Introduction
The role of aldosterone in cardiovascular disease
has received increasing attention (1–8). It
has been reported that elevated concentrations of plasma
aldosterone cause endothelial dysfunction and trigger a series of
vascular lesions (9), however, the
underlying molecular mechanism remains to be elucidated. Poly
(ADP-ribose) polymerase (PARP)1 is a nuclear localization protein
with a molecular weight of 116kDa, and has low levels of activity
in normal cell growth (10–12).
The predominant function of PARP1 is in single strand DNA damage
repair, to avoid changing the genetic information of cells
(10–12). When DNA damage occurs, PARP1 is
activated to repair the DNA and, if the damage increases further,
PARP1 is fully activated consuming high levels of energy,
eventually leading to cell death (13,14).
A previous investigation found that PARP-1 is
important in a variety of cardiovascular disease processes
(15) and that the catalytic
activity of PARP-1 increases significantly in the development of
these diseases. Previous studies, using animal models of
cardiovascular disease, demonstrated that PARP inhibitors have a
positive and effective role in alleviating the symptoms of
cardiovascular disease (14).
However, the underlying molecular mechanism remains to be
elucidated. It was hypothesized that changes in the activity of
PARP1 involved in the apoptosis of endothelial cells were caused by
aldosterone. In the present study, primary cultured human umbilical
vein endothelial cells (HUVECs) were selected as models of
endothelial cells. The cultured cells were treated with aldosterone
for identical durations or at the same concentration for different
durations and the levels of proliferation and apoptosis were
assessed. The PARP1 activity in the cells was simultaneously
detected.
Eplerenone is a novel, selective aldosterone
receptor inhibitor with advantages, including low levels of
interaction with androgen and progesterone receptors, longer
half-life, low incidence of side effects and good tolerance,
compared with other aldosterone receptor inhibitors (16,17).
Once daily oral administration effectively controls high blood
pressure and reduces damage to the heart, brain, kidney and other
target organs. Since its introduction, eplerenone has demonstrated
the ability to control hypertension, prevent target organ damage
associated with cardiovascular disease and improve the prognosis of
patients with hypertension (18–20).
To ascertain whether the damage caused by
aldosterone on endothelial cells derives from the direct effects of
aldosterone on aldosterone receptors, eplerenon, an aldosterone
receptor antagonist with fewer side effects and improved
specificity, was selected to treat cells in combination with
aldosterone. The present study aimed to determine whether
eplerenone was involved in offsetting the endothelial cell
apoptosis and increased activity of PARP1 caused by the
extracellular signaling molecule, aldosterone and, ultimately, to
investigate how aldosterone affects intracellular apoptosis and
PARP1 activity.
Materials and methods
Cell preparation
HUVECs were collected from the neonatal umbilical
cord by perfusion digestion (Yantaishan Hospital, Yantai, China)
according to a previously reported method (21). The cells were cultured in RPMI-1640
complete medium (Shanghai Sangon, Biological Engineering Co., Ltd.,
Shanghai, China) and then were incubated in a culture flask
(1×106/ml), followed by culture in 5% CO2 and
at 37°C. The medium was refreshed every 24 h. Once the growth
density of primarily cultured cells reached more than 80%, the
culture fluid was discarded. Following washing with
phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA)
for 2–3 times, 0.25% trypsin (Fuzhou Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) was added for digestion at
37°C for 1 min. The mixture was centrifuged at 200 x g for 5 min.
The supernatant was removed, and new culture fluid was added for
continued culture. The second to fifth-generation cells were
obtained for the following experiments.
Apoptosis detection
Apoptotic cells were detected using Terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; KGI
Biotechnology Development Co., Ltd., Nanjing, China), according to
the manufacturer’s instructions, and were observed using an IX81
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Apoptosis was also detected by flow cytometry
(FACSCanto; BD Biosciences, Inc., Franklin Lakes, NJ, USA). The
cells (5×103/ml) were collected by trypsin digestion,
centrifuged to precipitate the cells at 200 x g for 5 min at 4°C
and washed twice with PBS, prior to fixing in 2 ml 70% ethanol
(Sigma-Aldrich) at 4°C overnight. The cells were washed twice with
PBS, as described above, and 200 ml RNaseA (Fuzhou Maixin
Biotechnology Development Co., Ltd.) at a final concentration of 25
g/ml, was added to the cells and incubated at 37°C in a water bath
(DFY-10; Changzhou JieBo sen Instrument Co., Ltd., Changzhou,
China) for 30 min. Propidium iodide (PI; Sigma-Aldrich) solution
(500 ml) at a concentration of 50 g/ml was added to the cells for
30 min at 4°C in the dark, prior to being filtered using a 300 mesh
nylon mesh (Shanghai Tesheng Filters Material Co., Ltd., Shanghai,
China) and detected by flow cytometry. The number of detected cells
required was ≥5×104, the excitation wavelength was 488
nm and the emission wavelength was 620 nm. A histogram plotting the
intensity of the PI signal and a scattergram demonstrating the
front scattered light against the side-scattered light were plotted
using CellQuest software (BD Biosciences, Inc.).
Total cellular protein extracts
Following treatment, the cell culture was removed
and the cells (5×103/ml) were gently washed three times
with PBS. The adherent cells were scraped off the culture plates
using a cell scraper and collected by centrifugation at 1,049 x g
for 3 min at 4°C. The supernatant was carefully removed and 100 ml
lysis buffer (1 volume of sediment:3 volumes of buffer), pre-cooled
at 0°C, was added at a volume three times that of the volume of
sedimentation. The cells were repeatedly pipetted (5 times) and the
cell suspension was disrupted by agitation with glass beads
(0.4–0.5 mm) at 4°C for 30 min. The cell lysates were collected and
centrifuged at 1,049 x g for 10 min at 4°C and the supernatant,
containing the total cellular protein was preserved at −80°C for
subsequent use.
