Introduction
In the immune system, excessive innate immunity in
defense against bacterial or viral infections is a response to a
variety of pathological conditions, such as chronic inflammation
(1,2). During the inflammatory process,
numerous pro-inflammatory mediators are generated and the major
mediators of inflammatory events are members of the cyclooxygenase
(COX) family (3). Two major COX
isoforms, COX-1 and COX-2, catalyze the first step of the synthesis
of prostaglandin E2 (PGE2), which is the
transformation of arachidonic acid (4). In particular, COX-2 expression is
enhanced by stimuli from inflammatory mediators, including
lipopolysaccharide (LPS) and several pro-inflammatory cytokines
(5–8). In addition, COX-2 is located in the
perinuclear membrane and exerts pathological effects though the
biosynthesis of prostaglandins several hours after the stimuli
(9,10).
Nuclear transcription factor kappa-B (NF-κB), one of
the most important transcription factors, has critical roles in
inflammation and immunity as well as cell proliferation,
differentiation and survival (11). The activation of NF-κB involves the
phosphorylation of inhibitor of NF-κB (IκB). Once IκB is
phosphorylated, the resulting free NF-κB then translocates to the
nucleus, where it binds to κB binding sites in the promoter regions
of target genes and induces the transcription of pro-inflammatory
mediators, including inducible nitric oxide synthase (iNOS), COX-2
and tumor necrosis factor (TNF)-α (2,10,12).
It is well known that brain inflammation has a
crucial role in various diseases of the central nervous system
(CNS), including Alzheimer's disease and epilepsy (13,14).
The hippocampus in the mammalian brain, which is important in
memory function (15), is a
vulnerable to certain types of brain damage (16–20).
In particular, it is highly sensitive to various insults, including
inflammation induced by exo/endotoxin stimuli (5,21–24).
Tetanus toxin (TeT), an exotoxin, has a capacity for neuronal
binding and internalization (25–27).
When systematically administered to animals, TeT reaches the CNS
via retrograde transportation through nerve axons (28). A previous study by our group
reported that systemic administration of TeT caused responses in
the mouse hippocampus, including the secretion of certain
inflammatory cytokines and glial activation, while neuronal death
was not observed (27,29). However, in studies regarding TeT,
few have focussed upon the effect of TeT treatment on alterations
in inflammatory mediators in the hippocampus. Therefore, to further
investigate changes of inflammatory mediators induced by TeT, the
present study observed changes in the immunoreactivities and
protein levels of COX-2 and NF-κB/p65 in the mouse hippocampus
after the systemic administration of TeT.
Materials and methods
Experimental animals
A total of 56 male ICR mice (BW; weight, 25–30 g;
age, eight weeks) were purchased from the Jackson Laboratory
(Maine, ME, USA). The animals were housed under standard conditions
with a 12-h light/dark cycle at 23±3°C, 55±5% relative humidity and
free access to food and water. All animal care and experimental
procedures were performed according to the National Institutes of
Health (NIH) guidelines (NIH Guide for the Care and Use of
Laboratory Animals; NIH publication no. 85–23, 1985) and were
approved by the Institutional Animal Care and Use Committee (IACUC)
at Kangwon National University (approval no. KIACUC-140409-1;
Chuncheon, Republic of Korea). All efforts were made to minimize
animal suffering, as well as the number of animals used.
Treatment with TeT
The mice were intraperitoneally injected with a low
dose of TeT (100 ng/kg; Dawinbio, Seoul, Republic of Korea) and the
control animals were injected with the same volume of saline (pH
7.4). The mice (n=14 at each time-point) were sacrificed at 6, 12
and 24 h following treatment with TeT.
Preparation of tissue samples for
histology
Animals were anesthetized with sodium pentobarbital
(40 mg/kg; JW Pharmaceutical Co., Ltd., Seoul, Republic of Korea)
and transcardially perfused with 0.1 M phosphate-buffered saline
(PBS; pH 7.4; Sigma-Aldrich, St. Louis, MO, USA) followed by 4%
paraformaldehyde (Sigma-Aldrich) in 0.1 M PBS. The brains were
removed and post-fixed in 4% paraformaldehyde for 6 h. The brain
tissues were cryoprotected by infiltration with 30% sucrose
overnight (Sigma-Aldrich). Subsequently, tissues were frozen and
serially sectioned using a cryostat (Leica Microsystems GmbH,
Wetzlar, Germany) to obtain 30-µm coronal sections, which were then
collected in six-well plates containing PBS.
Immunohistochemical analysis
The expression of neuronal nuclei (NeuN), COX-2 and
NF-κB/p65 was determined using immunohistochemistry. Coronal
sections from control- and TeT-treated animals (n=7 for each
time-point) were incubated with with 0.3% hydrogen peroxide
(Sigma-Aldrich) in PBS for 30 min at room temperature. Subsequent
to washing three times with PBS (each for 10 min), the sections
were incubated with 10% normal goat serum (Vector Laboratories,
Inc., Burlingame, CA, USA) in 0.05 M PBS for 30 min at room
temperature. The samples were subsequently incubated with
polyclonal rabbit anti-NeuN (ABN78; 1:1,000; Chemicon; EMD
Millipore, Billerica, MA, USA), polyclonal rabbit anti-COX-2
(160126; 1:500; Chemicon) or polyclonal rabbit anti-NF-κB/p65
(sc-372; 1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibody overnight at 4°C. Subsequent to washing three times with
PBS (each for 10 min), the sampels were exposed to biotinylated
goat anti-rabbit immunoglobulin (Ig)G (BA-1000) and
streptavidin-biotinylated horseradish peroxidase complex (SA-5004)
(1:200; Vector Laboratories, Inc.) for 2 h at room temperature.
