Introduction
Premature ovarian failure (POF) is defined as the
occurrence of amenorrhea, hypergonadotropinemia and estrogen
deficiency in women <40 years of age, which is accompanied by
decreased levels of estrogen and increased levels of gonado tropin
(1,2). It is a major cause of female
infertility (3,4) and it has been reported that females
diagnosed with POF have almost a two-fold age-specific increase in
mortality rate, and are at increased risk of cardiovascular
disease, neurocognitive disorders, including Parkinson’s disease,
and endocrine and autoimmune disorders (5–13).
Notably, the early loss of ovarian function has significant
psychosocial sequelae and health implications (5). Although often considered a rare
disorder, POF affects 0.0001% of femals by the age of 20 years,
0.001% by 30 years and 0.01% by 40 years (14). In addition, the relevance of POF is
continuously increasing as females are tending to conceive more
frequently in their thirties and forties. A previous multi ethnic
population, cross sectional study revealed that the prevalence of
POF was 1.0% in Caucasian females, 1.4% in African American
females, 1.4% in Hispanic females, 0.5% in Chinese females and 0.1%
in Japanese females, suggesting significant differences in the
frequency of POF among ethnic groups (P=0.01) (15). In China, the incidence of POF is
estimated to be 1–3.8% in females aged <40 years (16).
A number of hypotheses have been suggested to
explain the development of POF (17). It has been reported that POF can be
induced by chemotherapy, radiotherapy, autoimmune disorders,
ovarian or other pelvic surgery, and genetic disorders, including
Turner syndrome and Fragile X syndrome (18). In addition, POI has been revealed
to have a significant genetic component (19). Although the underlying cause of POF
remains to be elucidated in the majority of cases, mutations in
certain candidate genes have been reported to be associated with
the risk of POF (20–26). In the present study, single
nucleotide polymorphisms (SNPs) of the growth differentiation
factor 9 (GDF9), bone morphogenetic protein 15 (BMP15), inhibin βB
(INHBB) and follicle stimulating hormone receptor (FSHR) genes were
investigated in a Chinese Hui population in Ningxia, western China,
and their association with POF was evaluated.
Patients and methods
Patients
Patients of Chinese Hui ethnicity, with a diagnosis
of POF were recruited from the Center for Reproductive Medicine,
the General Hospital of Ningxia Medical University (Yinchuan,
China) during the period between February 2010 and October 2011 for
investigation in the present study. All participants met the
following inclusion criteria: i) ≤40 years old; ii) duration of
amenorrhea ≥6 months; iii) serum FSH level ≥40 IU/l or serum
luteinizing hormone (LH) level ≥30 IU/l on two or more occasions;
iv) serum estradiol (E2) level ≤25 pg/ml in two
occasions in two consecutive months, with the presence of
amenorrhea; v) B mode ultrasound indicating no follicle reserves in
either ovary; vi) no autoimmune diseases, endocrine, liver or
kidney dysfunctions, or impaired glucose tolerance. Individuals
with a history of ovarian surgery, radiotherapy, chemotherapy or
other factors, which may damage ovarian functions were excluded. A
total of 63 patients were enrolled in the present study, among
which two were biological sisters, with normal karyotypes. The
patients had a mean age of 29.82±6.0 years (range, 17–39
years).
A total of 58 women of childbearing age of the
Chinese Hui population, who were admitted to the Center for
Reproductive Medicine, General Hospital of Ningxia Medical
University due to tubal factor or male factor infertility over the
same period, were recruited as controls. These individuals had a
mean age of 29.25±4.43 years (range, 22–41 years). All the control
individuals had a regular menstrual cycle, and normal reproductive
hormone levels and chromosomes. Transvaginal B mode sonography
(Aloka SSD 1400 Ultrasound system; Hitachi Aloka Medical, Ltd.,
Tokyo, Japan) of the uterus and bilateral annex revealed no organic
diseases, and the ovarian antral follicles were normal.
Ethical considerations
The present study was approved by the Ethics Review
Committee of the General Hospital of Ningxia Medical University
(permission no. NZ-IRB-2010017). Written informed consent was
obtained from all participants following a detailed description of
the potential benefits of the investigation.
DNA extraction, polymerase chain reaction
(PCR) assay and sequencing
Following overnight fasting, a 5 ml venous blood
sample was collected from the elbow, anticoagulated with EDTA
Na2 (Amresco, LLC, Solon, OH, USA) and stored at −80°C
for the subsequent experiments. Genomic DNA was extracted from the
blood using a TIANamp genomic DNA kit (Tiangen Biotech, Beijing,
China), according to the manufacturer’s instructions, and the
concentration was determined using ultraviolet spectrophotometry
(UV3100; Hitachi, Ltd., Tokyo, Japan). PCR amplification of the
GDF9, BMP15, FSHR and INHBB genes was
performed using the primers shown in Table I, in a 25 μl reaction volume
containing 1 μl DNA template, 12.5 μl PCR mix, 0.75
μl forward and reverse primers, 0.25 μl Taq DNA
polymerase and 10.5 μl ddH2O, under the PCR
amplification conditions shown in Table I. The PCR was conducted on an ABI
3100 sequencer (Applied Biosystems, Foster City, CA, USA). The PCR
products were confirmed using gel electrophoresis on a 2% agarose
gel (Sangon Biotech Co., Ltd., Shanghai, China). All PCR reagents,
primers and kits were purchased from Sangon Biotech Co., Ltd.
Subsequently, the PCR products were sequenced for GDF9, the
BMP15 gene protein coding region, INHBB gene exon
2 and the FSHR Ala307Thr and Ser680Asn variants
at the Beijing Genomics Institute (Beijing, China) using a dideoxy
chain termination method (27).
 | Table IPrimers and protocols used for PCR
amplification of GDF9, BMP15, FSHR and
INHβB. |
Table I
Primers and protocols used for PCR
amplification of GDF9, BMP15, FSHR and
INHβB.
| Gene | Location | Primer
sequence | PCR amplification
protocol |
|---|
| GDF9 | Exon 1 | F,
5′-TAGTCCACCCACACACCTGA-3′; R, 5′-CCAAAAGCTTGGTGGAACAG-3′ | 94°C for 4 min,
followed by 30 cycles at 94°C for 60 sec, 58°C for 45 sec and 72°C
for 50 sec, then finally 72°C for 7 min |
| Exon 2 | F,
5′-CCAGTAAGGTTGCTGGGAAT-3′; R, 5′-TCCCCCACTAAATGATCAGC-3′ | 94°C for 4 min,
followed by 30 cycles at 94°C for 60 sec, 58°C for 45 sec and 72°C
for 50 sec, then finally 72°C for 7 min |
| BMP15 | Exon 1 | F,
5′-CTCGTTCGCTCGCCTGGCGC-3′; R, 5′-GAAGTTGCGCATCATGCTGT-3′ | 94°C for 4 min,
followed by 30 cycles at 94°C for 60 sec, 58°C for 45 sec and 72°C
for 50 sec, then finally 72°C for 7 min |
| Exon 2 | F,
5′-ATGCGCTTCTCCAGCTTTGT-3′; R, 5′-TATGGAGCACACCTCACCTG-3′ | 94°C for 4 min,
followed by 30 cycles at 94°C for 60 sec, 58°C for 45 sec and 72°C
for 50 sec, then finally 72°C for 7 min |
| FSHR | Exon 10 | F,
5′-GAGTGTCACGCCTTCTCCTC-3′; R, 5′-GAGTGTCACGCCTTCTCCTT-3′ | 94°C for 4 min,
followed by 15 cycles at 94°C for 30 sec, 62°C for 30 sec and 72°C
for 30 sec, then finally 72°C for 8 min |
| INHBB | Exon 2 | F,
5′-TTTCCGATCAGTGGCCACGC-3′; R, 5′-AAGATGGTTTCCCCAGTGAC-3′ | 94°C for 4 min,
followed by 15 cycles at 94°C for 30 sec, 62°C for 30 sec and 72°C
for 30 sec, then finally 72°C for 8 min |
Statistical analysis
The sequencing chromatograms were assessed using the
Chromas 2.23 program (Technelysium, Tewantin, QLD, Australia), and
the sequences of the GDF9, BMP15, INHBB and
FSHR genes were aligned to those registered in GenBank
(http://www.ncbi.nlm.nih.gov/genbank/)
for the identification of mutatnt loci. All data were entered into
Microsoft Excel 2007 (Microsoft Corporation; Redmond, WA, USA), and
all statistical analyses were performed using SPSS version 17.0
statistical software (SPSS, Inc., Chicago, IL, USA). The
differences in proportions were assessed for statistical
significance using a χ2 test. P<0.05 was considered
to indicate a statistically significant difference.
