Introduction
Cervical carcinoma is one of the most frequent types
of cancer encountered in women (1). Currently, 5-fluorouracil (5-FU), a
commonly used antitumor chemotherapeutic drug, has been shown to be
one of the standard chemoradiotherapy regimens in the treatment of
many carcinomas including cervical cancer (2–4).
However, tumor recurrence and metastasis occur in a large number of
cancer patients mainly due to limited drug sensitivity and an
increased drug resistance (5).
Therefore, novel strategies of gene therapy for enhancing the
sensitivity of cancer cells to drug-induced growth inhibition and
apoptosis have been intensively explored (6–8).
Telomerase is an attractive molecular target for
cancer gene therapy due to its high prevalence in tumor cells
(9–11). Human telomerase is a
ribonucleoprotein complex, which is composed of an RNA component
hTR and telomerase reverse transcriptase (TERT), the catalytic
protein subunit of telomerase (12). The enzyme TERT is critical for
maintaining the telomere length and prolonging the life span of
cells, thereby contributing to uncontrolled cell proliferation
(10). Telomerase activity is
markedly increased in the majority of tumors but not detected in
the majority of normal cells. The reactivation of telomerase during
carcinogenesis is a common hallmark in the majority of human
tumors. In our previous study, hTERTC27, a 27 kDa C-terminal
polypeptide of hTERT, was reported to clearly decrease cell
proliferation and inhibit the tumorigenicity of human cervical
carcinoma and glioblastoma by promoting chromosome end-to-end
fusion during anaphase and by inducing telomere dysfunction
(13–16). However, hTERTC27 treatment alone
may not be capable of achieving a long-term benefit for cancer
therapy due to the unstable expression of this polypeptide, and the
problem of therapeutic gene integrating into the genome of tumor
cells with a rapidly dividing nature. However, it is noteworthy
that ectopic overexpression of this polypeptide markedly enhances
the sensitivity of HeLa cells to H2O2
treatment. Therefore, we hypothesized that hTERTC27 overexpression
is a potentially effective strategy for sensitizing cervical tumor
cells to chemotherapeutics and enhancing the antitumor effect of
5-FU chemotherapy.
To the best of our knowledge, the present is the
first study to report the antitumor effects of 5-FU and
overexpressed hTERTC27 on cervical carcinoma HeLa cells.
Overexpressed hTERTC27 increased the sensitivity of the HeLa cells
to 5-FU and significantly inhibited the HeLa cell proliferation
with 5-FU treatment. In addition, hTERTC27 overexpression clearly
promoted 5-FU-induced apoptosis by upregulating the expression of
activated caspase-3 and -9, and downregulating the expression of
B-cell lymphoma 2 (Bcl-2). These results indicate that
overexpression of hTERTC27 may provide a novel approach for
enhancing the sensitivity of 5-FU chemotherapy in the treatment of
cervical carcinoma.
Materials and methods
Cell culture
Human cervical cancer HeLa cells were obtained from
ATCC (Manassas, VA, USA) and maintained in Dulbecco’s modified
Eagle’s medium (DMEM; Hyclone Laboratories, Inc., South Logan, UT,
USA), supplemented with 10% fetal bovine serum (FBS; Hyclone
Laboratories, Inc.), 100 μg/ml penicillin (Ameresco, Middletown,
CT, USA) and 100 μg/ml streptomycin (Ameresco). The cells were
cultured at 37°C in a humidified atmosphere with 5%
CO2.
Transfection
The pcDNA3.1-hTERTC27 plasmid was maintained in our
laboratory. cDNAs of a 27 kDa hTERT C-terminal polypeptide
(hTERTC27, amino acid residues 882–1132) was constructed
downstream. The HeLa cells were transfected with pcDNA3.1 vectors
(known as control-HeLa cells) or pcDNA3.1-hTERTC27 vectors (termed
C27-HeLa cells) using Lipofectamine 2000 transfection reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and following the
manufacturer’s instructions. The untransfected HeLa cells were
designated as mock-HeLa cells. Twenty-four hours after
transfection, the cells were treated with 5-FU and experiments were
subsequently performed.