Antibodies were then visualized using 3,3′-diaminobenzidine
tetrachloride (Sigma-Aldrich) in 0.1 M Tris-HCl buffer (pH 7.2) and
samples were mounted on gelatin-coated slides. Following
dehydration by immersion in serial dilutions of ethanol, the
sections were mounted in Canada balsam (Kanto Chemical, Tokyo,
Japan). In order to test the specificity of the immunostaining, a
negative control sample was prepared using pre-immune serum instead
of primary antibody (data not show).
Eight sections per animal were selected to
quantitatively assess immunoreactivity for COX-2 and NF-κB/p65.
Digital images of the hippocampus proper and dentate gyrus were
captured using an AxioM1 light microscope (Carl Zeiss, Oberkochen,
Germany) equipped with a digital camera (Axiocam MRc 5; Carl Zeiss)
connected to a PC monitor. According to the method used in previous
studies by our group (27,30), immunostaining intensities were
semi-quantified using digital image analysis software (MetaMorph
4.01; Universal Imaging Corp., Bedford Hills, NY, USA). The level
of the immunoreactivity was scored as (−), (±), (+), (++) or (+++)
representing no staining (gray scale value ≥200), weakly positive
(gray scale value, 150–199), moderate (gray scale value, 100–149),
high (gray scale value, 50–99) or very high (gray scale value ≤49),
respectively.
Western blot analysis
The protein expression of COX-2 and NF-κB/p65 in the
hippocampus of the control- and TeT-treated animals (n=7 at each
time point) was determined by western blot analysis. Subsequent to
sacrifice by cervical dislocation, mice were decapitated and the
brains were removed. The brains were then serially and transversely
cut into 400-µm section using a vibratome (Leica
Microsystems GmbH), and the hippocampal region was dissected using
a surgical blade. The tissues were homogenized in 50 mM PBS (pH
7.4) containing 0.1 mM ethylene glycol tetraacetic acid (pH 8.0),
0.2% Nonidet P-40, 10 mM ethylendiamine tetraacetic acid (pH 8.0),
15 mM sodium pyrophosphate, 100 mM β-glycerophosphate, 50 mM NaF,
150 mM NaCl, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl
fluoride and 1 mM dithiothreitol (DTT) (All from Sigma-Aldrich).
Subsequent to centrifugation at 16,000 × g for 20 min, the protein
concentration of the supernatants was determined using a Micro
Bicinchoninic Acid protein assay kit with bovine serum albumin as a
standard (Pierce Biotechnology, Inc., Rockford, IL, USA). Aliquots
containing 50 µg total protein were boiled in loading buffer
containing 150 mM Tris (pH 6.8), 3 mM DTT, 6% SDS (Sigma-Aldrich),
0.3% bromophenol blue (Sigma-Aldrich) and 30% glycerol (Junsei
Chemical Co., Ltd., Tokyo, Japan). Aliquots were then subjected to
5% SDS-PAGE and electrotransferred to nitrocellulose membranes
(Pall Corp, Port Washington, NY, USA). To reduce background
staining, the membranes were incubated with 5% non-fat dry milk
(Sigma-Aldrich) in PBS containing 0.1% Tween 20 (Sigma-Aldrich) for
45 min and subsequently incubated with rabbit polyclonal anti-COX-2
(160126; 1:1,000; Chemicon), rabbit polyclonal NF-κB/p65 (sc-372;
1:1,000; Santa Cruz Biotechnology, Inc.) and rabbit polyclonal
anti-β-actin (ab8227; 1:2,000; Abcam, Cambridge, UK) overnight at
4°C. Subsequently, the membranes were washed three times with
PBS/0.1% Tween 20 (each for 10 min), followed by incubation with
the peroxidase-conjugated goat anti-rabbit IgG (A6154; 1:10,000;
Sigma-Aldrich) secondary antibody for 1 h at room temperature.
Antibodies were visualized using an enhanced chemiluminescence kit
(Pierce Biotechnology, Inc.). The blots were exposed to X-ray film
(X-max; Kodak, Rochester, NY, USA) and scanned using a Hewlett
Packard ScanJet 3200C at 300dpi (HP, Inc., Palo Alto, CA, USA).
Subsequently, densitometric analysis was conducted using Scion
Image software (Scion Corp., Frederick, MD, USA) in order to
quantify the bands, with normalization to β-actin.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. SPSS software, version 17.0 (SPSS, Inc., Chicago, IL,
USA) was used for statistical analysis. Differences between groups
were assessed using one-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
TeT does not cause neuronal damage in
hippocampi of mice
In the present study, the neuronal damage/death in
the hippocampi of mice treated with TeT was observed by
immunostaining for NeuN (Fig. 1).
NeuN-immunoreactive cells were observed in the hippocampus proper
(CA1-3 regions) and dentate gyrus of the control group (Fig. 1A–D). In the TeT-treated groups, the
distribution pattern of NeuN-immunoreactive cells was not different
from that in the control group at any time-point after TeT
treatment (Fig. 1E–P).