Caspase-3 activity assay
The cells (5×103/ml) from each
experimental group (0, 0.01, 0.1, 1, 10, 100 and 1,000
µmol/l aldosterone treatment) were collected, washed twice
with PBS and the total cell protein was obtained by dissociating
and extracting, as mentioned in the previous paragraph. Reaction
buffer (2X), containing 10 mM dithiothreitol and 1 mM caspase-3
tetrapeptide fluorogenic substrate (acetyl Asp-Glu-Val-Asp
7-amido-4-methylcoumarin; Ac-DEVD-AMC) (Fuzhou Maixin Biotechnology
Development Co., Ltd.) was added to the protein lysate and
incubated in 96-well plates at 37°C to react for 60 min.
Fluorescence analysis was performed using a DG5033A microplate
reader (Shanghai Precision & Scientific Instrument Co., Ltd.,
Shanghai, China) with an excitation wavelength of 380 nm and an
emission wavelength of 430–460 nm. Depending on the fluorescence
intensity of AMC, the activity of caspase-3 was measured to reflect
the degree of activated caspase-3.
PARP activity detection
The activity of PARP was detected in cultured
endothelial cells using a PARP Detection kit (Trevigen,
Gaithersburg, MD, USA), according to the manufacturer’s
instructions. The assessment required at least three repeats for
each sample to ensure reliable detection results.
RNA extraction and reverse
transcription
The first-strand cDNA was generated using a
TransScript First-Strand cDNA Synthesis SuperMix kit (Transgen,
Beijing, China). The first-strand cDNA was diluted 10-fold as a
template for polymerase chain reaction (PCR) or quantitative
(q)PCR.
qPCR
The qPCR was performed following the addition of
SYBR premix EX taq kit (Takara Bio Inc., Otsu, Shiga, Japan) and
gently mixing the plates. The quantity of the cDNA sample was 2
µl, the primer sequences used are presented in Table I and the cycling conditions were as
follows: 40 cycles of 95°C for 30 sec, 95°C for 5 sec and 60°C for
34 sec.
 | Table IPrimer sequences used for reverse
transcription quantitative polymerase chain reaction. |
Table I
Primer sequences used for reverse
transcription quantitative polymerase chain reaction.
| Target gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| β-actin |
GTTGTCGACGACGAGCG |
GCACAGAGCCTCGCCTT |
| Poly (ADP-ribose)
polymerase 1 |
TCTGCCTTGCTACCAATTCC |
GATGGGTTCTCTGAGCTTCG |
Immunoblotting
The proteins were separated by molecular weight
using 10% SDS-PAGE gels (Sigma-Aldrich) and were transferred onto a
nitrocellulose membrane (Advantec MFS, Inc., Dublin, CA, USA). The
membrane was subsequently blocked in 5% non-fat milk at room
temperature for 60 min.
The membranes were then incubated with the primary
antibody (rabbit anti-human PARP polyclonal antibody; #9664; Cell
Signaling Technology, Inc., MA, USA) diluted in 5% non-fat milk
(Fuzhou Maixin Biotechnology Development Co., Ltd.) overnight at
4°C. Following washing in Tris-buffered saline containing Tween-20
(TBST; Shanghai Sangon, Biological Engineering Co., Ltd.) three
times for 10 min on a horizontal shaker (HZ-9611K; Hualida
Experiment Equipment Company, Nanjing, China), the membranes were
incubated with the secondary antibody
(horseradish-peroxidase-labeled goat anti-rabbit IgG; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
diluted in 5% non-fat milk at room temperature for 60 min and were
washed with TBST and stained with DAB coloration liquid
(Sigma-Aldrich), followed by developing with the ECL system (GE
Healthcare Life Sciences, Livingston, NJ, USA) according to the
manufacturer’s instructions.
Results
Effect of different concentrations of
aldosterone on apoptosis
The subcultured HUVECs were treated with aldosterone
(0, 0.01, 0.1, 1, 10, 100 and 1,000 µmol/l; Fluka, St.
Louis, MO, USA) for 48 h. The 0 µmol/l concentration was
used as the control group. Following treatment, the apoptotic cells
were detected by a variety of methods.