Western blot analysis
The expression of hTERTC27 was detected at 12, 24,
48 and 72 h after transfection. The expression of caspase-3, -9 and
Bcl-2 was detected 48 h after 5-FU treatment. Western blot analysis
was performed as described previously (17). Antibodies against the hTERT
COOH-terminal fragment (1:250; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), cleaved caspase-3 (1:1,000) and cleaved
caspase-9 (1:1,000; both Cell Signaling Technology, Inc., Danvers,
MA, USA) and Bcl-2 (1:500; Santa Cruz Biotechnology, Inc.) were
used. The bound antibodies were located by further incubation with
horseradish peroxidase-conjugated goat anti-rabblit IgG (1:5,000;
Santa Cruz Biotechnology, Inc.). β-actin (1:500; Santa Cruz
Biotechnology, Inc.) was used as a loading control. Blots were
developed with an ECL plus kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA), and exposed to Kodak film (Eastman Kodak
Company, Rochester, NY, USA). The National Institutes of Health
Image program was used to quantify the intensity of the resulting
bands.
MTT assay
In total, 2×103 HeLa cells (mock, control
and C27) were seeded in a 96-well plate 24 h after transfection.
After 12 h, the cells were treated with different concentrations
(in μg/ml) of 5-FU of 0, 10, 20, 30, 40 or 50, respectively. The
MTT assay was then performed at 12, 24, 48 and 72 h after 5-FU
treatment. Briefly, 20 μl of 5 mg/ml MTT (Sigma, St. Louis, MO,
USA) in PBS was added and the cells were incubated for 4 h at 37°C
with 5% CO2. The cells were washed twice with PBS and
the pellet was then solubilized in 150 μl of 100% dimethylsulfoxide
(Sigma) by agitating for 5 mins. The absorbance was measured on a
microplate reader (Bio-Rad, Hercules, CA, USA) at a wavelength of
570 nm. The rate of cell viability was calculated according to the
equation: A570(Cd)/A570(C0) ×
100.
A570 (C0) is the absorbance of
the HeLa cells (mock, control and C27) without 5-FU treatment and
A570 (Cd) is the absorbance of the cells exposed to the
different concentrations of 5-FU. All the experiments were
performed in triplicate.
Colony formation assay
Twelve hours after transfection, control-HeLa and
C27-HeLa cells were exposed to 20 μg/ml 5-FU, known as the 5-FU and
C27+5-FU groups, respectively. The control-HeLa cells were exposed
to PBS as a control, designated as the group control. After 12 h,
the medium containing 5-FU was replaced. The cells were allowed to
grow for 10 days, fixed with methanol for 10 min and stained with
Giemsa for 15 min. The plates were washed and dried, and the
colonies were counted. The experiments were performed in
triplicate.
Flow cytometric analysis of
apoptosis
For a quantitative analysis of apoptosis, the cells
were harvested 48 h after treatment with 20 μg/ml 5-FU, washed once
in ice-cold PBS, incubated with annexin V-FITC/PI (Boehringer,
Mannheim, Germany) in calcium containing HEPES (Sigma) buffer, and
then immediately analyzed with a flow cytometry machine
(Becton-Dickinson, Bedford, MA, USA).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling (TUNEL) assay
The presence of apoptotic cells in the HeLa cells
was detected by the TUNEL assay using the in situ cell death
detection kit (POD; Roche Diagnostics, Mannheim, Germany) according
to the manufacturer’s instructions. The apoptotic cells were
analyzed under a fluorescence microscope (Olympus Corporation,
Tokyo, Japan).
Statistical analysis
Data were presented as the mean ± SD. The results
were compared by two-way analysis of variance. All the statistical
calculations were performed with the SPSS11.0 software package
(SPSS, Inc., Chicago, IL, USA). P<0.05 was used to indicate a
statistically significant difference.