Results
GDF9 SNPs
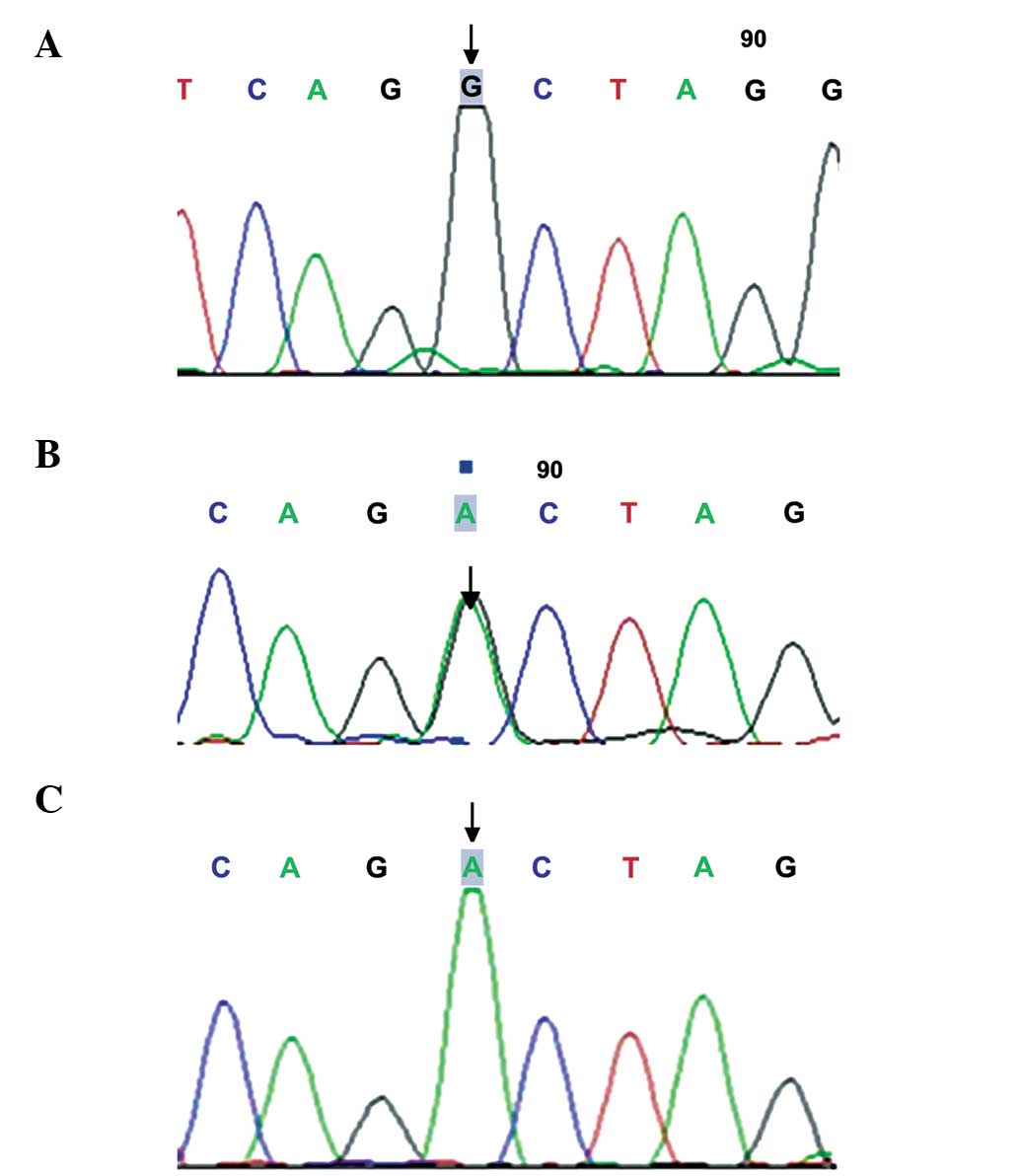
A total of four SNPs were genotyped across the
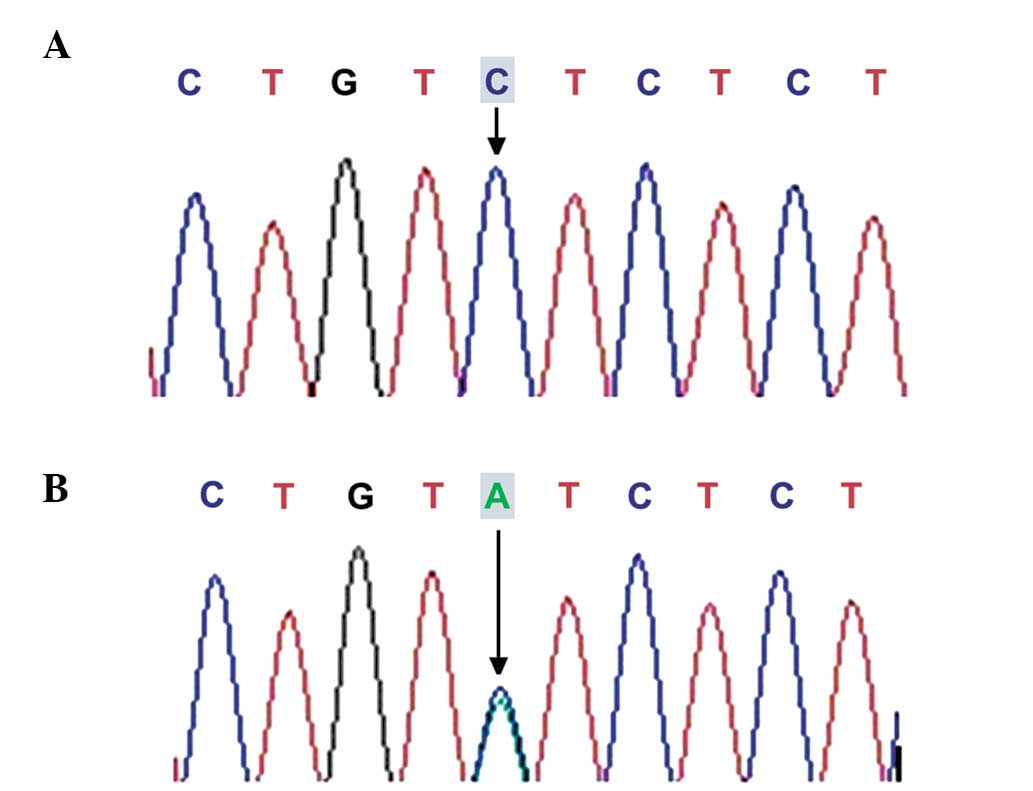
GDF9 locus using sequencing analysis, including D57Y
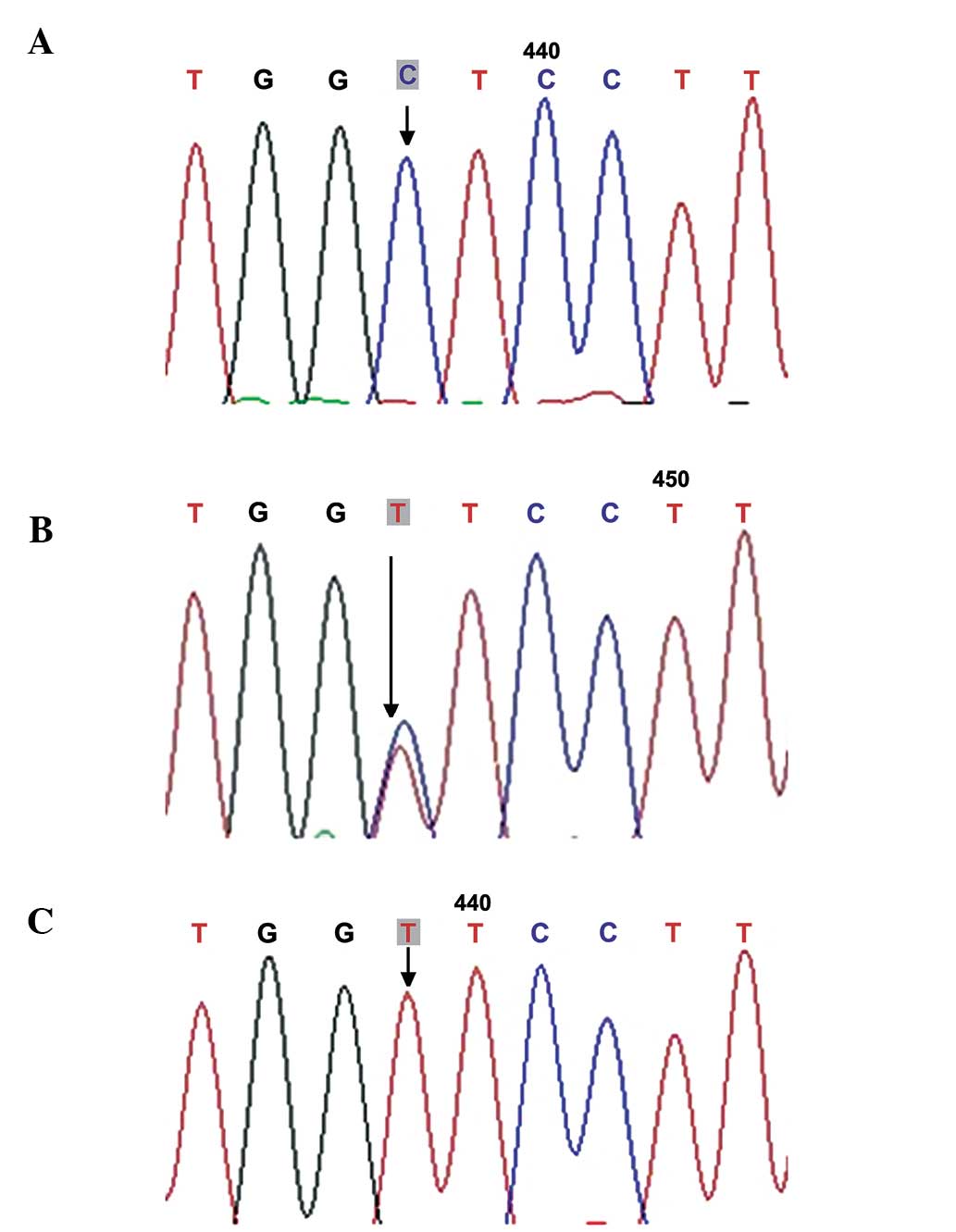
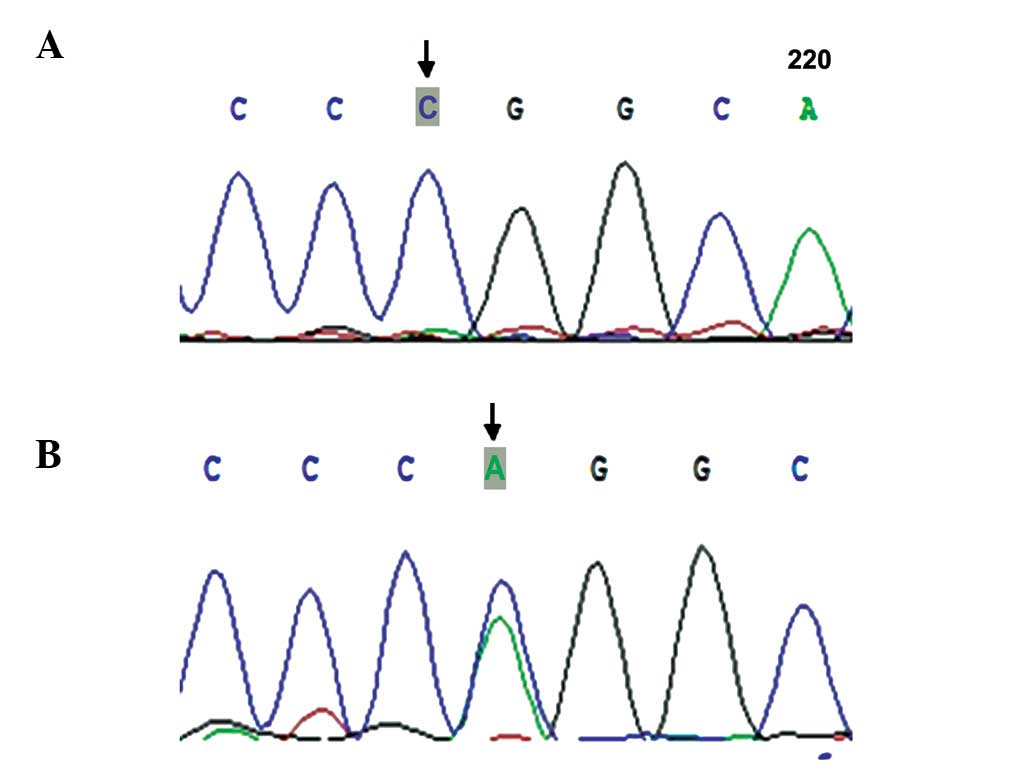
(169G>T; Fig. 1), rs1049127
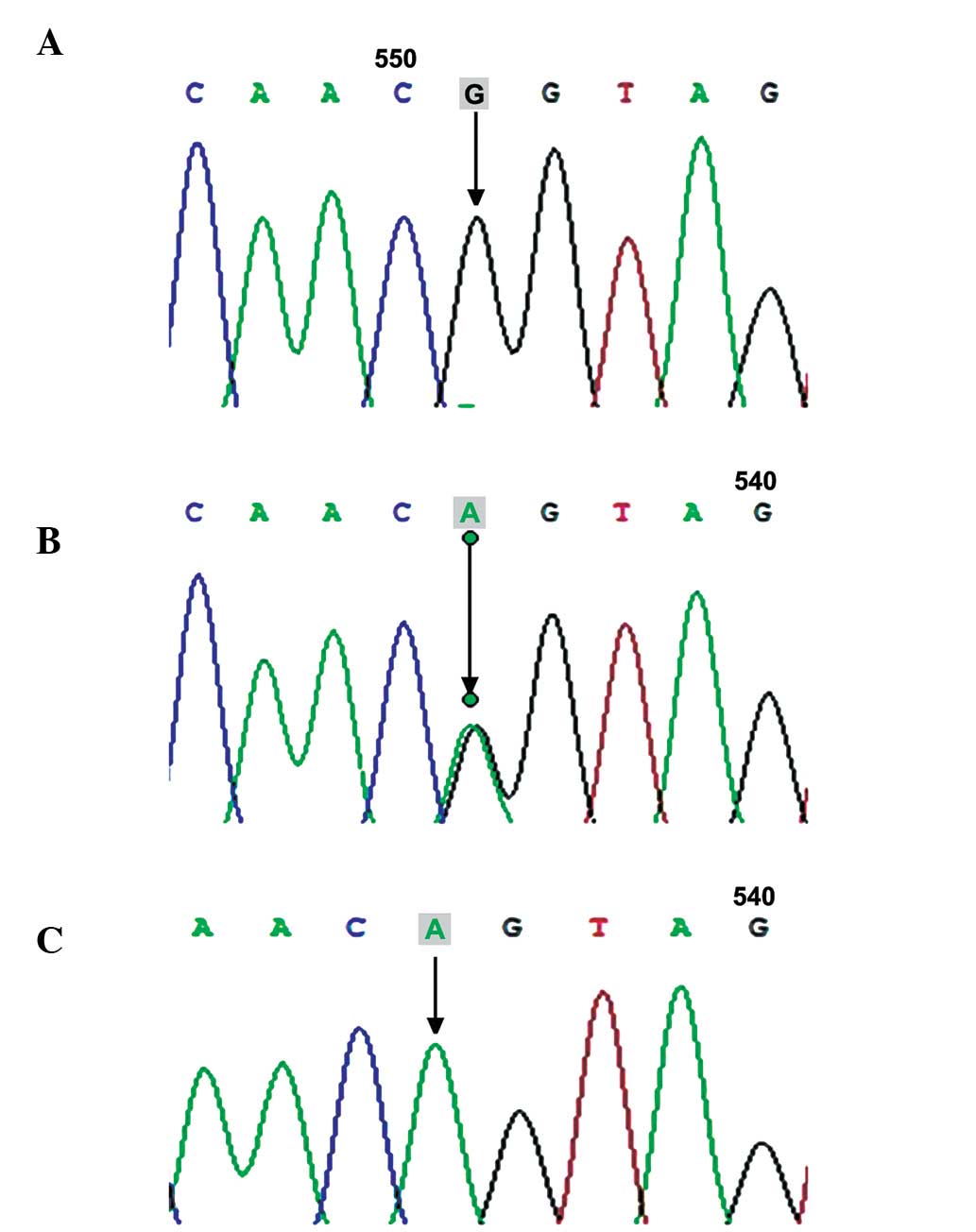
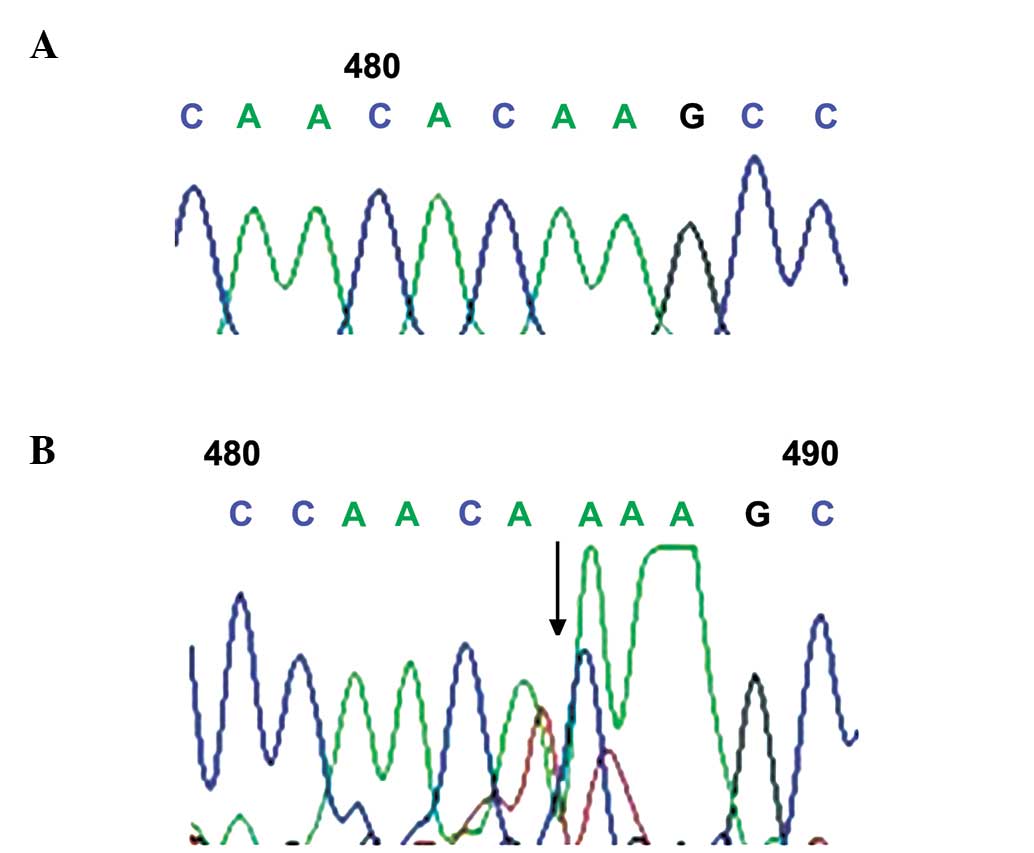
(546G>A; Fig. 2), rs254286
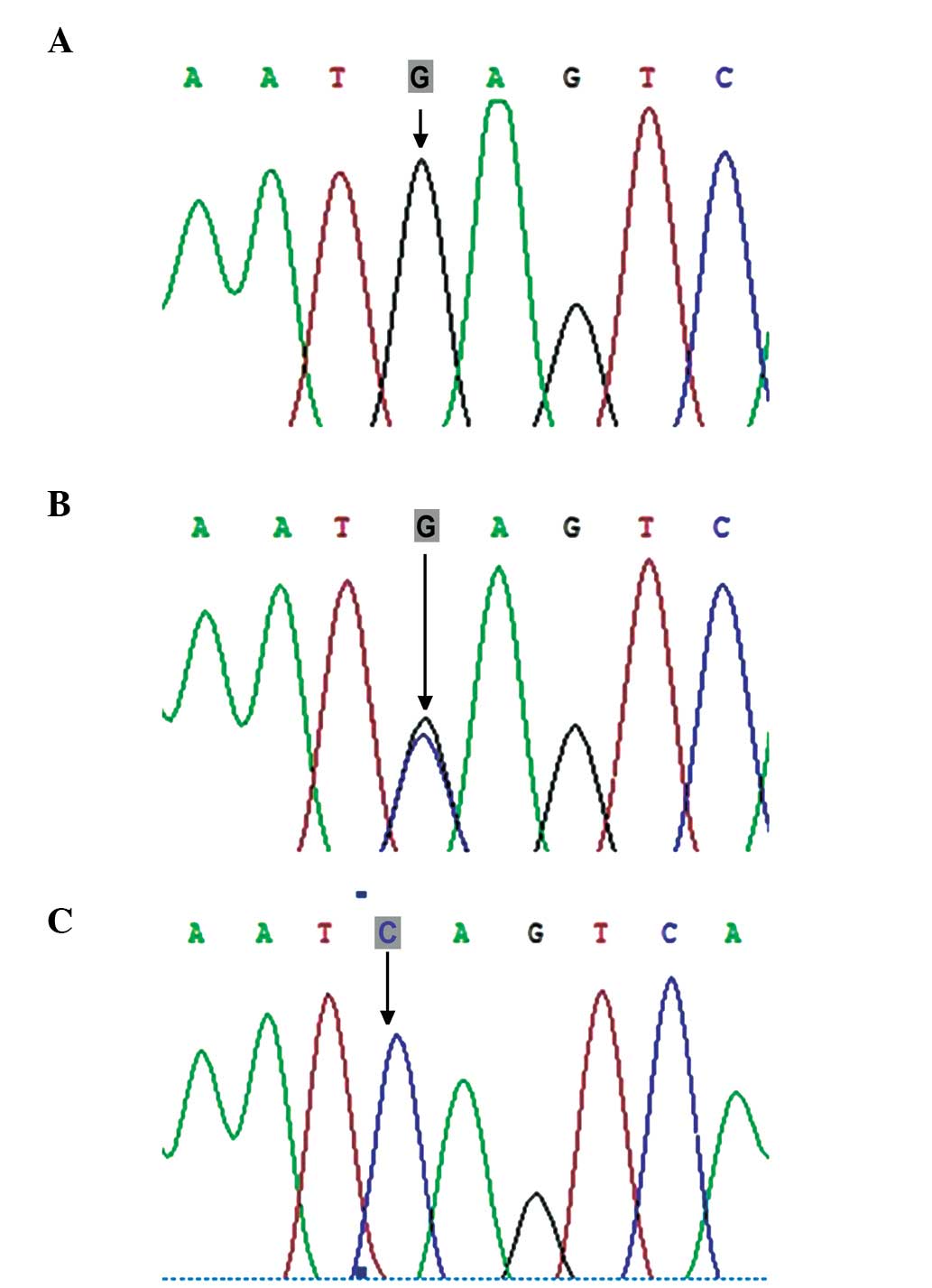
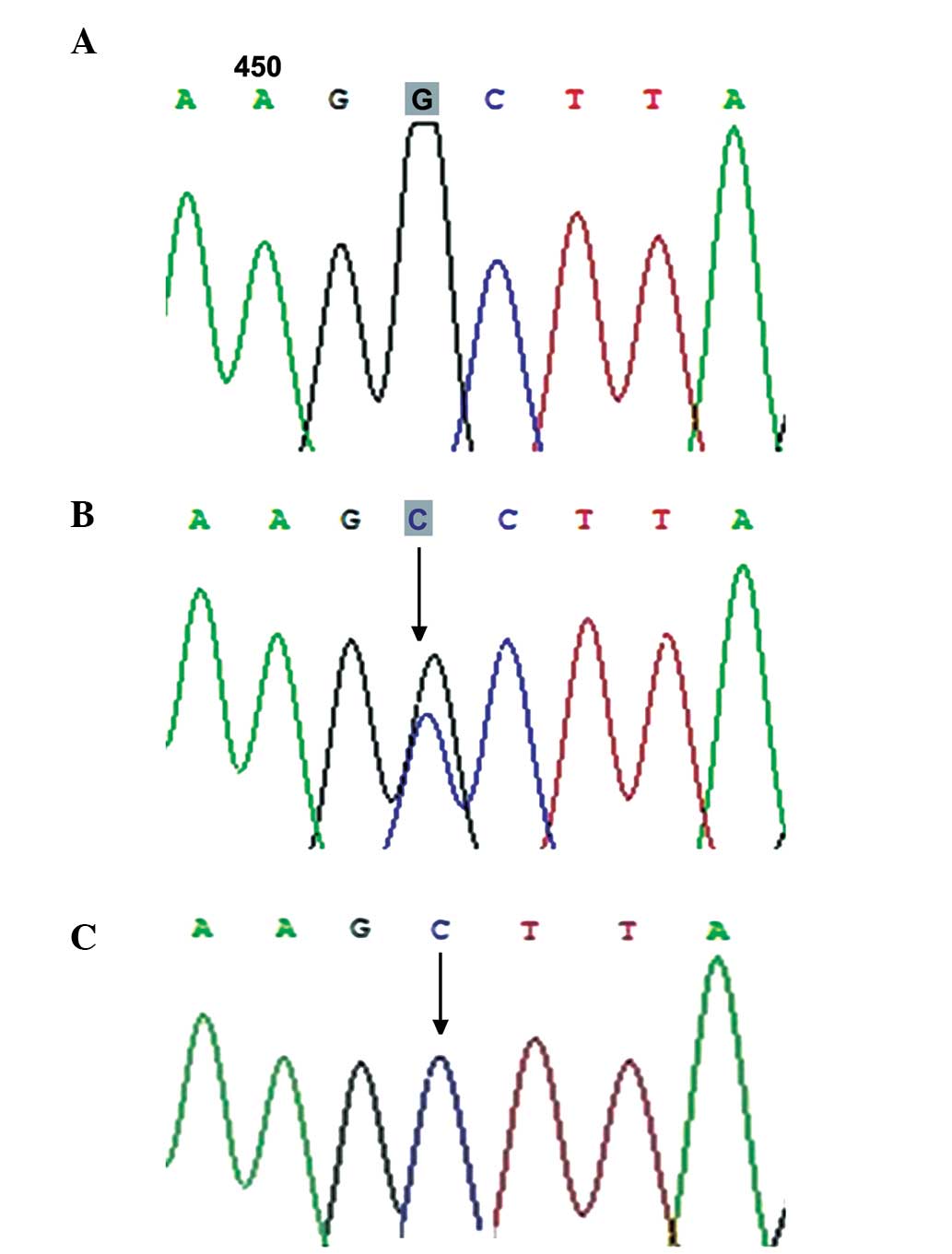
(447C>T; Fig. 3) and rs254285
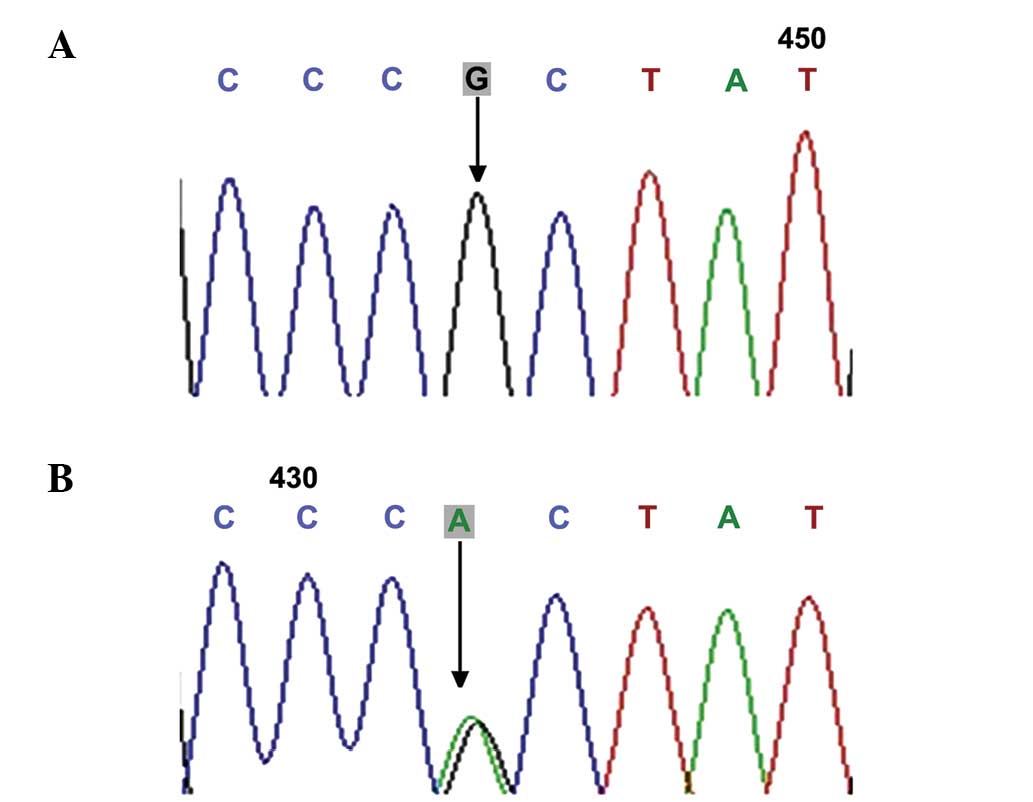
(969C>G; Fig. 4). The
