prolong the survival of patients with EBV-related
lymphoproliferative disease (18). In
addition, EBV-specific CTLs may eradicate untreated as well as
rituximab-resistant lymphoma and EBV-lymphoproliferative disease
(19). Adoptive immunotherapy with
the cell products has led to in vivo expansion of EBV-CTLs
in 80% of the patients, and a clinical response in 70% of the
patients (20). Consistent with these
previous findings, our study also demonstrated the feasibility of
generating and infusing EBV-specific CTLs in NHL mouse models. In
the present study, we demonstrated that EBV-specific cellular
immunity may be effectively detected and expanded in vitro.
EBV-specific CTLs were generated by tumor antigen stimulation and
induced to proliferate by cytokines (Fig.
1). These CTLs possess potent specific lysis ability when
abundantly proliferated in vitro (Fig. 2). EBV-specific CTLs may also exert
potent antitumor effects in vivo, which achieved a superior
treatment response ratio in mice (Fig.
3). However, the cellular monotherapy did not achieve a
significantly superior outcome compared with traditional
chemotherapy (Fig. 3A), which
suggests that cancer treatment, at least in cases with lymphoma, is
not be completely replaceable by CTL immunotherapy. However, along
with the advances in cancer immunotherapy research, several novel
engineering methods for specific CTLs, such as chimeric antigen
receptor T cells, have attracted attention and represent a
promising clinical application prospect.
As mentioned above, the tumor microenvironment has
been proven to be associated with lymphoma initiation and
progression. Cytokines and cells in the tumor microenvironment play
crucial roles in modulating the biological behavior of malignant
tumors, such as proliferation, differentiation and angiogenesis. In
a molecular pathogenesis study on Hodgkin's lymphoma, the
expression of a variety of cytokines and chemokines by the tumor
cells were considered to be the driving force behind the abnormal
immune response. The malignant tumor cells and
lymphocyte-predominant T cells modulate the microenvironment,
permitting cells to develop the fully malignant phenotype and evade
host immune surveillance (21). The
tumor microenvironment was also found to control tumor growth in a
human primary effusion lymphoma mouse xenograft model (22). High numbers of tumor-infiltrating
regulatory T cells (Tregs) predicted improved survival of
follicular lymphoma patients, while a marked reduction in Treg
numbers was a predictor of transformation to DLBCL (23). Apart from NHL, an increased number of
CD68+ tumor-associated macrophages was found to be
closely associated with shortened survival in patients with classic
Hodgkin's lymphoma and provided a new biomarker for risk
stratification (24). In the present
study, the infused EBV-specific CTLs were found to accumulate in
the tumor sites where they exerted their cytotoxic effects
(Fig. 4A). Furthermore, the
F4/80+ TIMs were also elevated in tumors following
immunotherapy (Fig. 4B), which may be
attributed to tumor microenvironment alterations. We hypothesized
that there are two possible mechanisms underlying TIM upregulation:
i) Certain cytokines released by EBV-specific CTLs stimulated and
induced peripheral blood PBMCs to differentiate into macrophages
and, under the effect of cytokines, these macrophages were
recruited to the tumor site; and ii) abundant tumor-associated
antigens (TAAs) were released when the EBV-specific CTLs were
transfused into the mice, provoking a related innate immune
response at the tumor site. However, there are currently no
concrete data or proof to support these hypotheses. Therefore,
further studies are required and more efforts should be focused on
elucidating the role of TIMs in NHL treatment.
In cocnclusion, our present study demonstrated that
adoptive transfer of EBV-activated T cells enhanced EBV-related NHL
treatment response rate in mice. EBV-specific CTLs may indirectly
promote lymphoma immune reaction by activating TIM proliferation,
in addition to their direct tumor cytotoxic effect. These results
may provide a basis for further research on EBV-related NHL
immunotherapy.
Acknowledgements
This study was supported by the Hospital Management
Research Foundation of the PLA General Hospital of Chengdu Military
Region (grant no. 2013YG-B045).
References
|
1
|
Heslop HE: Biology and treatment of
Epstein-Barr virus-associated non-Hodgkin lymphomas. Hematology Am
Soc Hematol Educ Program. 2005:260–266. 2005. View Article : Google Scholar
|
|
2
|
Doherty PC and Christensen JP: Accessing
complexity: The dynamics of virus-specific T cell responses. Annu
Rev Immunol. 18:561–592. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Russell JH and Ley TJ: Lymphocyte-mediated
cytotoxicity. Annu Rev Immunol. 20:323–370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Iglesias E, Samri A, Kamkamidze G,
Decoville T, Carcelain G and Autran B: A systematic comparison of
methods to measure HIV-1 specific CD8 T cells. J Immunol Methods.
272:23–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Subklewe M, Chahroudi A, Schmaljohn A,
Kurilla MG, Bhardwaj N and Steinman RM: Induction of Epstein-Barr
virus-specific cytotoxic T-lymphocyte responses using dendritic
cells pulsed with EBNA-3A peptides or UV-inactivated, recombinant
EBNA-3A vaccinia virus. Blood. 94:1372–1381. 1999.PubMed/NCBI
|
|
6
|
Tabi Z, Moutaftsi M and Borysiewicz LK:
Human cytomegalovirus pp65 and immediate early 1 antigen-specific
HLA class I-restricted cytotoxic T cell responses induced by
cross-presentation of viral antigens. J Immunol. 166:5695–5703.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rehermann B, Chang K-M, McHutchinson J,
Kokka R, Houghton M, Rice CM and Chisari FV: Differential cytotoxic
T-lymphocyte responsiveness to the hepatitis B and C viruses in
chronically infected patients. J Virol. 70:7092–7102.