Results
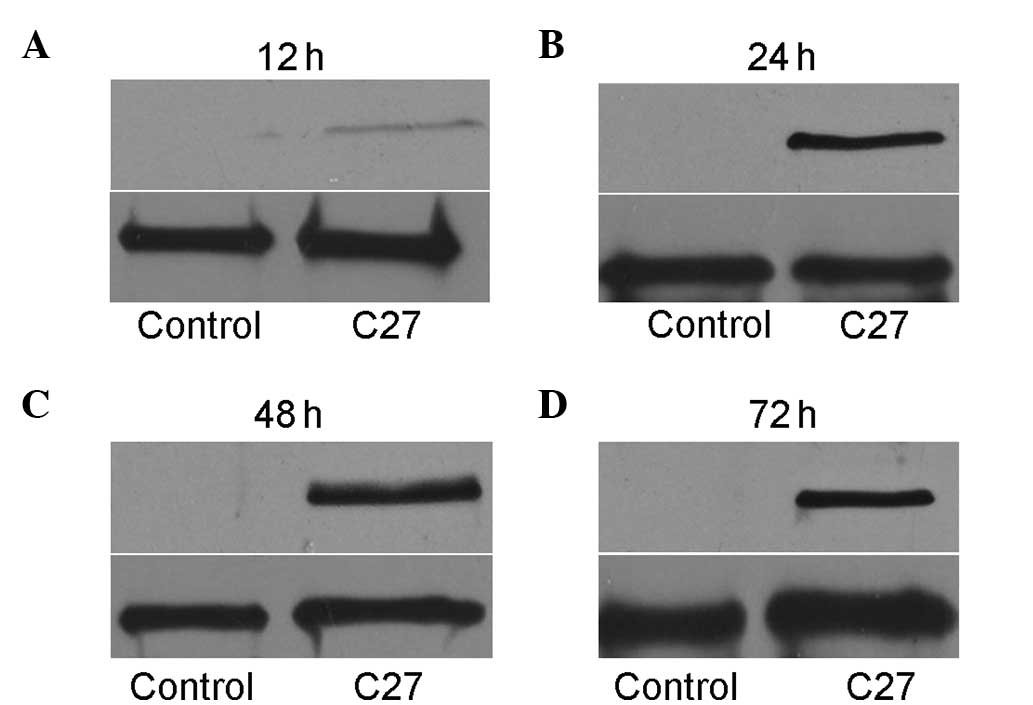
hTERTC27 expression in HeLa cells
following transfection
Exogenous hTERTC27 protein was detected by western
blot analysis at 12, 24, 48 and 72 h after transfection. As shown
in Fig. 1A, the cells transfected
with a recombinant plasmid containing hTERTC27 cDNA expressed
hTERTC27 protein (27 kDa) slightly 12 h after transfection. After
24 h, the cells expressed the hTERTC27 protein in abundance.
However, no exogenous hTERTC27 protein was detected in the
non-transfected HeLa cells, and empty vector-transfected cells.
Thus, the cells were treated with 5-FU 24 h following transfection
in the subsequent experiments.
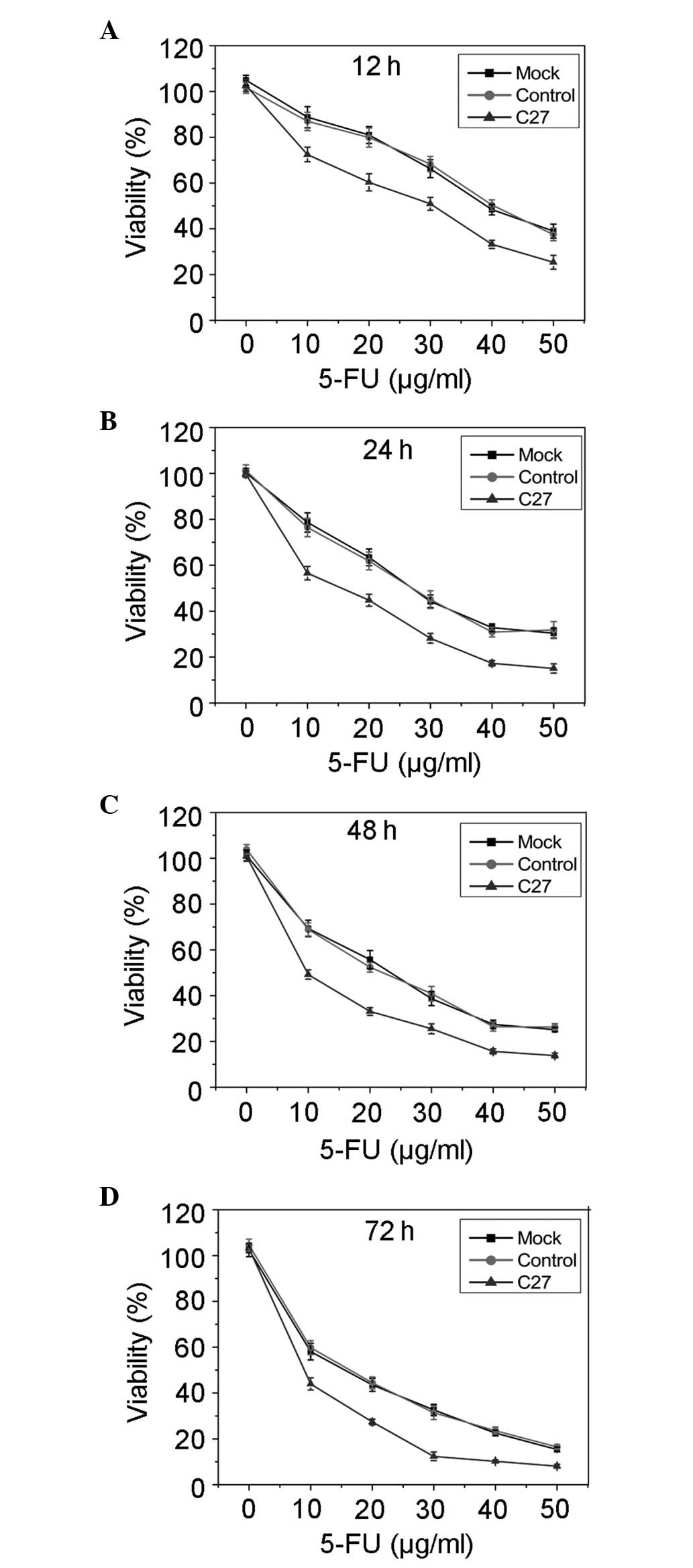
hTERTC27 enhances HeLa cell sensitivity
to 5-FU treatment
The effect of overexpressed hTERTC27 polypeptide on
cell viability following treatment with different concentrations of
5-FU was determined at indicated time points by the MTT assay. As
shown in Fig. 2, the cell
viability was markedly inhibited in the C27 group when compared
with that in the mock group (P<0.01) and control (P<0.01).