c.169G>T (D57Y) missense mutation has not been detected
previously, and occurred in two patients with POF, while the
mutation was not detected in the control individuals. The
frequencies of the 546G>A genotype and allele A were
significantly higher in the POF group than in the normal control
group (34.92, vs. 6.90% and 19.05, vs. 3.23%, respectively;
P<0.05), while no significant differences were observed in the
frequencies of the c.447C>T and c.969C>G mutations between
the two groups (60.32, vs. 50% and 50.79, vs. 55.17%; P>0.05;
Table II).
 | Table IIMutations of GDF9,
BMP15 and INHβB in patients with POF and control
individuals. |
Table II
Mutations of GDF9,
BMP15 and INHβB in patients with POF and control
individuals.
| Gene | Mutation | Exon | Sequence
variation | Amino acid
variation | Mutation rate (%)
| P-value |
|---|
| POF (n=63) | Control (n=58) |
|---|
| GDF9 | novel | 1 | c.169G>T | p.Asp57Tyr | 3.17 | – | – |
| rs10491279 | 2 | c.546G>A | p.Glu182Glu | 34.92 | 6.90 | <0.05 |
| rs254286 | 2 | c.447C>T | p.Thr149Thr | 60.32 | 50 | >0.05 |
| rs254285 | 1 | c.969C>G | silent | 50.79 | 55.17 | >0.05 |
| rs3810682 | 1 | c.−9C>G | silent | 7.94 | 6.90 | >0.05 |
| BMP15 | common | 1 | 788insTCT | 262insLeu | 3.17 | – | – |