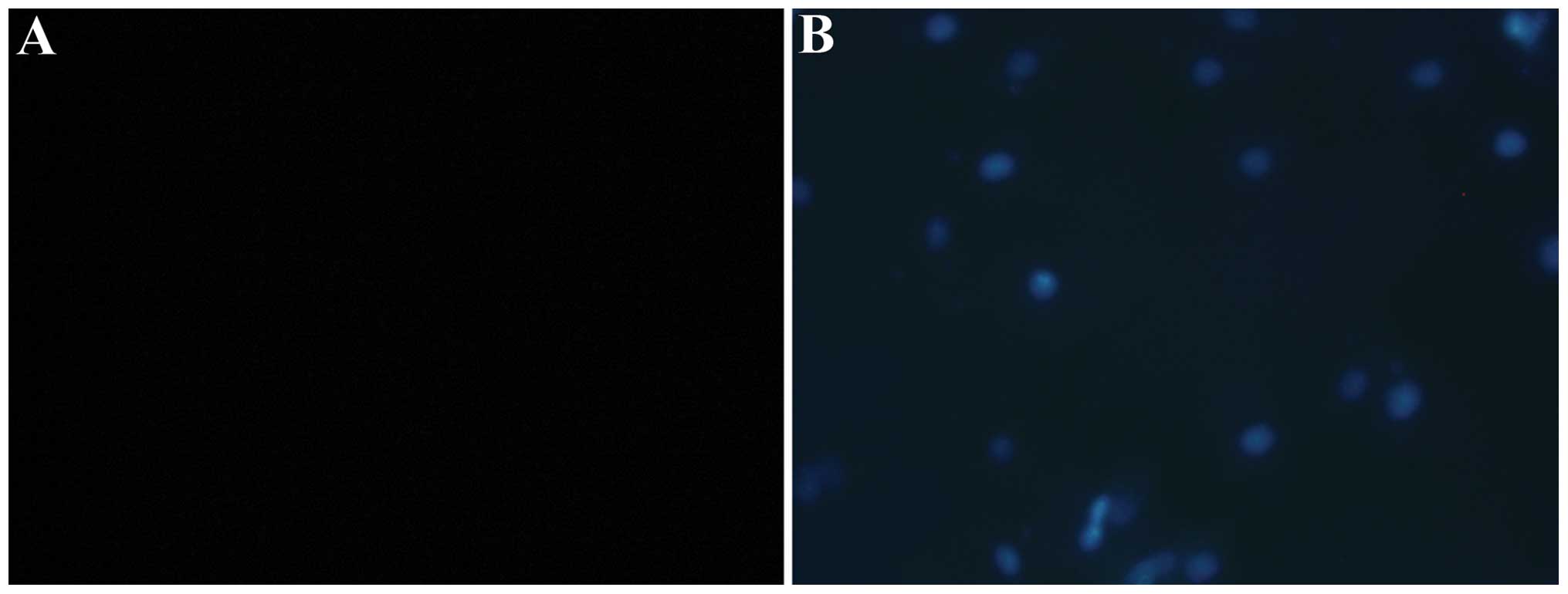
TUNEL was used to determine the number of
endothelial cells undergoing apoptosis. No positive staining was
detected following treatment of the HUVECs with 0, 0.01, 0.1, 1 or
10 µmol/l aldosterone, similar to the control group.
However, when the concentration of aldosterone increased to 100
µmol/l, a number of cells exhibited positive TUNEL staining,
indicating that free 3′ hydroxyl groups were produced due to DNA
cleavage in these cells, suggesting that several cells have entered
apoptosis. When the concentration was further increased to 1,000
µmol/l, the number of positive TUNEL stained cells
increased, indicating that a number of cells had entered the
apoptotic process (Fig. 1).
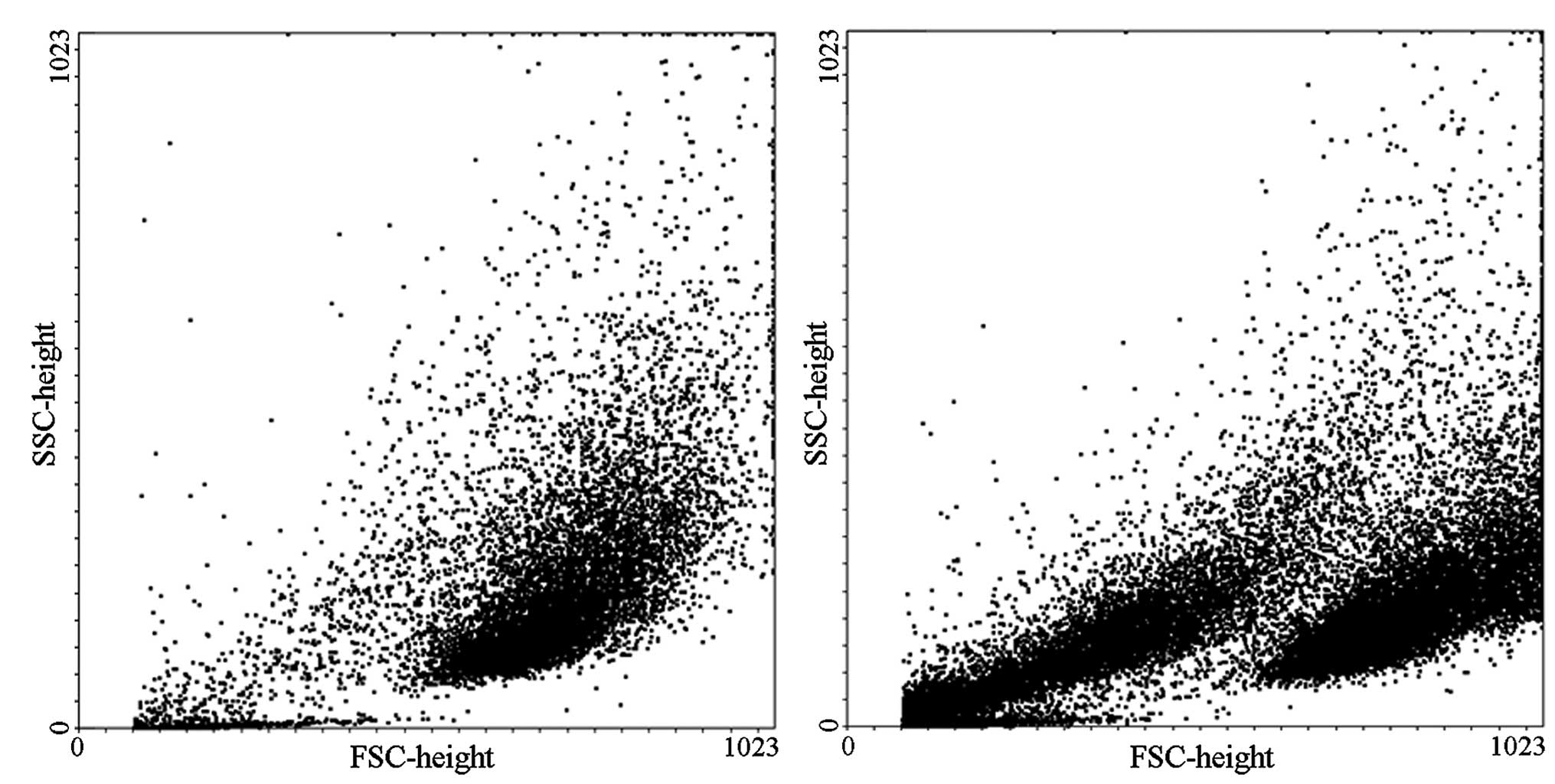
Flow cytometry was used to detect and quantify the
number of apoptotic cells. The results demonstrated that dense
endothelial cells treated with low concentrations of aldosterone
(0.01, 0.1, 1 and 10 µmol/l) exhibited no differences in the
number of apoptotic cells compared with the 0 µmol/l control
group. When the concentration was increased to 100 µmol/l, a
marked increase of apoptosis was observed compared with the control
group (0.64, vs. 18.3%; P<0.01). The effect on apoptosis was
more pronounced when the concentration of aldosterone was increased
to 1,000 µmol/l compared with the control group (0.64, vs.