However, no significant difference was observed between the mock
group and the control (P>0.01). These results suggested that
overexpressed hTERTC27 enhances the sensitivity of HeLa cells to
5-FU treatment and promotes 5-FU-induced growth inhibition. In
order to perform subsequent experiments, a final concentration of
20 μg/ml 5-FU was selected to observe the effects of overexpressed
hTERTC27 and 5-FU on HeLa cell proliferation and apoptosis.
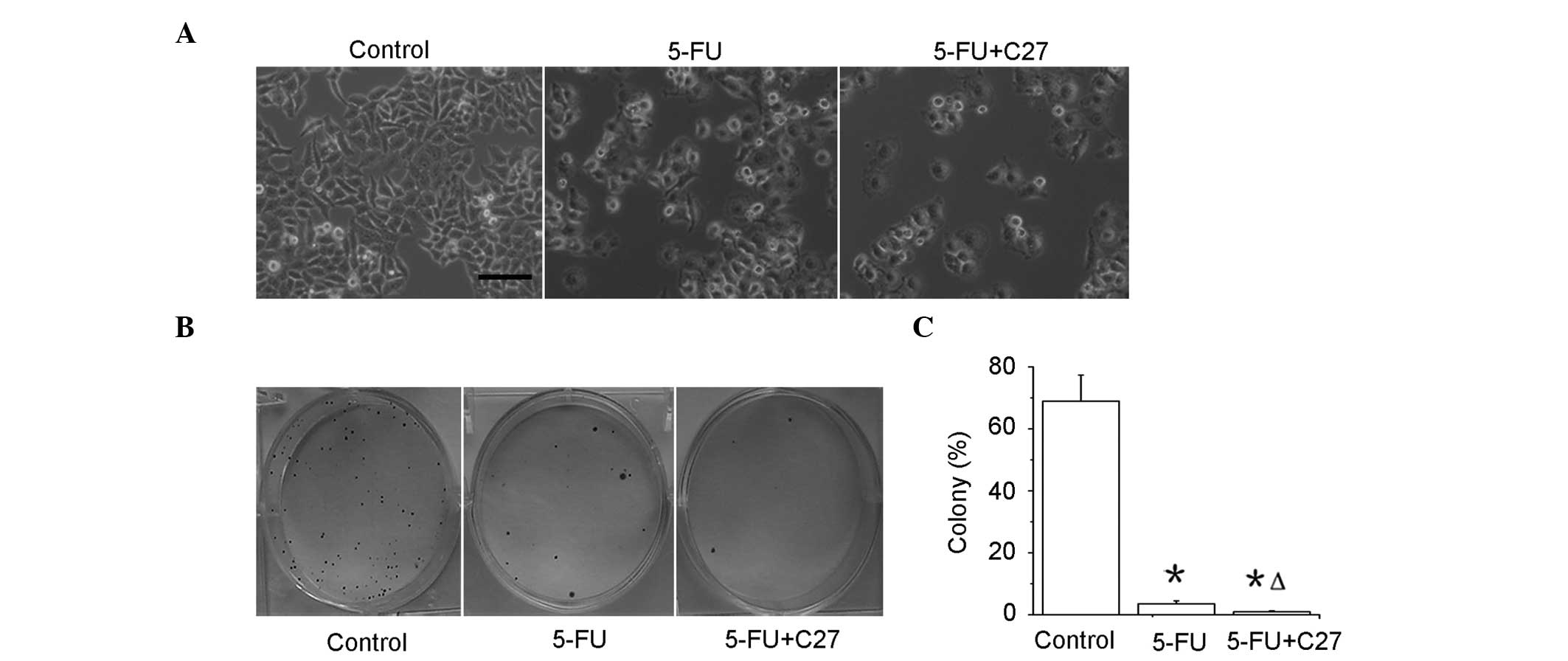
hTERTC27 promotes inhibition of
proliferation of HeLa cells with 5-FU treatment
The control-HeLa and C27-HeLa cells were exposed to
20 μg/ml 5-FU (5-FU and C27+5-FU groups), and control-HeLa cells
were exposed to PBS as a control (control group). As shown in
Fig. 3A, the cell numbers were
significantly decreased in the C27+5-FU (P<0.01) and 5-FU
(P<0.01) groups when compared with that in the control group.