| rs17003221 | 2 | c.852C>T | p.Ser284Ser | 4.76 | 3.45 | >0.05 |
| novel | 2 | c.1095C>A | p.Por 365Por | 30.16 | 22.41 | >0.05 |
| INHBB | rs6165 | 10 | c.919 G>A | p.Ala307Thr | 92.06 | 91.38 | >0.05 |
| FSHR | rs6166 | 10 | c.2039 G>A | p.Ser680Asn | 96.83 | 93.10 | >0.05 |
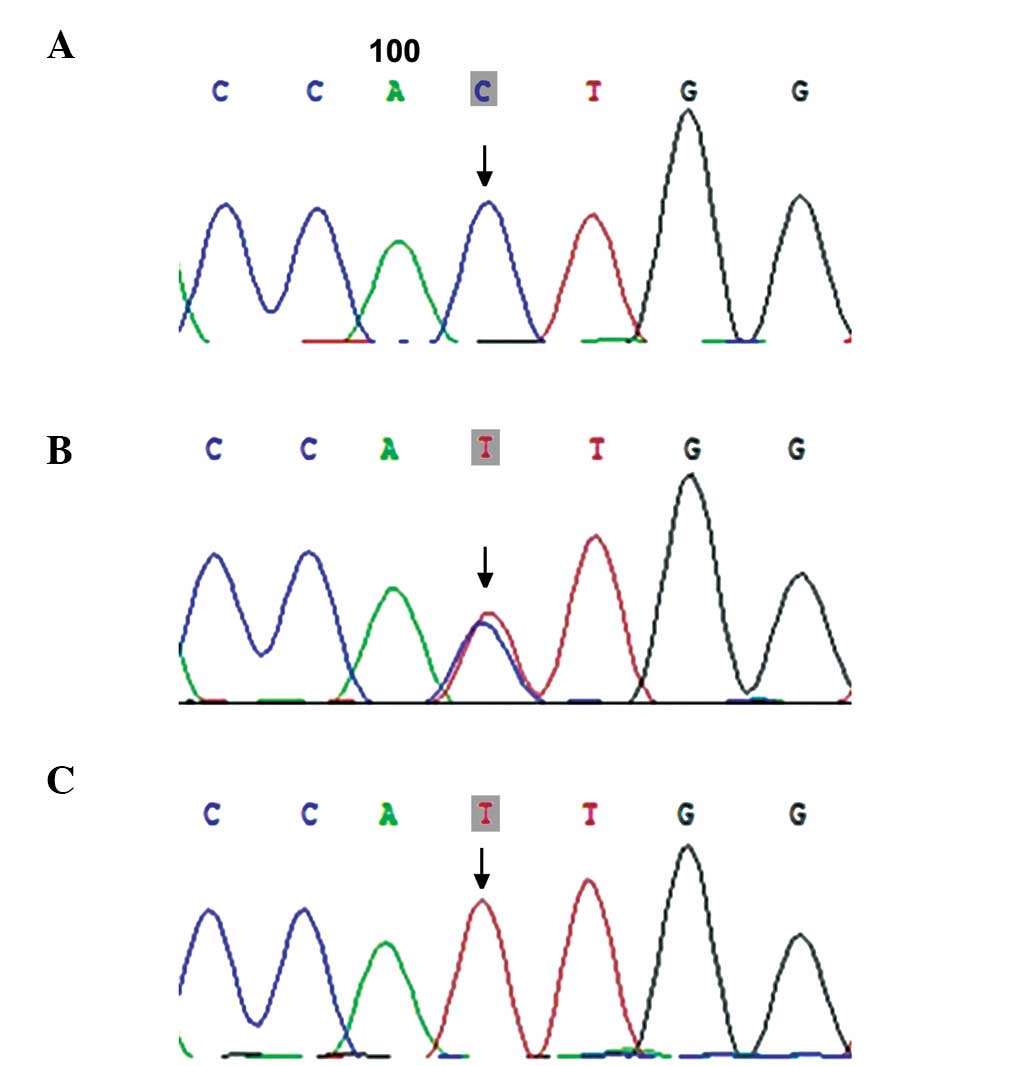
BMP15 SNPs
The present study detected three SNPs within the
BMP15 gene, including rs79377927 (788_789insTCT; Fig. 5), rs3810682 (−9C>G; Fig. 6), and rs17003221 (852C>T;
Fig. 7), and the frequencies of
the 9C>G and 852C>T genotypes did not vary significantly
between the POF and control groups (7.94, vs. 6.90% and 4.76, vs.