42.5%; P<0.01; Fig. 2).
Effect of aldosterone treatment duration
on apoptosis
The subcultured HUVECs were treated with aldosterone
at a final concentration of 1,000 µmol/l for 24, 48 or 72 h.
An untreated group of cells was used as a control.
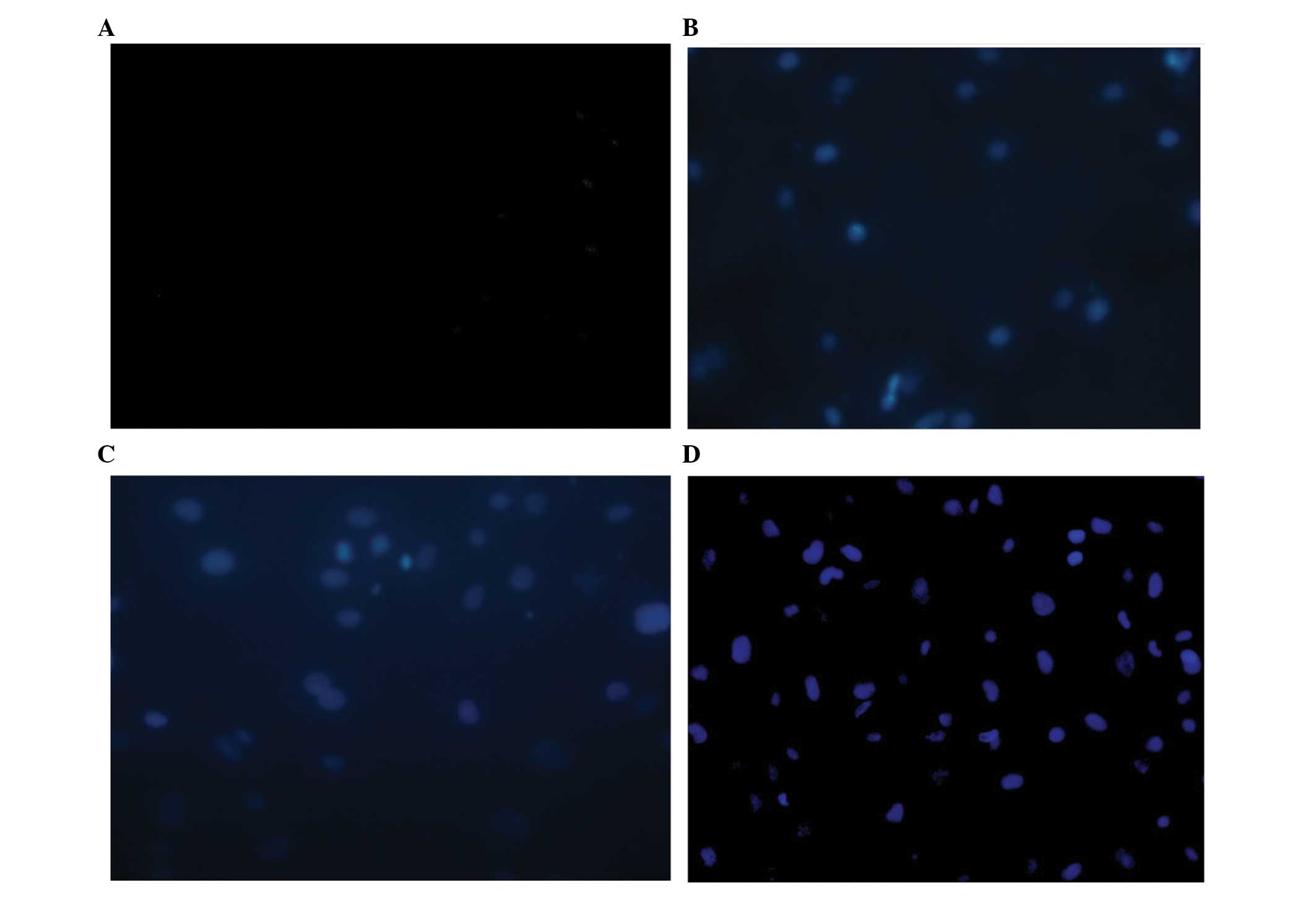
TUNEL was used to determine endothelial cell
apoptosis. When the duration of aldosterone treatment reached 24 h,
a small quantity of TUNEL-positive cells were detected. The number
of positive TUNEL stained cells further increased at 48 h and
markedly increased until 72 h (Fig.