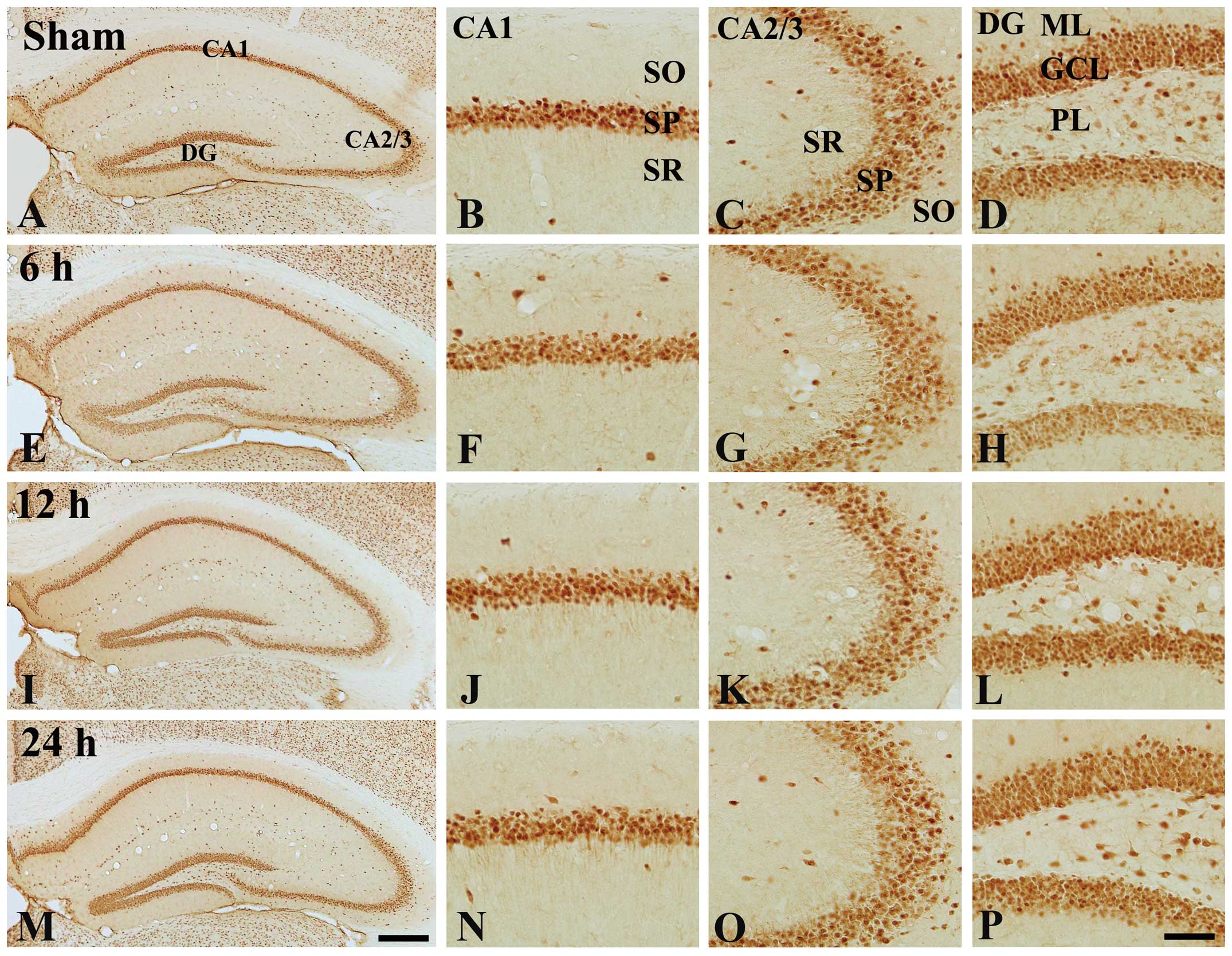 | Figure 1Immunohistochemical analysis of NeuN
in the hippocampi of (A–D) the control mice and (E–P) 100 ng/kg
TeT-treated mice. Numbers of NeuN-immunoreactive cells in the
TeT-treated groups were similar to those in the control-group. CA,
cornu ammonis; DG, dentate gyrus; GCL, cell layer; ML, molecular
later; PL, polymorphic layer; SO, stratum oriens; SP, stratum
pyramidal; SR, stratum radiatum. Scale bar, 400 µm (A, E, I
and M) or 50 µm (B–D, F, G, H, J–L, N–P). TeT, tetanus
toxin; NeuN, neuronal nuclei. |
TeT increases COX-2 expression in mouse
hippocampi
In the control group, COX-2 immunoreactivity
observed in the stratum pyramidal of the CA1 region was low and
that in the stratum pyramidal of the CA2/3 region was moderate;
furthermore, low COX-2 immunoreactivity was observed in the granule
cell layer of the dentate gyrus (Table
I; Fig. 2A–C).