Notably, the C27+5-FU group demonstrated a lower cell proliferation
rate compared with that of the 5-FU group (P<0.01). A colony
formation assay revealed that the colony formation rates in the
5-FU (2.63 ± 0.58%) and C27+5-FU (0.32 ± 0.09%) groups were
significantly lower compared with that in the control (68.74 ±
8.62%) group (P<0.05) (Fig. 3B and
C). Moreover, the combined therapeutic group of C27+5-FU (0.3 ±
0.1%) demonstrated an additional decrease in the colony formation
rate when compared with the 5-FU group alone. These results
indicate that overexpressed hTERTC27 evidently inhibits the
proliferation and colony formation in HeLa cells with 5-FU
treatment.
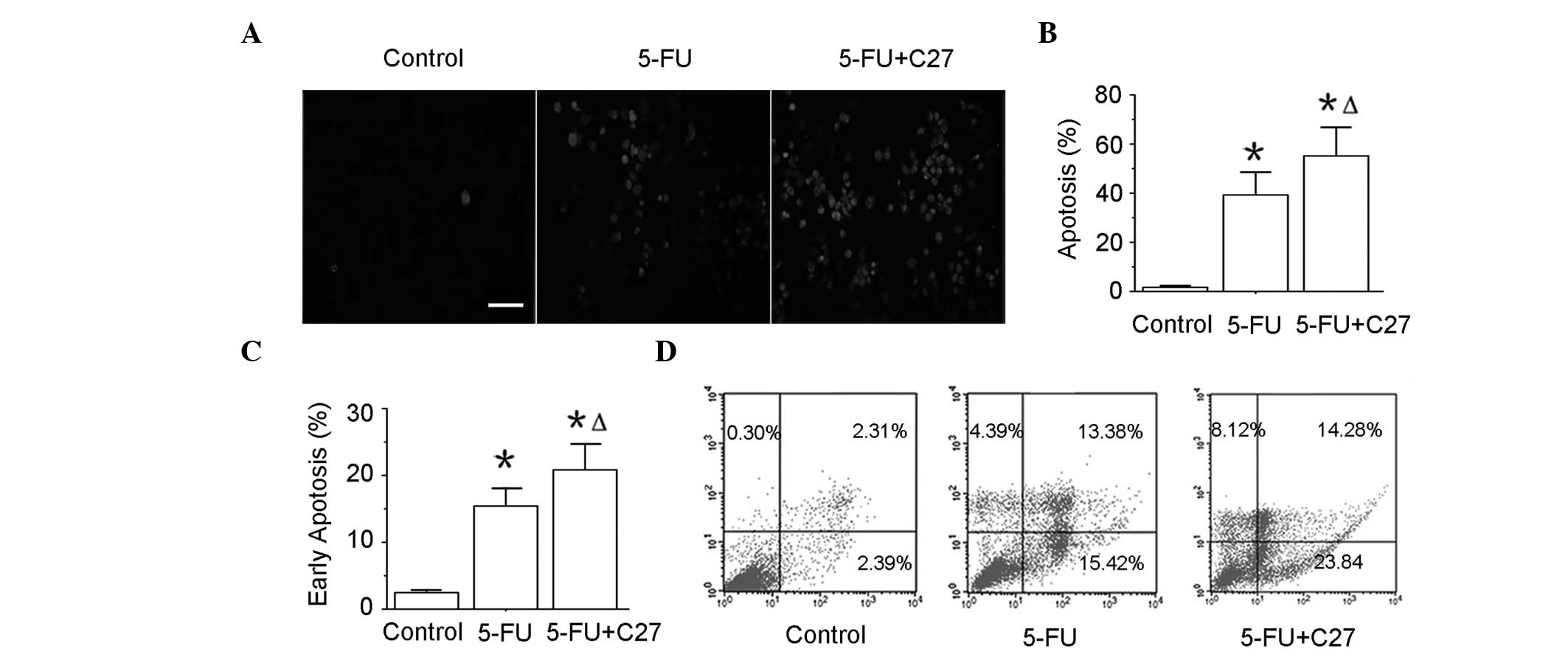
hTERTC27 promotes apoptosis of HeLa cells
with 5-FU treatment
To determine whether hTERTC27 promotes apoptosis of
HeLa cells, the TUNEL assay and annexin-V/PI double staining
followed by a flow cytometry assay were performed. The apoptotic
death (TUNEL positive) of the 5-FU group (39.28 ± 9.25%) was highly
increased compared with that of the control group (1.66 ± 0.34%;