3).
Apoptosis was also detected and quantified using
flow cytometry. The results demonstrated that the ability of
aldosterone (1,000 µmol/l) to induce the apoptosis of
endothelial cells with high cell density increased with increasing
treatment durations. When the treatment duration reached 72 h, the
majority of cells had died and the number of cells was
significantly less compared with any of the other experimental
groups, suggesting that several cells had been lysed into cell
fragments, which cannot be used for further PI staining or
fluorescent activated cell sorting analysis.
Effects of aldosterone on the activity of
caspase-3
The effect of aldosterone at different
concentrations on the activity of caspase-3 in cells was
investigated. On the basis of the preceding detection result of
cell apoptosis, the cells were treated with aldosterone (100 or
1,000 µmol/l) for 48 h at different cell densities and the
cells (5×103/ml) were collected and dissociated prior to
extracting the total cellular protein for a caspase-3 activity
assay.
The caspase-3 activity results from the treatment of
HUVECs at higher or lower densities were consistent with the
results from the apoptotic assays. The cells treated with 100
µmol/l aldosterone increased the activity of caspase-3 and,
following treatment with 1,000 µmol/l aldosterone, a more
marked increase in the activity of caspase-3 was observed. The
activity of caspase-3 was more markedly increased in the high cell
density group compared with the low cell density group.
The effect of aldosterone treatment duration on the
vitality of caspase-3 in HUVECs was also investigated. According to
the apoptosis results, the cells were detected at lower densities
and treated with 1,000 µmol/l aldosterone for 24, 48 or 72
h. The cells were collected and dissociated at the end of the
treatment and the total cellular protein was extracted for the
detection of caspase-3 activity. The results were consistent with
those of apoptosis. Caspase-3 activity increased as the treatment
duration increased and the rate of increased gradually.
Effects of aldosterone treatment on the
activity of PARP1
The effect of different concentrations of
aldosterone on the activity of intracellular PARP1 was subsequently
investigated. Based on the preceding results of apoptosis, the
cells were treated with 100 and 1,000 µmol/l aldosterone for
48 h at different cell densities, and the cells were collected,
dissociated and the total cellular protein was extracted to detect
PARP1 activity. Treatment with 100 µmol/l aldosterone
upregulated the activity of PARP1, while 1,000 µmol/l
aldosterone treatment upregulated the activity of PARP1 more
markedly. The increased range of PARP1 activity were increased in
the cells at a high density compared with those at a low
density.
The effect of the duration of aldosterone treatment
on the activity of PARP1 in HUVECs was also assessed. According to
the results of apoptosis, the cells were selected to be detected at
lower densities, treated with 1,000 µmol/l aldosterone for
24, 48 or 72 h. The cells were collected and dissociated following
treatment, and the total cellular protein was extracted for
detection of PARP1 activity. As the duration of treatment
increased, the vitality of PARP1 increased and this rate of
increase was gradual (Fig. 4).
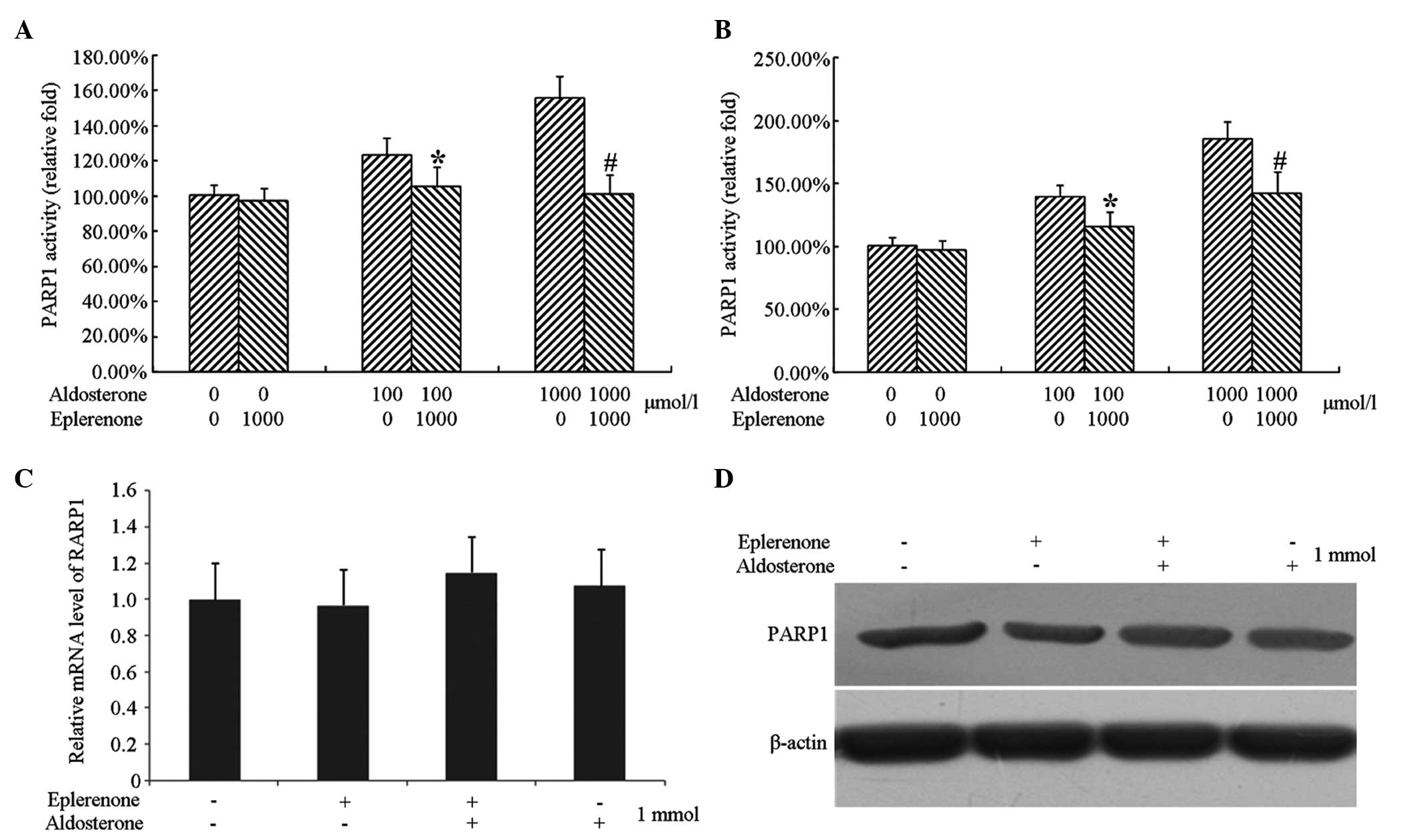
Inhibition of eplerenone on the
activation of caspase-3 by aldosterone
Processing HUVECs at a lower cell density and
treating with 1,000 µmol/l eplerenone completely inhibited
the activation of caspase-3 by aldosterone. However, in the case of
high cell density, 1,000 µmol/l eplerenone did not
completely suppress the activation of caspase-3 by aldosterone and
only partially reduced the activation of caspase-3.