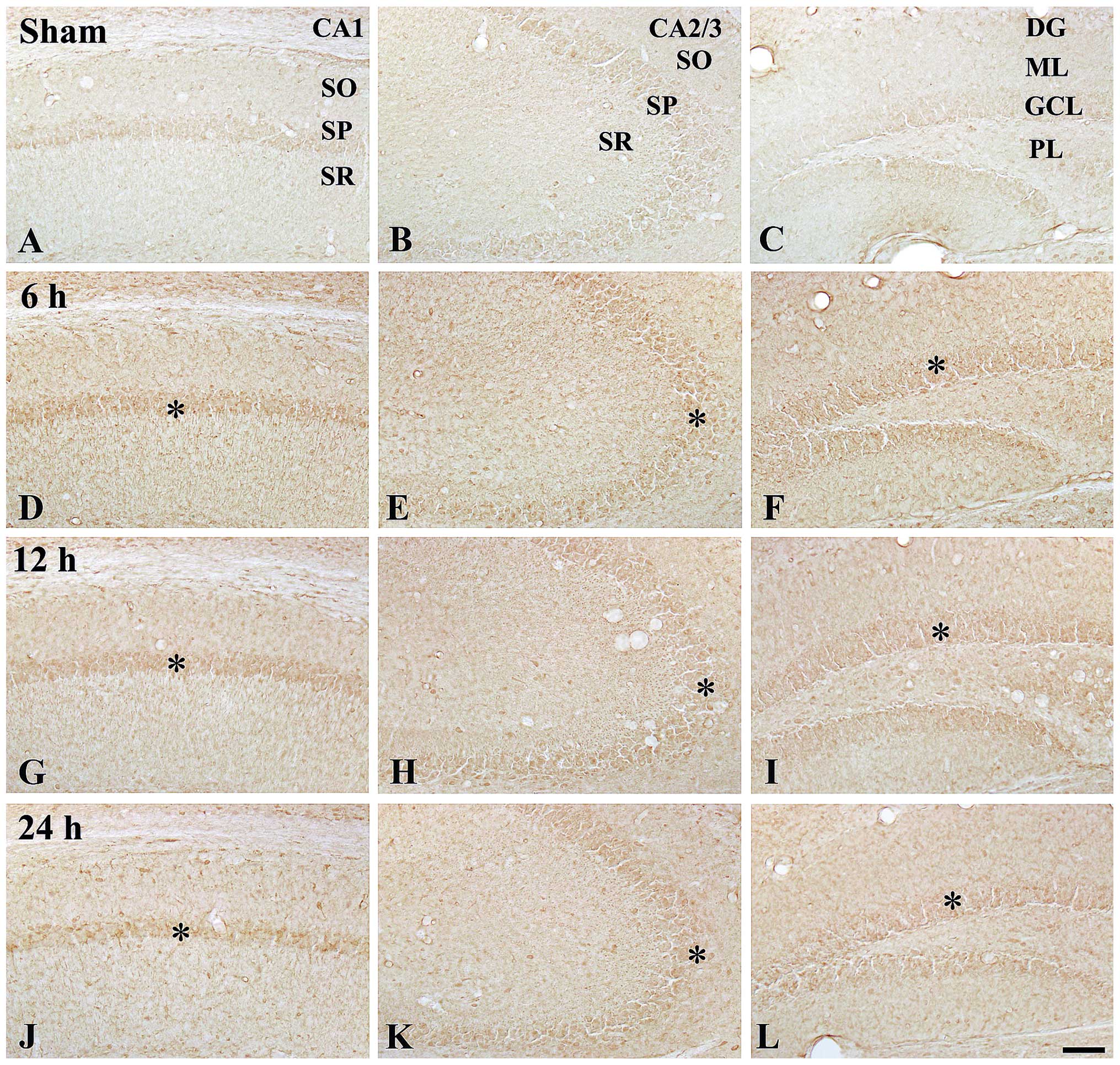
 | Figure 2Immunohistochemical detection of
COX-2 in the hippocampi of (A–C) the control mice and (D–L) 100
ng/kg TeT-treated mice. At 24 h post-treatment, COX-2
immunoreactivity was distinctively increased in the SP (asterisks)
and GCL (asterisk and arrows). TeT, tetanus toxin; COX,
cyclooxygenase; SP, striatum pyramidal; GCL, granule cell layer;
SO, stratum oriens; SR, stratum radiatum; ML, molecular later; PL,
polymorphic layer. Scale bar, 50 µm. |
 | Table ISemi-quantitative analysis of
cyclooxygenase-2 immunoreactivity in hippocampal regions after
treatment of mice with 100 ng/kg TeT. |
Table I
Semi-quantitative analysis of
cyclooxygenase-2 immunoreactivity in hippocampal regions after
treatment of mice with 100 ng/kg TeT.
| Region | Layer | Time after TeT
treatment
|
|---|
| Control | 6 h | 12 h | 24 h |
|---|
| CA1 | SO | − | ± | ± | + |
| SP | ± | + | + | ++ |
| SR | − | ± | ± | + |
| CA2-3 | SO | − | ± | ± | + |
| SP | + | ++ | ++ | +++ |
| SR | − | ± | ± | + |
| DG | ML | − | ± | ± | ± |
| GCL | ± | + | + | ++ |
| PL | − | ± | ± | + |
At 6 h post-treatment, COX-2 immunoreactivity was
slightly increased in the stratum pyramidal and granule cell layer
in all the hippocampal sub-regions compared with that in the
control group (Table I; Fig. 2D–F). COX-2 immunoreactivity at 12 h
post-treatment was similar to that at 6 h post-treatment (Table I, Fig.
2G–I). Of note, at 24 h post-treatment, COX-2 immunoreactivity
in the stratum pyramidal and granule cell layer was significantly
increased compared with that at 12 h post-treatment; in particular,
the immunoreactivity in the stratum pyramidal of the CA2/3 region
was high (Table I; Fig. 2J–L).