3.45%; P>0.05), while the 788_789insTCT genotype was detected in
two patients with POF (Table
II).
INHBB SNPs
Exon 2 of the INHBB gene was sequenced and
the novel synonymous mutation locus Por365Por (Fig. 8) was identified. However, no
significant difference was observed in the occurrence of the
mutation between the POF and control groups (30.16, vs. 22.41;
P>0.05; Tables II and III).
 | Table IIIComparison of genotype frequency of
GDF9, FSHR, and INHβB between patients with
POF and controls. |
Table III
Comparison of genotype frequency of
GDF9, FSHR, and INHβB between patients with
POF and controls.
| Gene | Codon | Genotype | Frequency (%)
| OR (95% CI) |
χ2-value | P-value |
|---|
| POF (n=63) | Control (n=58) |
|---|
| GDF9 | 546 | G/G | 65.08 | 93.10 | 0.138
(0.044–0.432) | 14.058 | <0.05 |
| | G/A | 31.75 | 6.90 | 6.279
(1.997–19.748) | 11.728 | <0.05 |
| | A/A | 3.17 | 0 | – | – | – |
| | G | 80.95 | 96.77 | 0.152
(0.051–0.452) | 14.364 | <0.05 |
| | A | 19.05 | 3.23 | 6.588
(2.211–19.634) | 14.364 | <0.05 |
| 447 | C/C | 39.68 | 50.00 | 0.658
(0.320–1.353) | 1.301 | >0.05 |
| | C/T | 47.62 | 46.55 | 1.044
(0.511–2.133) | 0.014 | >0.05 |
| | T/T | 12.70 | 3.45 | 4.073
(0.828–20.042) | 3.408 | >0.05 |
| | C | 63.49 | 75.00 | 0.634
(0.367–1.097) | 2.665 | >0.05 |
| | T | 36.51 | 25.00 | 1.572
(0.911–2.728) | 2.665 | >0.05 |
| 969 | C/C | 47.61 | 44.83 | 1.154
(0.563–2.366) | 0.153 | >0.05 |
| | C/G | 39.68 | 43.10 | 0.892
(0.431–1.844) | 0.095 | >0.05 |
| | G/G | 11.11 | 12.07 | 0.927
(0.304–2.827) | 0.018 | >0.05 |
| | C | 69.05 | 68.55 | 1.104
(0.643–1.895) | 0.129 | >0.05 |
| | G | 30.95 | 31.45 | 0.906
(0.528–1.555) | 0.129 | >0.05 |
| FSHR | 307 | G/G | 7.94 | 8.62 | 0.914
(0.250–3.334) | 0.019 | >0.05 |
| | G/A | 39.68 | 43.10 | 0.868
(0.421–1.792) | 0.146 | >0.05 |
| | A/A | 52.38 | 48.28 | 1.179
(0.577–2.407) | 0.204 | >0.05 |
| | G | 27.80 | 30.20 | 0.890
(0.510–1.552) | 0.168 | >0.05 |
| | A | 72.20 | 69.80 | 1.123
(0.644–1.959) | 0.168 | >0.05 |
| 680 | G/G | 3.17 | 6.90 | 0.443
(0.078–2.513) | 0.888 | >0.05 |
| | G/A | 47.61 | 37.93 | 1.488
(0.720–3.072) | 1.157 | >0.05 |
| | A/A | 49.22 | 55.17 | 0.787
(0.385–1.610) | 0.431 | >0.05 |
| | G | 27.00 | 25.90 | 1.059
(0.598–1.878) | 0.039 | >0.05 |
| | A | 73.0 | 74.10 | 0.944
(0.533–1.673) | 0.039 | >0.05 |
| INHBB | 365 | C/C | 69.84 | 77.59 | 0.669
(0.295–1.517) | 0.931 | >0.05 |
| | C/A | 30.16 | 22.41 | 1.495
(0.659–3.390) | 0.931 | >0.05 |
| | A/A | 0 | 0 | – | – | – |
| | C | 84.90 | 88.80 | 0.711
(0.334–1.513) | 0.789 | >0.05 |
| | A | 15.10 | 11.20 | 1.407
(0.661–2.995) | 0.789 | >0.05 |
FSHR SNPs
A total of two SNPs of rs6165 (919G>A) and rs6166
(2039G>A) were detected in exon 10 of the FSHR gene
(Figs. 9 and 10); however, the frequency of these two
mutations exhibited no significant difference between the POF and
control groups (92.06, vs. 91.38% and 96.83, vs. 93.10%; P>0.05;
Table II). In addition, no
significant differences were observed in the genotype frequency of
the FSHR gene between the two groups (P>0.05; Table III).
Analysis of the interaction between the FSHR
SNPs and GDF9 SNPs revealed no significant differences in
the frequencies of the FSHR 307-GDF9 546, FSHR
680-GDF 546 and FSHR 307-680 combined genotypes
between the POF and control groupa (P>0.05; Tables IV–VI).
 | Table IVComparison of the frequency of the
FSHR 307 GDF9 546 combined genotype between patients
with POF and control individuals. |
Table IV
Comparison of the frequency of the
FSHR 307 GDF9 546 combined genotype between patients
with POF and control individuals.
| FSHR
307-GDF9 546 combined genotype | Frequency in POF
patients (n=63) n (%) | Frequency in
controls (n=58) n (%) | OR (95% CI) |
χ2-value | P-value |
|---|
| G/G-G/G | 3 (4.76) | 3 (5.17) | 1.000
(reference) | | |
| G/G-G/A | 1 (1.59) | 1 (1.72) | 1.000
(0.041–24.547) | 0.000 | >0.05 |
| G/A-A/A | – | – | – | – | – |
| G/A-G/A | 5 (7.94) | 9 (15.52) | 1.800
(0.259–12.502) | 0.010 | >0.05 |
| AnyA-G/G | 41 (65.08) | 43 (74.14) | 1.049
(0.200–5.497) | 0.000 | >0.05 |
| AnyA-G/A | 17 (26.98) | 9 (15.52) | 0.529
(0.088–3.179) | 0.055 | >0.05 |
| G/G-AnyA | 1 (1.59) | 1 (1.72) | 1.000
(0.041–24.547) | 0.000 | >0.05 |
| G/A-AnyA | 8 (12.70) | 4 (6.90) | 0.500
(0.068–3.696) | 0.029 | >0.05 |
| AnyA-AnyA | 18 (28.57) | 10 (17.24) | 0.556
(0.094–3.285) | 0.036 | >0.05 |
 | Table VIComparison of frequency of
FSHR 307 680 combined genotype between patients with POF and
controls. |
Table VI
Comparison of frequency of
FSHR 307 680 combined genotype between patients with POF and
controls.
| FSHR 307-680
combined genotype | Frequency in POF
patients (n=63) n (%) | Frequency in
controls (n=58) n (%) | OR (95% CI) |
χ2-value | P-value |
|---|
| G/G-G/G | 2 (3.17%) | 3 (5.17) | 1.000
(reference) | | |
| G/G-G/A | 3 (4.76%) | – | – | – | – |
| G/A-A/A | 2 (3.17%) | 4 (6.90) | 1.333
(0.113–15.704) | 0 | >0.05 |
| G/A-G/A | 23 (36.51%) | 21 (36.21) | 0.609
(0.092–4.007) | 0.271 | >0.05 |
| AnyA-G/G | – | 1 (1.72) | – | – | – |
| AnyA-G/A | 27 (42.86%) | 22 (37.93) | 0.543
(0.083–3.545) | 0.416 | >0.05 |
| G/G-AnyA | – | 2 (3.45) | – | – | – |
| G/A-AnyA | 2 (3.17%) | 3 (5.17) | 1.000
(0.08–12.557) | 1.000 | >0.05 |
| AnyA-AnyA | 58 (92.06%) | 53 (91.38) | 0.609
(0.098–3.788) | 0.006 | >0.05 |
Discussion
GDF9, a member of the transforming growth
factor-β superfamily, is expressed in oocytes and is considered to
be a requirement for ovarian folliculogenesis (28). GDF9 mutations have been
associated with POF (29). Dixit
et al (30) reported that
two rare missense mutations, c.199A>C and c.646G>A, in Indian
females with ovarian failure, were associated with ovarian failure,
and the presence of the c.447>T mutation was considered to infer
a higher risk for POF. In 203 patients with POF, the heterozygous
transversion, 557C>A, in exon 2 within the GDF9 gene was
associated with POF (31). Zhao
et al (32) identified four
SNPs across the GDF9 coding regions in 100 Chinese females
with POF, including c.436C>T (p.Arg146Cys), c.588A>C
(silent), c.712A>G (p.Thr238Ala), and c.1283G>C
(p.Ser428Thr), and the nonsynonymous SNPs c.436C>T and
c.1283G>C were also detected in 96 control individuals. The
c.712A>G perturbation, which results in a missense mutation
(p.Thr238Ala), was not detected in any of the control
individuals.