Inhibition of eplerenone on the
activation of PARP1 by aldosterone
Processing HUVECs at a lower cell density and
treating with 1,000 µmol/l eplerenone completely inhibited
the activation of PARP1 by aldosterone. However, in the case of
high cell density, 1,000 µmol/l eplerenone did not
completely suppress the activation of PARP1 by aldosterone and only
partially reduced the activation of PARP1.
Detection of the protein expression of
PARP1
Endothelial cells cultured at a low growth density
were treated with 1,000 µmol/l eplerenone and/or 1,000
µmol/l aldosterone for 48 h and cells without any treatment
were used as a control group. The cells were collected following
treatment and were divided into two groups, one for extracting RNA
to determine the mRNA expression of PARP1 by qPCR, and another for
extracting total cellular protein to determine the expression of
PARP1 by immunobloting.
The results revealed that neither treatment of cells
with eplerenone or aldosterone alone or together affected the mRNA
and protein expression levels of PARP1 (Fig. 5).
Inhibition of ABT-888 on
aldosterone-induced apoptosis
The subcultured HUVECs were treated with aldosterone
at a final concentration of 0, 100 or 1,000 µmol/l in the
corresponding cell wells as a control group and ABT-888
(Sigma-Aldrich) was added to the experimental group. Following
culturing for 48 h, the culture supernatant from the cells was
collected and all the adherent cells were digested using trypsin,
aggregated and fixed with 70% ethanol, prior to staining with PI
for flow cytometric analysis.
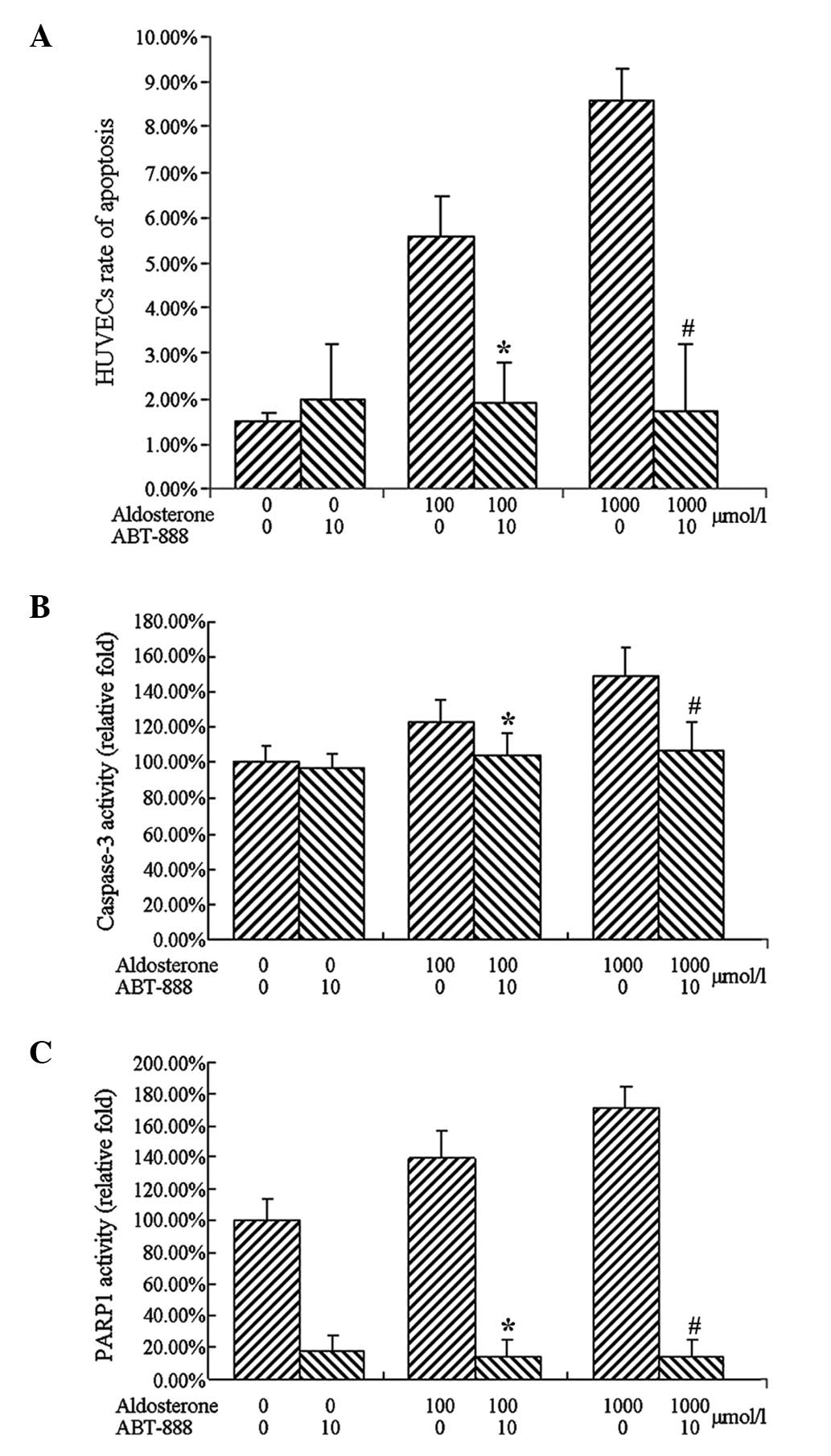
The results demonstrated that the cells treated with
aldosterone at a final concentrations of 100 and 1,000
µmol/l induced apoptosis significantly, which was consistent
with the previous results. Treatment with ABT-888 (10
µmol/l) and aldosterone (100 and 1,000 µmol/l),
completely inhibited aldosterone-induced apoptosis (Fig. 6).