TeT increases NF-κB/p65 expression in
mouse hippocampi
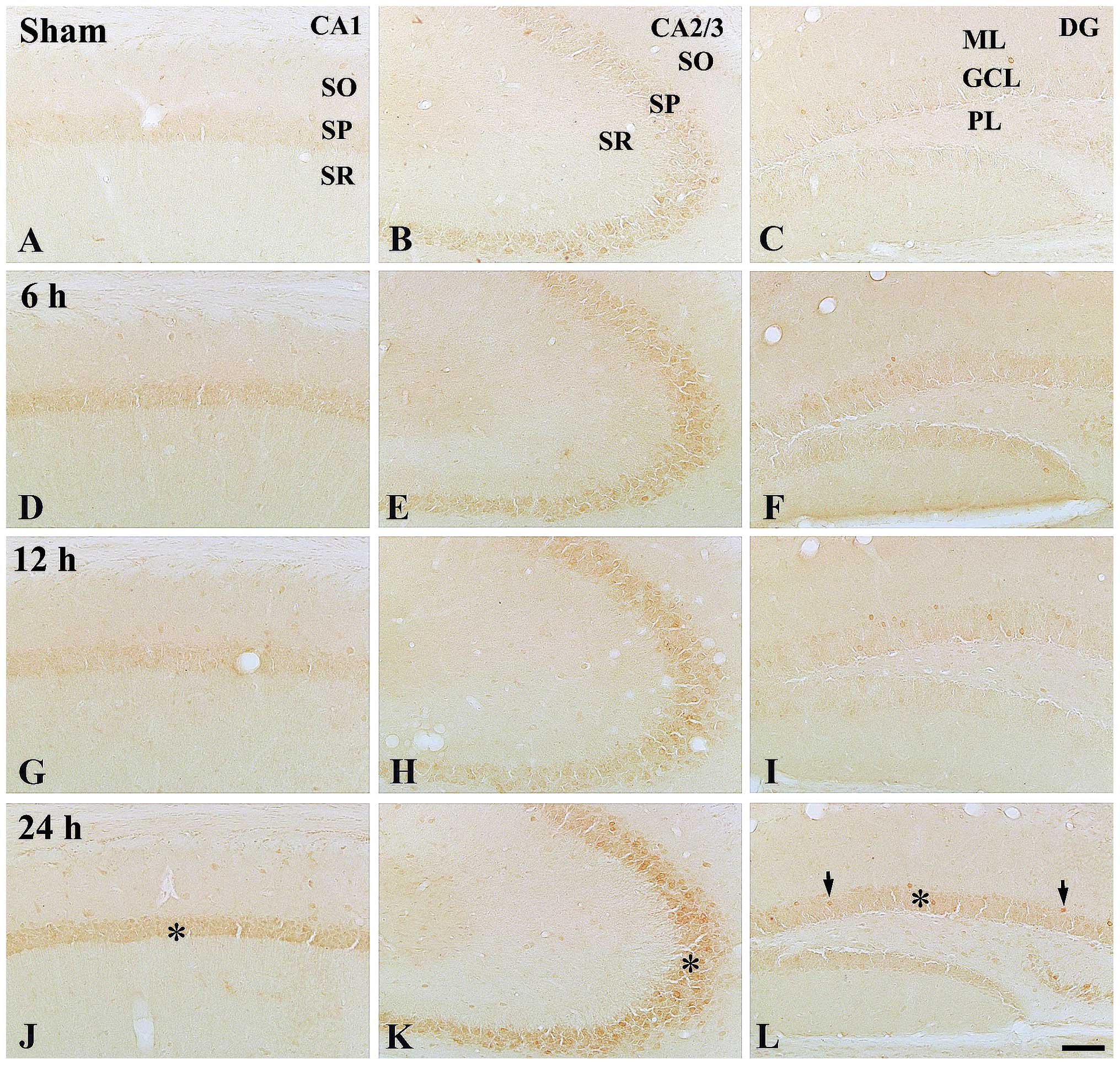
In the control group, moderate NF-κB/p65
immunoreactivity was detected in the stratum pyramidal of the CA1-3
regions, while low NF-κB/p65 immunoreactivity was identified in the
granule cell layer of the dentate gyrus (Table II; Fig. 3A–C).
 | Table IISemi-quantitative analysis of nuclear
factor-κB/p65 immunoreactivity in hippocampal regions after
treatment with 100 ng/kg TeT. |
Table II
Semi-quantitative analysis of nuclear
factor-κB/p65 immunoreactivity in hippocampal regions after
treatment with 100 ng/kg TeT.
| Area | Layer | Time after TeT
treatment (h)
|
|---|
| Control | 6 | 12 | 24 |
|---|
| CA1 | SO | ± | + | + | + |
| SP | + | ++ | ++ | ++ |
| SR | ± | + | + | ++ |
| CA2-3 | SO | ± | + | + | + |
| SP | + | ++ | ++ | ++ |
| SR | ± | + | + | + |
| DG | ML | – | + | + | + |
| GCL | ± | ++ | ++ | ++ |
| PL | ± | + | + | + |
At 6 h post-treatment, NF-κB/p65 immunoreactivity
was markedly increased in all layers of all hippocampal sub-regions
compared with those in the control group (Table II; Fig. 3D–F). At 12 h post-treatment, the
pattern of NF-κB/p65 immunoreactivity was similar to that at 6 h
post-treatment (Table II;
Fig. 3G–I). At 24 h
post-treatment, NF-κB/p65 immunoreactivity in all layers was not
significantly changed compared with that at 12 h post-treatment;
however, the immunoreactivity was higher than that in the control
group (Table II; Fig. 3J–L).