(P<0.01). In addition, the combinatorial group of C27+5-FU
(55.14 ± 11.67%) revealed a further increase in the TUNEL-positive
rate when compared with the 5-FU group (P<0.05). To confirm this
result, early apoptosis (annexin-V+/PI−) was
assessed by flow cytometry (Fig. 4C
and D). Additionally, ~23% HeLa cells in group C27+5-FU were
found to undergo early apoptosis, while only ~15% in the 5-FU and
2% in the control groups. These data indicate that hTERTC27
overexpression may promote the HeLa cell apoptosis induced by
5-FU.
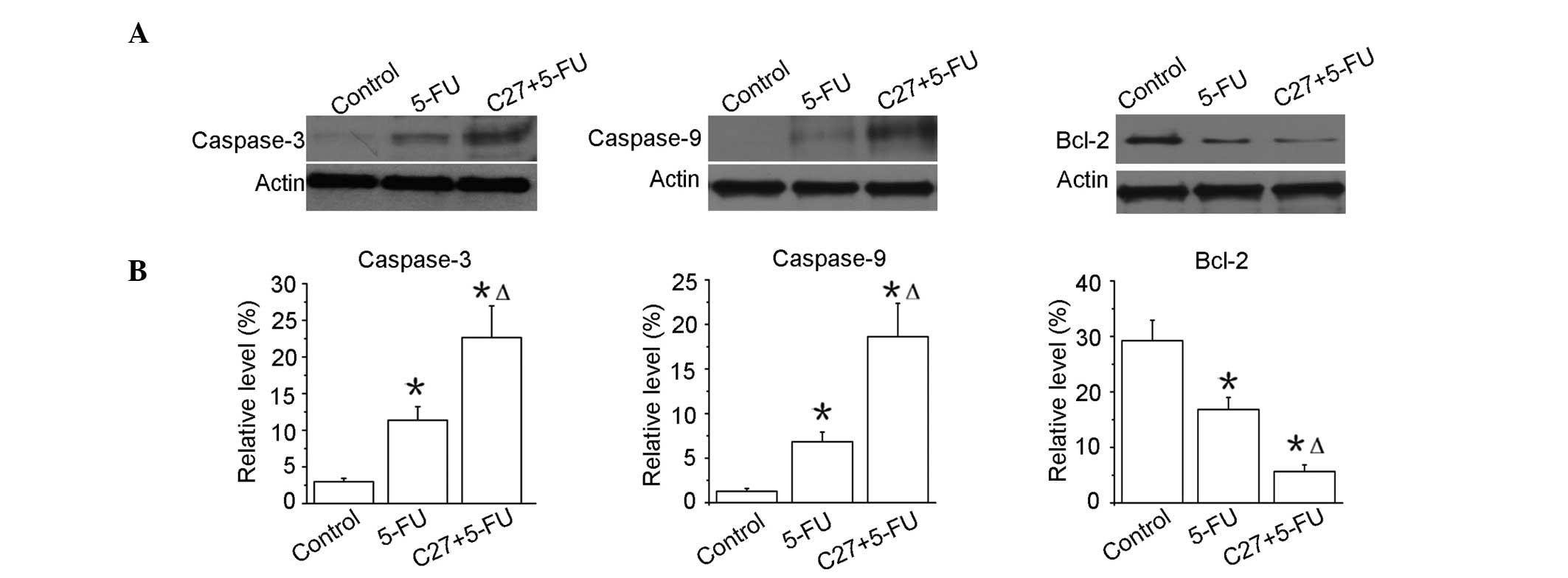
hTERTC27 promotes the activation of
caspases and the downregulation of Bcl-2 in HeLa cells with 5-FU
treatment
Apoptosis is known to be characterized by the
activation of caspases. Thus, the effect of hTERTC27 and 5-FU was
examined on the activation of caspase-3 and -9. As shown in
Fig. 5, the amount of caspase-3
activation was evidently enhanced in the C27+5-FU group when
compared with that in the 5-FU group (P<0.01). Similar results
were also observed in the activation of caspase-9. The changes in
the signalling proteins were evaluated to be relevant to apoptosis.