ABT-888 inhibits the activation of
caspase-3 by aldosterone
Based on the results demonstrating that ABT-888
inhibited aldosterone-induced apoptosis, low density cells were
treated with aldosterone (100 or 1,000 µmol/l) and ABT-888
(10 µmol/l) for 48 h. The cells were collected and
dissociated following treatment, and the total cellular protein was
extracted for caspase-3 activity assay.
The results of caspase-3 activity were consistent
with the apoptosis, revealing that treatment with 100 µmol/l
aldosterone increased that activity of caspase-3. A more marked
increase in the activity of caspase-3 was observed following
treatment with 1,000 µmol/l aldosterone. Treatment with 10
µmol/l ABT-888, led to complete inhibition of the activation
of caspase-3 by aldesterone.
Impact of ABT-888 on PARP1 activity
To confirm the inhibitory effect on the cell
viability PARP1 following treatment with ABT-888, the treated cells
were collected, dissociated and the total cell protein was
extracted to detect PARP1 activity.
Treatment with 100 or 1,000 µmol/l
aldosterone upregulated the activity of PARP1 and cotreatment with
10 µmol/l ABT-888 significantly reduced PARP1 activity
(Fig. 7).
Discussion
The activity of the PARP1 protease is catalyzed by
poly ADP-ribosyl in eukaryotic cells and this protein is involved
in DNA repair following DNA damage or fracture. The PARP enzyme
activity accounts for >90% of the enzyme activity in the protein
family (19). PARP1 is important
in DNA repair and apoptosis. Deletion of PARP1 renders cells more
sensitive to DNA damage and causes a marked accumulation of genetic
mutations, inducing tumorigenesis (20,22).
The accumulation of DNA damage often leads to cancer
and genetic mutations, whereas excessive DNA damage can lead to
apoptosis. Breast cancer susceptibility gene (BRCA)1 and BRCA2 are
DNA double-strand break repair proteins and deletion of either
protein causes the accumulation of DNA mutations, leading to breast
cancer. PARP1 inhibitors are used to treat breast cancer exhibiting
deletion of BRCA1 or BRCA2. With this deletion, a large number of
cells exhibiting DNA damage and inhibiting the activity of PARP1
further increased DNA damage, leading to apoptosis and ultimately
treating tumors (23).
Numerous PARP1-specific inhibitors are available for
the clinical treatment of cancer. Previous studies have
demonstrated that elevated PARP1 activity in normal cells leads to
damage of endothelial function (24). The present study confirmed that the
abnormal elevation of plasma aldosterone concentration induced
apoptosis of endothelial cells, thereby triggering endothelial
dysfunction. It was demonstrated that this effect was transmitted
into the cells by aldosterone receptors. Aldosterone also increased
the activity of PARP1 in cells, however, the activation of PARP1
was not caused by regulating mRNA or protein expression levels.
The PARP1 inhibitor, ABT-888, is commonly used with
aldosterone to process cells and it has been revealed that the
increase in PARP1 activity mediated the effect of aldosterone on
the apoptosis of endothelial cells. Specific concentrations of
ABT-888 reduced PARP1 activity to low levels and completely
inhibited the aldosterone-stimulated apoptosis of HUVECs.
ABT-888 also acted on the nucleoprotein PARP1
directly through the cell membrane, this confirmed the effect of
aldosterone stimulating apoptosis through the aldosterone receptor.
The stimulation of aldosterone was transferred between the
extracellular and intracellular environment through its receptors,
while ABT-888 directly inhibited intracellular PARP1 activity.
Inhibition of intracellular PARP1 protein inhibited the activity of
the extracellular signal, which confirmed that PARP1 apoptosis was
mediated by aldosterone.
The present study investigated the molecular
mechanisms underlying aldosterone-induced endothelial cell damage
through the activation of PARP1 activity, leading to cell
apoptosis. However, the mechanisms underlying the activation of
PARP1 by the aldosterone activating aldosterone receptor requires
further investigation. It was found that the activation and role of
aldosterone on PARP1 was not due to altering the mRNA or protein
levels and, therefore, the mechanisms require further
investigation.