1996.PubMed/NCBI
|
|
8
|
Nijmeijer BA, Mollevanger P, van
Zelderen-Bhola SL, Kluin-Nelemans HC, Willemze R and Falkenburg JH:
Monitoring of engraftment and progression of acute lymphoblastic
leukemia in individual NOD/SCID mice. Exp Hematol. 29:322–329.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markasz L, Skribek H, Uhlin M, Otvos R,
Flaberg E, Eksborg S, Olah E, Stuber G and Szekely L: Effect of
frequently used chemotherapeutic drugs on cytotoxic activity of
human cytotoxic T-lymphocytes. J Immunother. 31:283–293. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sánchez-Aguilera A, Montalbán C, de la
Cueva P, Sánchez-Verde L, Morente MM, García-Cosío M, García-Laraña
J, Bellas C, Provencio M, Romagosa V, et al: Spanish Hodgkin
Lymphoma Study Group: Tumor microenvironment and mitotic checkpoint
are key factors in the outcome of classic Hodgkin lymphoma. Blood.
108:662–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guedez L, McMarlin AJ, Kingma DW, Bennett
TA, Stetler-Stevenson M and Stetler-Stevenson WG: Tissue inhibitor
of metalloproteinase-1 alters the tumorigenicity of Burkitt's
lymphoma via divergent effects on tumor growth and angiogenesis. Am
J Pathol. 158:1207–1215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori F, Ishida T, Ito A, Sato F, Masaki A,
Takino H, Ri M, Kusumoto S, Komatsu H, Ueda R, et al: Potent
antitumor effects of bevacizumab in a microenvironment-dependent
human lymphoma mouse model. Blood Cancer J. 2:e672012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Canioni D, Salles G, Mounier N, Brousse N,
Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard
C, et al: High numbers of tumor-associated macrophages have an
adverse prognostic value that can be circumvented by rituximab in
patients with follicular lymphoma enrolled onto the GELA-GOELAMS
FL-2000 trial. J Clin Oncol. 26:440–446. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mohammad RM, Wall NR, Dutcher JA and
Al-Katib AM: The addition of bryostatin 1 to cyclophosphamide,
doxorubicin, vincristine, and prednisone (CHOP) chemotherapy
improves response in a CHOP-resistant human diffuse large cell
lymphoma xenograft model. Clin Cancer Res. 6:4950–4956.
2000.PubMed/NCBI
|
|
15
|
Israel BF and Kenney SC: Virally targeted
therapies for EBV-associated malignancies. Oncogene. 22:5122–5130.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Frisan T, Sjöberg J, Dolcetti R, Boiocchi
M, De Re V, Carbone A, Brautbar C, Battat S, Biberfeld P, Eckman M,
et al: Local suppression of Epstein-Barr virus (EBV)-specific
cytotoxicity in biopsies of EBV-positive Hodgkin's disease. Blood.
86:1493–1501. 1995.PubMed/NCBI
|
|
17
|
Sing AP, Ambinder RF, Hong DJ, Jensen M,
Batten W, Petersdorf E and Greenberg PD: Isolation of Epstein-Barr
virus (EBV)-specific cytotoxic T lymphocytes that lyse
Reed-Sternberg cells: Implications for immune-mediated therapy of
EBV+ Hodgkin's disease. Blood. 89:1978–1986. 1997.PubMed/NCBI
|
|
18
|
Heslop HE, Slobod KS, Pule MA, Hale GA,
Rousseau A, Smith CA, Bollard CM, Liu H, Wu MF, Rochester RJ, et
al: Long-term outcome of EBV-specific T-cell infusions to prevent
or treat EBV-related lymphoproliferative disease in transplant
recipients. Blood. 115:925–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doubrovina E, Oflaz-Sozmen B, Prockop SE,
Kernan NA, Abramson S, Teruya-Feldstein J, Hedvat C, Chou JF,
Heller G, Barker JN, et al: Adoptive immunotherapy with unselected
or EBV-specific T cells for biopsy-proven EBV+ lymphomas after
allogeneic hematopoietic cell transplantation. Blood.
119:2644–2656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Icheva V, Kayser S, Wolff D, Tuve S,
Kyzirakos C, Bethge W, Greil J, Albert MH, Schwinger W, Nathrath M,
et al: Adoptive transfer of Epstein-Barr virus (EBV) nuclear
antigen 1-specific T cells as treatment for EBV reactivation and
lymphoproliferative disorders after allogeneic stem-cell
transplantation. J Clin Oncol. 31:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Steidl C, Connors JM and Gascoyne RD:
Molecular pathogenesis of Hodgkin's lymphoma: Increasing evidence
of the importance of the microenvironment. J Clin Oncol.
29:1812–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Staudt MR, Kanan Y, Jeong JH, Papin JF,
Hines-Boykin R and Dittmer DP: The tumor microenvironment controls
primary effusion lymphoma growth in vivo. Cancer Res. 64:4790–4799.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carreras J, Lopez-Guillermo A, Fox BC,
Colomo L, Martinez A, Roncador G, Montserrat E, Campo E and Banham
AH: High numbers of tumor-infiltrating FOXP3-positive regulatory T
cells are associated with improved overall survival in follicular
lymphoma. Blood. 108:2957–2964. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|