Effects of TeT on COX-2 and NF-κB p65
protein levels
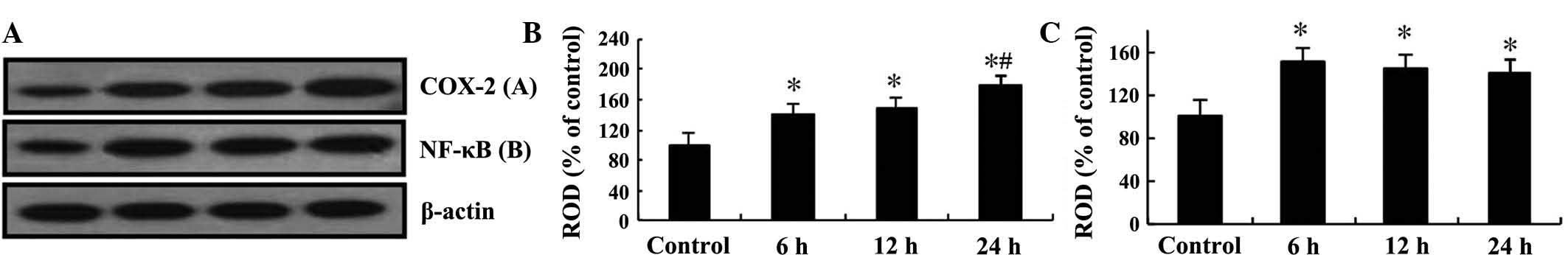
Western blot analysis was performed to confirm the
changes in the protein levels of COX-2 and NF-κB/p65 in the mouse
hippocampi after TeT treatment (Fig.
4). COX-2 protein levels steadily increased in a time-dependent
manner until 24 h post-treatment, while NF-κB/p65 levels were
significantly increased at 6 h post-treatment and then remained
constant until 24 h post-treatment (Fig. 4).
Discussion
TeT has been commonly used in experimental studies
on neurological disorders or animal models of diseases (31,32).
It was reported that intrahippocampal TeT injection induced
neuronal damage or death in certain brain regions, particularly in
the hippocampus (21,33). However, results of previous studies
regarding the induction of neuronal damage or death were
inconsistent due to differences in animals, dosages of TeT, routs
of administration and time of sacrifice (27,29,34).
In the present study, following intraperitoneal injection of TeT,
no neuronal damage or loss in any of the sub-regions of the
hippocampus was identified using immunohistochemical analysis of
NeuN expression.
In the present study, COX-2 immunoreactivity and
expression levels were significantly increased compared with those
in the control group at 24 h after injection of 100 ng/kg TeT; in
particular, enhanced COX-2 immunoreactivity was identified in the
stratum pyramidal of the hippocampus proper (CA1-3 regions) and in
the granule cell layer of the dentate gyrus. In analogy with this
finding, a previous study showed that systemic administration of
LPS increased COX-2 immuno-reactivity in the mouse hippocampus
(5). The brain and the immune
system are extensively interconnected and regulate each other
(35,36). The communication within the
hippo-campus contributes to region-specific vulnerability to
certain insults (35). In the
hippocampus, certain types of stimulation, such as bacterial toxin
stimuli, evoke a rapid immune response accompanied with specific
cellular and molecular changes (5,37).
For example, intraperitoneal treatment with LPS caused a reduction
in the mRNA levels of interleukin-1β and 6 in the cerebral cortex
and hippocampus of mice (38). In
addition, upon activation with LPS, microglia in the olfactory bulb
were shown to secrete a variety of cytokines in a rat model of
neuroinflammation (39). A
previous study also showed that microglia in rat hippocampi were
activated following injection of TeT into the ventral hippocampus
(40). Furthermore, recent studies
by our group revealed marked changes in inflammatory cytokines
accompanied with glial activation in all hippocampal sub-regions
after intraperitoneal administration of 100 ng/kg TeT (27,29).
NF-κB, a heterodimer of its p65 and p50 sub-units,
is located in the cytoplasm as an inactive complex bound to its
inhibitor, which is phosphorylated and subsequently degraded and
dissociated to produce activated NF-κB (10,12).
The present study observed changes of NF-κB/p65 immunoreactivity
and expression levels in mouse hippocampi after systemic
administration of TeT. NF-κB/p65 immunoreactivity was increased in
the cytoplasm of pyramidal neurons and granule cells after TeT
treatment. It was reported that NF-κB was activated by numerous
different types of stimuli and NF-κB regulated the expression of
COX-2 in the CNS (41). Therefore,
these results lead to the hypothesis that the increased COX-2
expression may be closely associated with the increase of NF-κB/p65
immunoreactivity in neurons after TeT treatment.
In conclusion, the present study suggested that the
systemic administration of 100 ng/kg TeT did not cause neuronal
damage; however, it markedly increased the expression of COX-2 and
NF-κB/p65 in mouse hippocampi after TeT treatment.
Acknowledgments
The authors would like to thank Mr. Seung Uk Lee
(Department of Neurobiology, School of Medicine, Kangwon National
University, Chuncheon, Republic of Korea) for his technical
assistance in the experiments of this study. The present study was
supported by the National Research Foundation of Korea (NRF) funded
by the Ministry of Education, Science and Technology (grant no.
2010-0010580) and by a 2014 Research Grant from Kangwon National
University (Chuncheon, Korea).