The present study confirmed that the PARP1
inhibitor, ABT-888, inhibited the effect of high concentrations of
aldosterone on the injury of cultured endothelial cells, suggesting
a novel direction in the treatment of cardiovascular diseases
caused by aldosterone. However, in vitro experiments do not
completely simulate in vivo conditions, and the feasibility
of the treatment programs also require further investigation in
animal experiments.
References
|
1
|
Hillaert MA, Lentjes EG, Beygui F,
Kemperman H, Asselbergs FW, et al: Measuring and targeting
aldosterone and renin in atherosclerosis-a review of clinical data.
Am Heart J. 162:585–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tomaschitz A, Pilz S, Ritz E, Meinitzer A,
Boehm BO and März W: Plasma aldosterone levels are associated with
increased cardiovascular mortality: the Ludwigshafen Risk and
Cardiovascular Health (LURIC) study. Eur Heart J. 31:1237–1247.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vantrimpont P, Rouleau JL, Ciampi A, et
al: Two-year time course and significance of neurohumoral
activation in the Survival and Ventricular Enlargement (SAVE)
Study. Eur Heart J. 19:1552–1563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun Y, Zhang J, Lu L, Chen SS, Quinn MT
and Weber KT: Aldosterone-induced inflammation in the rat heart:
role of oxidative stress. Am J Pathol. 161:1773–1781. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pitt B, Bakris G, Ruilope LM, DiCarlo L
and Mukherjee R; EPHESUS Investigators: Serum potassium and
clinical outcomes in the Eplerenone Post-Acute Myocardial
Infarction Heart Failure Efficacy and Survival Study (EPHESUS).
Circulation. 118:1643–1650. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palmer BR, Pilbrow AP, Frampton CM, Yandle
TG, Skelton L, Nicholls MG and Richards AM: Plasma aldosterone
levels during hospitalization are predictive of survival
post-myocardial infarction. Eur Heart J. 29:2489–2496. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calhoun DA: Aldosterone and cardiovascular
disease: smoke and fire. Circulation. 114:2572–2574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keidar S, Kaplan M, Pavlotzky E, Coleman
R, Hayek T, Hamoud S and Aviram M: Aldosterone administration to
mice stimulates macrophage NADPH oxidase and increases
atherosclerosis development: a possible role for
angiotensin-converting enzyme and the receptors for angiotensin II
and aldosterone. Circulation. 109:2213–2220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatakeyama H, Miyamori I, Fujita T, Takeda
Y, Takeda R and Yamamoto H: Vasular aldosterone. Biosynthesis and a
link to angiotensin II-induced hypertrophy of vascular
smooth-muscle cells. J Biol Chem. 269:24316–24320. 1994.PubMed/NCBI
|
|
10
|
Godon C, Cordelières FP, Biard D, Giocanti
N, Méqnin-Chanet F, Hall J and Favaudon V: PARP inhibition versus
PARP-1 silencing: different outcomes in terms of single-strand
break repair and radiation susceptibility. Nucleic Acids Res.
36:4454–4464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schultz N, Lopez E, Saleh-Gohari N and
Helleday T: Poly(ADP-ribose) polymerase (PARP-1) has a controlling
role in homologous recombination. Nucleic Acids Res. 31:4959–4964.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Claybon A, Karia B, Bruce C and Bishop AJ:
PARP1 suppresses homologous recombination events in mice in vivo.
Nucleic Acids Res. 38:7538–7545. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiarugi A and Moskowitz MA: Cell biology.
PARP-1 – a perpetrator of apoptotic cell death? Science.
297:200–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rho YH, Chung CP, Oeser A, et al:
Inflammatory mediators and premature coronary atherosclerosis in
rheumatoid arthritis. Arthritis Rheum. 61:1580–1585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pacher P and Szabó C: Role of
poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases:
the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev.
25:235–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki J, Iwai M, Mogi M, Oshita A, Yoshii
T, Higaki J and Horiuchi M: Eplerenone with valsartan effectively
reduces atherosclerotic lesion by attenuation of oxidative stress
and inflammation. Arterioscler Thromb Vasc Biol. 26:917–921. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takai S, Jin D, Muramatsu M, Kirimura K,
Sakonjo H and Miyazaki M: Eplerenone inhibits atherosclerosis in
nonhuman primates. Hypertension. 46:1135–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pitt B, Remme W, Zannad F, et al:
Eplerenone, a selective aldosterone blocker, in patients with left
ventricular dysfunction after myocardial infarction. N Engl J Med.
348:1309–1321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diefenbach J and Bürkle A: Introduction to
poly(ADP-ribose) metabolism. Cell Mol Life Sci. 62:721–730. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsifetaki N, Georqiadis AN, Alamanos Y,
Fanis S, Arqyropoulou MI and Drosos AA: Subclinical atherosclerosis
in scleroderma patients. Scand J Rheumatol. 39:326–329. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sand-Dejmek J, Adelmant G, Sobhian B,
Calkins AS, Marto J, Iqlehart DJ and Lazaro JB: Concordant and
opposite roles of DNA-PK and the “facilitator of chromatin
transcription” (FACT) in DNA repair, apoptosis and necrosis after
cisplatin. Mol Cancer. 10:742011. View Article : Google Scholar
|
|
23
|
Bryant HE, Schultz N, Thomas HD, et al:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haile WB, Echeverry R, Wu F, Guzman J, An
J, Wu J and Yepes M: Tumor necrosis factor-like weak inducer of
apoptosis and fibroblast growth factor-inducible 14 mediate
cerebral ischemia-induced poly(ADP-ribose) polymerase-1 activation
and neuronal death. Neuroscience. 171:1256–1264. 2010. View Article : Google Scholar : PubMed/NCBI
|