Bcl-2 is one of the most significant proteins of the antiapoptotic
family. As shown in Fig. 5, the
Bcl-2 expression was markedly reduced in the C27+5-FU group when
compared with that in the 5-FU group (P<0.01). These results
consistently demonstrated that the overexpression of hTERTC27
clearly promoted 5-FU-induced apoptotic death in HeLa cells.
Discussion
In the present study, the overexpression of
exogenous hTERTC27 was revealed to contribute to sensitizing HeLa
cells to chemotherapeutic drugs such as 5-FU by promoting
apoptosis. 5-FU, as an antimetabolic drug, is widely used in cancer
chemotherapy due to its easy administration and high efficiency
(3,18). However, long-term 5-FU chemotherapy
is usually not administered to patients due to a decreasing
sensitivity and side effects caused by the high dosage. Therefore,
enhancing the sensitivity of cancer cells to 5-FU has become a
crucial strategy for enhancing the antitumor effect of
chemotherapeutic agents.
A number of therapeutic approaches are currently
being explored to target telomere or telomerase for cancer therapy
since telomerase is highly expressed in the majority of the tumors
(11,19). The abnormal telomerase activity
protects telomere from being shortened and therefore leads to
uncontrollable cell proliferation ability and malignant
transformation (10). In our
previous study, the ectopic expression of hTERTC27 was reported to
lead to chromosome end-to-end fusion during anaphase and telomere
dysfunction (13). In the present
study, hTERTC27 overexpression was demonstrated to increase the
sensitivity of HeLa cells to 5-FU treatment by significantly
inhibiting the cell viability. These changes resulted from the
hTERTC27-induced increasing sensitivity of HeLa cells to the
oxidative stress caused by 5-FU, which is consistent with the
results of previous studies in which the overexpression of this
polypeptide was observed to markedly enhance the sensitivity of
HeLa cells to H2O2-induced injury (14). These results indicated that a low
concentration of 5-FU treatment in the presence of hTERTC27 may
achieve the same therapeutic effect as a higher 5-FU concentration.
This combinatorial strategy may decrease the therapeutic dose of
5-FU, and thus reduce the side effects of 5-FU chemotherapy.
The Bcl-2 family proteins are key regulators of cell
apoptosis (20,21), and sequential activation of
caspases has been revealed to be involved in cell apoptosis
(22). The activation of the
caspase-3 pathway is a hallmark of apoptosis, and is activated in
the apoptotic cell by extrinsic (death ligand) and intrinsic
(mitochondrial) pathways (23).
Caspase-9, an initiator caspase, has been connected to the
mitochondrial death pathway (24).
Once initiated, caspase-9 continues to cleave procaspase-3, leading
to multiple cellular target cleavage including poly-ADP ribose
polymerase (25). In the present
study it was found that 5-FU in combination with hTERTC27
significantly downregulated the expression of Bcl-2 and markedly
promoted the activation of caspase-3 and -9, which confirmed that
overexpression of the hTERTC27 polypeptide may enhance the
antitumor effects of 5-FU by promoting cell apoptosis.
In conclusion, to the best of our knowledge, the
present study reports for the first time the anticancer potential
of the combination of 5-FU treatment and hTERTC27 overexpression.
The overexpression of hTERTC27 was revealed to increase the
sensitivity of cancer cells to 5-FU treatment. Furthermore, the
combinatorial therapy was demonstrated to promote 5-FU-induced
activation of caspase-3 and -9, and downregulate the expression
levels of Bcl-2. Altogether, the present study has shown that the
combination of 5-FU treatment and hTERTC27 overexpression may
provide a potential clinical strategy for enhancing the sensitivity
of tumor cells to chemotherapy.
Acknowledgements
This study was supported by grants from National
Natural Science Foundation of China (NSFC; 81301318), Science and
Technology Project of Guangdong (2011B031800351), Shenzhen Basic
Research Project (JCYJ20120613170218654), and the Natural Science
Foundation of SZU (80100035901).