References
|
1
|
Chae HS, Kang OH, Lee YS, Choi JG, Oh YC,
Jang HJ, Kim MS, Kim JH, Jeong SI and Kwon DY: Inhibition of
LPS-induced iNOS, COX-2 and inflammatory mediator expression by
paeonol through the MAPKs inactivation in RAW 264.7 cells. Am J
Chin Med. 37:181–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim JB, Han AR, Park EY, Kim JY, Cho W,
Lee J, Seo EK and Lee KT: Inhibition of LPS-induced iNOS, COX-2 and
cytokines expression by poncirin through the NF-kappaB inactivation
in RAW 264.7 macrophage cells. Biol Pharm Bull. 30:2345–2351. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matheus AS, Coelho AM, Sampietre S,
Patzina R, Jukemura J, Cunha JE and Machado MC: Effect of
inhibition of prostaglandin E2 production on pancreatic infection
in experimental acute pancreatitis. HPB (Oxford). 9:392–397. 2007.
View Article : Google Scholar
|
|
4
|
Ji K and Tsirka SE: Inflammation modulates
expression of laminin in the central nervous system following
ischemic injury. J Neuroinflammation. 9:1592012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chung DW, Yoo KY, Hwang IK, Kim DW, Chung
JY, Lee CH, Choi JH, Choi SY, Youn HY, Lee IS and Won MH: Systemic
administration of lipopolysaccharide induces cyclooxygenase-2
immunoreactivity in endothelium and increases microglia in the
mouse hippocampus. Cell Mol Neurobiol. 30:531–541. 2010. View Article : Google Scholar
|
|
6
|
Fan LW, Kaizaki A, Tien LT, Pang Y, Tanaka
S, Numazawa S, Bhatt AJ and Cai Z: Celecoxib attenuates systemic
lipopolysaccharide-induced brain inflammation and white matter
injury in the neonatal rats. Neuroscience. 240:27–38. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng M, Wang YL, Wang FF, Chen C and Wang
CY: The cyclooxygenase-2 inhibitor parecoxib inhibits
surgery-induced proinflammatory cytokine expression in the
hippocampus in aged rats. J Surg Res. 178:e1–e8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuloaga KL, O'Connor DT, Handa RJ and
Gonzales RJ: Estrogen receptor beta dependent attenuation of
cytokine-induced cyclo-oxygenase-2 by androgens in human brain
vascular smooth muscle cells and rat mesenteric arteries. Steroids.
77:835–844. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi BK, Kim JH, Jung JS, Lee YS, Han ME,
Baek SY, Kim BS, Kim JB and Oh SO: Reduction of ischemia-induced
cerebral injury by all-trans-retinoic acid. Exp Brain Res.
193:581–589. 2009. View Article : Google Scholar
|
|
10
|
Sastre B and del Pozo V: Role of PGE2 in
asthma and nonasthmatic eosinophilic bronchitis. Mediators Inflamm.
2012:6453832012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oeckinghaus A and Ghosh S: The NF-kappaB
family of transcription factors and its regulation. Cold Spring
Harb Perspect Biol. 1:a0000342009. View Article : Google Scholar
|
|
12
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: From bench to bedside. Exp Biol Med
(Maywood). 233:21–31. 2008. View Article : Google Scholar
|
|
13
|
Giovannini MG, Scali C, Prosperi C,
Bellucci A, Pepeu G and Casamenti F: Experimental brain
inflammation and neurode-generation as model of Alzheimer's
disease: Protective effects of selective COX-2 inhibitors. Int J
Immunopathol Pharmacol. 16(Suppl 2): S31–S40. 2003.
|
|
14
|
Vezzani A and Granata T: Brain
inflammation in epilepsy: Experimental and clinical evidence.
Epilepsia. 46:1724–1743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bombardi C and Di Giovanni G: Functional
anatomy of 5-HT2A receptors in the amygdala and hippocampal
complex: Relevance to memory functions. Exp Brain Res. 230:427–439.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahmadi A, Sayyah M, Khoshkholgh-Sima B,
Choopani S, Kazemi J, Sadegh M, Moradpour F and Nahrevanian H:
Intra-hippocampal injection of lipopolysaccharide inhibits kindled
seizures and retards kindling rate in adult rats. Exp Brain Res.
226:107–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oltedal L, Haglerød C, Furmanek T and
Davanger S: Vesicular release of glutamate from hippocampal neurons
in culture: An immunocytochemical assay. Exp Brain Res.
184:479–492. 2008. View Article : Google Scholar
|
|
18
|
Ding Y, Chang C, Xie L, Chen Z and Ai H:
Intense exercise can cause excessive apoptosis and synapse
plasticity damage in rat hippocampus through Ca2+
overload and endoplasmic reticulum stress-induced apoptosis
pathway. Chin Med J (Engl). 127:3265–3271. 2014.
|
|
19
|
Feng J, Wu Q, Zhang D and Chen BY:
Hippocampal impairments are associated with intermittent hypoxia of
obstructive sleep apnea. Chin Med J (Engl). 125:696–701. 2012.
|
|
20
|
Li HY, Yuan ZY, Wang YG, Wan HJ, Hu J,
Chai YS, Lei F, Xing DM and DU LJ: Role of baicalin in regulating
Toll-like receptor 2/4 after ischemic neuronal injury. Chin Med J
(Engl). 125:1586–1593. 2012.
|
|
21
|
Bagetta G, Corasaniti MT, Nisticó G and
Bowery NG: Behavioural and neuropathological effects produced by
tetanus toxin injected into the hippocampus of rats.