In the present study, a novel missense mutation,
c.169G>T (D57Y), was detected in the GDF9 gene, which was
present in two patients with POF and was not detected in the
control individuals. This mutation was present in the proprotein
region of the GDF9 gene, and the G T mutation at position
169 led to the substitution of aspartic acid with glutamic acid at
position 57. To the best of our knowledge, this is the first time
that this mutant locus has been observed in patients with POF.
Previously, the GDF9 G169A was identified in Chinese females
with a diminished ovarian reserve, without abnormal protein
secretion or activity, which may contribute to the location of this
mutant locus in the non conserved sequence of the GDF9 protein
(33).
The c.546G>A (p.Glu182Glu) mutation occurs at
exon 2 of the GDF9 gene, which was initially identified in
Indian females with ovarian failure (30). Laissue et al (31) reported frequen cies of 23.15%
(47/203) and 54.26% (14/54) of the 546G>A mutation in French
patients with POF and normal controls, respectively, and this
546G>A variation was considered to be a common synonymous
mutation in the French population. Zhao et al (32) detected the c.546G>A heterozygous
point mutation in 26 of 100 patients with POF (26%) and 28 of 96
control individuals (29.17%), with no significant differences
observed. In the present study, the frequency of the c.546G>A
mutation was significantly higher in the patients with POF
(34.92%), compared with the control individuals (6.90%; P<0.05),
indicating that the c.546G>A mutation was closely associated
with POF in the Chinese Hui population.
In India, a case-control study revealed an 85.04%
genotype distribution of c.447C>T in females with POF, which was
higher than that in control individuals (χ2=5.93;
P=0.05) (30). In 203 women with
POF, recruited from several clinical centers in France, the
frequency of c.447C>T was 75.9% in patients with POF and 55.5%
in control individuals (31).
Considering these findings, the c.447C>T mutation was
hypothesized to correlate with a high risk of POF. In the present
study, the frequency of c.447C>T was higher in the patients with
POF than in the control individuals (60.32, vs. 50%, respectively),
however, no significant difference was observed (P>0.05). This
finding was consistent with previous investigations in Indian and
French populations (30,31). In total, four SNPs were identified
in the GDF9 gene in patients of the Chinese Hui population
with POF, and the c.169G>T and c.546G>A mutations within the
GDF9 gene were found to closely correlate with the
development of POF in these patients.
As a paracrine signaling molecule involved in oocyte
and follicular development, the BMP15 gene is considered to
be one of the important candidate genes involved in POF (33). However, various incidence rates of
the BMP15 mutation have been detected in patients with POF
of different ethnicities (34),
and the correlation between the BMP15 mutation and POF
development varies among them (35–37).
The c.985C>T missense mutation in the BMP15 gene, which
was initially identified in Chinese females with POF in 2010
(38), has been found to result in
alteration of the polar/positive amino acid, arginine, to the
nonpolar and neutral amino acid, cysteine, which is located in the
region of biologically active BMP15, and this c.985C>T
variant has been reported to be an important potential disease
associated mutation in BMP15 among patients with POF
(38). However, Zhang et al
(39) demonstrated rare mutations
in BMP15 exons, and changes in the BMP15 pro-peptide in
Chinese females with POF, and concluded that the two SNPs
rs17003221 (CT) in exon 2 and rs (3810682CG: ss16336587) in the
putative promoter region of exon 1 were not associated with POF. In
the present study, three SNPs were identified in the coding region
of the BMP15 gene, rs3810682 (−9C>G) and rs79377927
(788_789insTCT) in exon 1 and rs17003221 (852C>T) in exon 2,
which were detected in patients with POF and the control
individuals. The findings revealed a significantly higher frequency
of allele C, compared with allele G in the SNP-9C>G at exon 1 of
the BMP15 gene (95.97, vs. 4.03%; P<0.05), which was consistent
with a previous study (38). This
further demonstrated that, unlike European populations and other
Asian populations, the C allele was predominant in SNP-9C>G
within the BMP15 gene. No missense mutations, associated with the
development of POF, were identified in the coding region of
BMP15. As the coding region of the BMP15 protein is highly
conserved across species (40), it
was hypothesized that BMP15, although involved in the pathogenesis
of POF, may not be the major factor causing diminished ovarian
function, which may be explained by other specific mechanisms,
including disorders of pre-translation processing, including mRNA
splicing.
INH is a dimeric glycoprotein composed of an
α-subunit (INHA) and one of two possible β-subunits (INHBA or
INHBB), which has been defined as a gonadal hormone that exerts a
specific negative feedback action on the secretion of FSH from the
gonadotropic cells of the pituitary gland (41,42).
It has been reported that INH is a candidate gene for the
development of POF (43), and the
INHA gene has been found to closely correlate with POF
(44–46), while the INHBB and
INHBA genes are not associated with ovarian failure
(47). In the present study, a
synonymous mutation (c.1095C>A) at exon 2 of the INHBB
gene was identified. Generally, this mutation does not affect the
original amino acid sequence or the carrier’s ovarian function,
however, the present study reported a higher frequency of the
INHBB mutation in the patients with POF, compared with the
controls (30.16, vs. 22.41%, respectively; P>0.05), suggesting
that the INHBB mutation may be involved in the alteration of
ovarian function and ovarian reserve.
At present, the FSHR gene variants reported
to be associated with POF include Ala189Val, Asn191Ile,
Tle160Thr/Arg573Cys, Asp224Val/leu601Val and Pro348Arg
polymorphisms at exon 7 and a Phe591Ser polymorphism at exon 10
(48). In Brazilian females, the
presence of the Ala307Thr polymorphism is likely to be associated
with a more precocious onset of POF (49). In Argentinian females, mutations in
the FSHR gene are rare in females with POF, and the presence
of a particular FSHR isoform does not appear to be
associated with POF (50). Whitney
et al (51) reported that
FSHR gene deletions were uncommon in females with POF,
although the gene is polymorphic. In addition, no C566T mutation of
the FSHR gene has been detected in patients with POF and
control individuals, which appears rare in Chinese females with POF
(52). In the present study, two
SNPs of rs6165 (919G>A) and rs6166 (2039G>A) were detected in
the FSHR gene; however, the frequency of these two mutations
exhibited no significant difference between the POF group and the
control group, and no significant differences were observed in the
genotype frequency of the FSHR gene between the two
groups.
In addition, analysis of the interaction between the
FSHR SNPs and GDF9 SNPs revealed no significant
differences in the frequencies of the FSHR 307-GDF9
546, FSHR 680-GDF 546 or FSHR 307-680 combined
genotypes between the POF group and the control group (all
P>0.05). This may have been caused by the small sample size used
in the present study, which cannot accurately reflect the
interaction between the FSHR SNPs and GDF9 SNPs.
Therefore, further investigations with larger sample sizes are
required to examine the FSHR-GDF9 interactions in
females with POF.
In conclusion, GDF9 c.169G>T (D57Y) and
c.546G>A (rs1049127), and BMP15 rs79377927
(788_789insTCT) were found to be associated with POF in patients of
the Chinese Hui population. The results of the present study
identified regional variation in GDF9 and BMP15 SNPs
in POF, however the underlying mechanisms require further analysis.
The correlation between GDF9 and FSHR genes was not
detected in the present study, therefore further studies with
larger sample sizes are required to comprehensively evaluate the
interaction between POF and its associated genes. Screening of
known mutation locis is of great significance when investigating
the genetic mechanisms of molecules associated with POF. In
conclusion, exogenous oocyte derived cell growth factors may be
used to specifically rectify abnormal sites, and to improve the
quality of life, delay the age of menopause, and increase ovarian
reserve function in patients with POF.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360095), the
Natural Science Foundation of Ningxia Hui Autonomous Region (grant
no. NZ10116) and the Doctorate Program Foundation of Ningxia
Medical University (grant no. KF2010-47).
References
|
1
|
Rebar RW: Premature ovarian failure.
Obstet Gynecol. 113:1355–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goswami D and Conway GS: Premature ovarian
failure. Hum Reprod Update. 11:391–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalantaridou SN, Davis SR and Nelson LM:
Premature ovarian failure. Endocrinol Metab Clin North Am.
27:989–1006. 1998. View Article : Google Scholar
|
|
4
|
Nippita TA and Baber RJ: Premature ovarian
failure: a review. Climacteric. 10:11–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goswami D and Conway GS: Premature ovarian
failure. Horm Res. 68:196–202. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacobsen BK, Knutsen SF and Fraser GE: Age
at natural menopause and total mortality and mortality from
ischemic heart disease: the Adventist Health Study. J Clin
Epidemiol. 52:303–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Løkkegaard E, Jovanovic Z, Heitmann BL, et
al: The association between early menopause and risk of ischaemic
heart disease: influence of Hormone Therapy. Maturitas. 53:226–233.