References
|
1
|
Markman M: Chemoradiation in the
management of cervix cancer: current status and future directions.
Oncology. 84:246–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hemaiswarya S and Doble M: Combination of
phenylpropanoids with 5-fluorouracil as anti-cancer agents against
human cervical cancer (HeLa) cell line. Phytomedicine. 20:151–158.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto K, Fujiwara Y, Nishida T, et al:
Induction chemotherapy with docetaxel, 5-FU and CDDP (DFP) for
advanced gastric cancer. Anticancer Res. 29:4211–4215.
2009.PubMed/NCBI
|
|
4
|
Osaka Y, Shinohara M, Hoshino S, Ogata T,
Takagi Y, Tsuchida A and Aoki T: Phase II study of combined
chemotherapy with docetaxel, CDDP and 5-FU for highly advanced
esophageal cancer. Anticancer Res. 31:633–638. 2011.
|
|
5
|
Nagata M, Nakayama H, Tanaka T, et al:
Overexpression of cIAP2 contributes to 5-FU resistance and a poor
prognosis in oral squamous cell carcinoma. Br J Cancer.
105:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CJ, Stratmann J, Zhou ZG and Sun XF:
Suppression of microRNA-31 increases sensitivity to 5-FU at an
early stage, and affects cell migration and invasion in HCT-116
colon cancer cells. BMC Cancer. 10:6162010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karasawa H, Miura K, Fujibuchi W, et al:
Down-regulation of cIAP2 enhances 5-FU sensitivity through the
apoptotic pathway in human colon cancer cells. Cancer Sci.
100:903–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin G, Lin MC, Lin S, et al: Early growth
response protein-1 promoter-mediated synergistic antitumor effect
of hTERTC27 gene therapy and 5-fluorouracil on nasopharyngeal
carcinoma. Cancer Biother Radiopharm. 27:434–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gomez DE, Armando RG, Farina HG, Menna PL,
Cerrudo CS, Ghiringhelli PD and Alonso DF: Telomere structure and
telomerase in health and disease (Review). Int J Oncol.
41:1561–1569. 2012.PubMed/NCBI
|
|
10
|
Shay JW and Wright WE: Role of telomeres
and telomerase in cancer. Semin Cancer Biol. 21:349–353. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruden M and Puri N: Novel anticancer
therapeutics targeting telomerase. Cancer Treat Rev. 39:444–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Podlevsky JD and Chen JJ: It all comes
together at the ends: telomerase structure, function, and
biogenesis. Mutat Res. 730:3–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang JJ, Lin MC, Bai YX, et al: Ectopic
expression of a COOH-terminal fragment of the human telomerase
reverse transcriptase leads to telomere dysfunction and reduction
of growth and tumorigenicity in HeLa cells. Cancer Res.
62:3226–3232. 2002.
|
|
14
|
Huang J, Bai YX, Han SW, et al: A human
TERT C-terminal polypeptide sensitizes HeLa cells to
H2O2-induced senescence without affecting
telomerase enzymatic activity. Biochem Biophys Res Commun.
301:627–632. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ng SS, Gao Y, Chau DH, et al: A novel
glioblastoma cancer gene therapy using AAV-mediated long-term
expression of human TERT C-terminal polypeptide. Cancer Gene Ther.
14:561–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Y, Ng SS, Chau DH, et al: Development
of recombinant adeno-associated virus and adenovirus cocktail
system for efficient hTERTC27 polypeptide-mediated cancer gene
therapy. Cancer Gene Ther. 15:723–732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin G, Zhao L, Yin F, et al: TCF3 inhibits
F9 embryonal carcinoma growth by the down-regulation of Oct4. Oncol
Rep. 26:893–899. 2011.PubMed/NCBI
|
|
18
|
Afzal S, Jensen SA, Vainer B, et al: MTHFR
polymorphisms and 5-FU-based adjuvant chemotherapy in colorectal
cancer. Ann Oncol. 20:1660–1666. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lü MH, Liao ZL, Zhao XY, et al:
hTERT-based therapy: A universal anticancer approach (Review).
Oncol Rep. 28:1945–1952. 2012.PubMed/NCBI
|
|
20
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
García-Sáez AJ: The secrets of the Bcl-2
family. Cell Death Differ. 19:1733–1740. 2012.
|
|
22
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012.
|
|
25
|
Rosen A and Casciola-Rosen L:
Macromolecular substrates for the ICE-like proteases during
apoptosis. J Cell Biochem. 64:50–54. 1997. View Article : Google Scholar : PubMed/NCBI
|