Neuropharmacology. 29:765–770. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuhad A and Chopra K: Effect of sesamol on
diabetes-associated cognitive decline in rats. Exp Brain Res.
185:411–420. 2008. View Article : Google Scholar
|
|
23
|
Lindsay L, Liu P, Gliddon C, Zheng Y,
Smith PF and Darlington CL: Cytosolic glucocorticoid receptor
expression in the rat vestibular nucleus and hippocampus following
unilateral vestibular deafferentation. Exp Brain Res. 162:309–314.
2005. View Article : Google Scholar
|
|
24
|
Mao H, Toufexis D, Wang X, Lacreuse A and
Wu S: Changes of metabolite profile in kainic acid induced
hippocampal injury in rats measured by HRMAS NMR. Exp Brain Res.
183:477–485. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benn SC, Ay I, Bastia E, Chian RJ, Celia
SA, Pepinsky RB, Fishman PS, Brown RH Jr and Francis JW: Tetanus
toxin fragment C fusion facilitates protein delivery to CNS neurons
from cerebrospinal fluid in mice. J Neurochem. 95:1118–1131. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fishman PS, Parks DA, Patwardhan AJ and
Matthews CC: Neuronal binding of tetanus toxin compared to its
ganglioside binding fragment [H(c)]. Nat Toxins. 7:151–156. 1999.
View Article : Google Scholar
|
|
27
|
Yan BC, Park JH, Kim IH, Shin BN, Ahn JH,
Yoo KY, Lee DS, Kim MJ, Kang IJ and Won MH: Chronological changes
in inflammatory cytokines immunoreactivities in the mouse
hippo-campus after systemic administration of high dosage of
tetanus toxin. Exp Brain Res. 223:271–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Indrawattana N, Sookrung N, Kulkeaw K,
Seesuay W, Kongngoen T, Chongsanguan M, Tungtrongchitr A and
Chaicumpa W: Human monoclonal ScFv that inhibits cellular entry and
metalloprotease activity of tetanus neurotoxin. Asian Pac J Allergy
Immunol. 28:85–93. 2010.PubMed/NCBI
|
|
29
|
Park SM, Yan BC, Park JH, Choi JH, Yoo KY,
Lee CH, Baek YY, Kim YM, Kang IJ and Won MH: Gliosis in the mouse
hippo-campus without neuronal death after systemic administration
of high dosage of tetanus toxin. Cell Mol Neurobiol. 32:423–434.
2012. View Article : Google Scholar
|
|
30
|
Lee CH, Park JH, Cho JH, Ahn JH, Yan BC,
Lee JC, Shin MC, Cheon SH, Cho YS, Cho JH, et al: Changes and
expressions of Redd1 in neurons and glial cells in the gerbil
hippocampus proper following transient global cerebral ischemia. J
Neurol Sci. 344:43–50. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Halliday AJ, Campbell TE, Nelson TS,
McLean KJ, Wallace GG and Cook MJ: Levetiracetam-loaded
biodegradable polymer implants in the tetanus toxin model of
temporal lobe epilepsy in rats. J J Clin Neurosci. 20:148–152.
2013. View Article : Google Scholar
|
|
32
|
Jiruska P, Shtaya AB, Bodansky DM, Chang
WC, Gray WP and Jefferys JG: Dentate gyrus progenitor cell
proliferation after the onset of spontaneous seizures in the
tetanus toxin model of temporal lobe epilepsy. Neurobiol Dis.
54:492–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bagetta G, Nistico G and Bowery NG:
Characteristics of tetanus toxin and its exploitation in
neurodegenerative studies. Trends Pharmacol Sci. 12:285–289. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee CL, Hannay J, Hrachovy R, Rashid S,
Antalffy B and Swann JW: Spatial learning deficits without
hippocampal neuronal loss in a model of early-onset epilepsy.
Neuroscience. 107:71–84. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Urra X, Obach V and Chamorro A: Stroke
induced immunodepression syndrome: From bench to bedside. Curr Mol
Med. 9:195–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Williamson LL and Bilbo SD: Chemokines and
the hippocampus: A new perspective on hippocampal plasticity and
vulnerability. Brain Behav Immun. 30:186–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Quan N, Whiteside M and Herkenham M: Time
course and localization patterns of interleukin-1beta messenger RNA
expression in brain and pituitary after peripheral administration
of lipopolysaccharide. Neuroscience. 83:281–293. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Henry CJ, Huang Y, Wynne A, Hanke M,
Himler J, Bailey MT, Sheridan JF and Godbout JP: Minocycline
attenuates lipo-polysaccharide (LPS)-induced neuroinflammation,
sickness behavior and anhedonia. J Neuroinflammation. 5:152008.
View Article : Google Scholar
|
|
39
|
Doursout MF, Schurdell MS, Young LM,
Osuagwu U, Hook DM, Poindexter BJ, Schiess MC, Bick DL and Bick RJ:
Inflammatory cells and cytokines in the olfactory bulb of a rat
model of neuro-inflammation; insights into neurodegeneration? J
Interferon Cytokine Res. 33:376–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shaw JA, Perry VH and Mellanby J: Tetanus
toxin-induced seizures cause microglial activation in rat
hippocampus. Neurosci Lett. 120:66–69. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
O'Neill LA and Kaltschmidt C: NF-kappa B:
A crucial transcription factor for glial and neuronal cell
function. Trends Neurosci. 20:252–258. 1997. View Article : Google Scholar : PubMed/NCBI
|