2006. View Article : Google Scholar
|
|
8
|
Uygur D, Sengül O, Bayar D, et al: Bone
loss in young women with premature ovarian failure. Arch Gynecol
Obstet. 273:17–19. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LaBarbera AR, Miller MM, Ober C, et al:
Autoimmune etiology in premature ovarian failure. Am J Reprod
Immunol Microbiol. 16:115–122. 1998.
|
|
10
|
Schmidt PJ, Cardoso GM, Ross JL, et al:
Shyness, social anxiety and impaired self esteem in Turner syndrome
and premature ovarian failure. JAMA. 295:1374–1376. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Der Voort DJ, van Der Weijer PH and
Barentsen R: Early menopause: increased fracture risk at older age.
Osteoporos Int. 14:525–530. 2004. View Article : Google Scholar
|
|
12
|
Shuster LT, Rhodes DJ, Gostout BS, et al:
Premature menopause or early menopause: long term health
consequences. Maturitas. 65:161–166. 2010. View Article : Google Scholar
|
|
13
|
Rocca WA, Grossardt BR, de Andrade M, et
al: Survival patterns after oophorectomy in premenopausal women: a
population based cohort study. Lancet Oncol. 7:821–828. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coulam CB, Adamson SC and Annegers JF:
Incidence of premature ovarian failure. Obstet Gynecol. 67:604–606.
1986.PubMed/NCBI
|
|
15
|
Luborsky JL, Meyer P, Sowers MF, et al:
Premature menopause in a multi ethnic population study of the
menopause transition. Hum Reprod. 18:199–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma LK and Lin SQ: Premature ovarian
failure and resistant/insensitive ovarian syndrome. Shiyong
Fuchanke Zazhi. 19:198–200. 2003.In Chinese.
|
|
17
|
Vujovic S: Aetiology of premature ovarian
failure. Menopause Int. 15:72–75. 2009.PubMed/NCBI
|
|
18
|
Beck Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shelling AN: Premature ovarian failure.
Reproduction. 140:633–641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Caburet S, Arboleda VA, Llano E, et al:
Mutant cohesin in premature ovarian failure. N Engl J Med.
370:943–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pouresmaeili F and Fazeli Z: Premature
ovarian failure: a critical condition in the reproductive potential
with various genetic causes. Int J Fertil Steril. 8:1–12.
2014.PubMed/NCBI
|
|
22
|
Qin Y, Jiao X, Dalgleish R, et al: Novel
variants in the SOHLH2gene are implicated in human premature
ovarian failure. Fertil Steril. 101:1104–1109. 2014. View Article : Google Scholar
|
|
23
|
Simpson CM, Robertson DM, Al Musawi SL, et
al: Aberrant GDF9 expression and activation are associated with
common human ovarian disorders. J Clin Endocrinol Metab.
99:E615–E624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pyun JA, Kim S, Cha DH, et al:
Polymorphisms within the FANCAgene associate with premature ovarian
failure in Korean women. Menopause. 21:530–533. 2014. View Article : Google Scholar
|
|
25
|
Pyun JA, Kim S, Cha DH, et al: Epistasis
between IGF2R and ADAMTS19 polymorphisms associates with premature
ovarian failure. Hum Reprod. 28:3146–3154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pierce SB, Gersak K, Michaelson Cohen R,
et al: Mutations in LARS2, encoding mitochondrial leucyl tRNA
synthetase, lead to premature ovarian failure and hearing loss in
Perrault syndrome. Am J Hum Genet. 92:614–620. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sanger F, Nicklen S and Coulson AR: DNA
sequencing with chain terminating inhibitors. Proc Natl Acad Sci
USA. 74:5463–5467. 1977. View Article : Google Scholar
|
|
28
|
Aaltonen J, Laitinen MP, Vuojolainen K, et
al: Human growth differentiation factor 9 (GDF 9) and its novel
homolog GDF 9B are expressed in oocytes during early
folliculogenesis. J Clin Endocrinol Metab. 84:2744–2750.
1999.PubMed/NCBI
|
|
29
|
Kovanci E, Rohozinski J, Simpson JL, et
al: Growth differen tiating factor 9 mutations may be associated
with premature ovarian failure. Fertil Steril. 87:143–146. 2007.
View Article : Google Scholar
|
|
30
|
Dixit H, Rao LK, Padmalatha V, et al:
Mutational screening of the coding region of growth differentiation
factor 9 gene in Indian women with ovarian failure. Menopause.
12:749–754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Laissue P, Christin Maitre S, Touraine P,
et al: Mutations and sequence variants in GDF9 and BMP15 in
patients with premature ovarian failure. Eur J Endocrinol.
154:739–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao H, Qin Y, Kovanci E, et al: Analyses
of GDF9mutation in 100 Chinese women with premature ovarian
failure. Fertil Steril. 88:1474–1476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dixit H, Rao LK, Padmalatha VV, et al:
Missense mutations in the BMP15gene are associated with ovarian
failure. Hum Genet. 119:408–415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Persani L, Rossetti R and Cacciatore C:
Genes involved in human premature ovarian failure. J Mol
Endocrinol. 45:257–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Di Pasquale E, Rossetti R, Marozzi A, et
al: Identification of new variants of human BMP15gene in a large
cohort of women with premature ovarian failure. J Clin Endocrinol
Metab. 91:1976–1979. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rossetti R, Di Pasquale E, Marozzi A, et
al: BMP15mutations associated with primary ovarian insufficiency
cause a defective production of bioactive protein. Hum Mutat.
30:804–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tiotiu D, Alvaro Mercadalm B, Imbert R, et
al: Variants of the BMP15gene in a cohort of patients with
premature ovarian failure. Hum Reprod. 25:1581–1587. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang B, Wen Q, Ni F, et al: Analyses of
growth differentiation factor 9 (GDF9) and bone morphogenetic
protein 15 (BMP15) mutation in Chinese women with premature ovarian
failure. Clin Endocrinol (Oxf). 72:135–136. 2010. View Article : Google Scholar
|
|
39
|
Zhang P, Shi YH, Wang LC, et al: Sequence
variants in exons of the BMP 15 gene in Chinese patients with
premature ovarian failure. Acta Obstet Gynecol Scand. 86:585–589.
2007. View Article : Google Scholar
|
|
40
|
Clelland E, Kohli G, Campbell RK, et al:
Bone morphogenetic protein 15 in the zebrafish ovary: complementary
deoxyribo nucleic acid cloning, genomic organization, tissue
distribution and role in oocyte maturation. Endocrinology.
147:201–209. 2006. View Article : Google Scholar
|
|
41
|
Burger HG: Inhibin: definition and
nomenclature, including related substances. J Endocrinol.
117:159–160. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Jong FH: Inhibin. Physiol Rev.
68:555–607. 1988.PubMed/NCBI
|
|
43
|
Shelling AN, Burton KA, Chand AL, et al:
Inhibin: a candidate gene for premature ovarian failure. Hum
Reprod. 15:2644–2649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marozzi A, Porta C, Vegetti W, et al:
Mutation analysis of the inhibin alpha gene in a cohort of Italian
women affected by ovarian failure. Hum Reprod. 17:1741–1745. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chand AL, Harrison CA and Shelling AN:
Inhibin and premature ovarian failure. Hum Reprod Update. 16:39–50.
2010. View Article : Google Scholar
|
|
46
|
Woad KJ, Pearson SM, Harris SE, et al:
Investigating the asso ciation between inhibin alpha gene promoter
polymorphisms and premature ovarian failure. Ferti Steril.
91:62–66. 2009. View Article : Google Scholar
|
|
47
|
Dixit H, Deendayal M and Singh L:
Mutational analysis of the mature peptide region of inhibin genes
in Indian women with ovarian failure. Hum Reprod. 19:1760–1764.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Desai SS, Roy BS and Mahale SD: Mutations
and polymorphisms in FSH receptor: functional implications in human
reproduction. Reproduction. 146:R235–R248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vilodre LC, Kohek MB and Spritzer PM:
Screening of follicle stimulating hormone receptor gene in women
with premature ovarian failure in southern Brazil and associations
with phenotype. J Endocrinol Invest. 31:552–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sundblad V, Chiauzzi VA, Escobar ME, et
al: Screening of FSH receptor gene in Argentine women with
premature ovarian failure (POF). Mol Cell Endocrinol. 222:53–59.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Whitney EA, Layman LC, Chan PJ, et al: The
follicle stimulating hormone receptor gene is polymorphic in
premature ovarian failure and normal controls. Fertil Steril.
64:518–524. 1995.PubMed/NCBI
|
|
52
|
Chen XN, Chen GA and Li MZ: Follicular
stimulating hormone receptor gene C566T mutation in premature
ovarian failure. Zhonghua Fu Chan Ke Za Zhi. 41:315–318. 2006.In
Chinese. PubMed/NCBI
|