Introduction
Hepatitis B virus (HBV) infection is a serious
public health problem worldwide, with ~400 million chronic HBV
surface antigen (HBsAg) carriers (1). In the majority of patients, the virus
is quiescent or replicating at low levels. However, chemotherapy
may disrupt the balance between the host immune responses and viral
replication, and enhanced viral replication is a universal
phenomenon in clinical cases (2).
The mechanisms of HBV reactivation are not
completely understood. The majority of studies consider there to be
at least two mechanisms responsible for this phenomenon. One is the
suppression and reconstitution of the host immune response
following chemotherapy, which is pivotal in controlling HBV
infection and replication (1).
Another is the direct stimulatory effect anticancer agents,
including glucocorticoids, may have on viral replication.
Corticosteroids increase HBV expression in vitro by binding
to the glucocorticoid-responsive element and augmenting the HBV
enhancer I (3,4). However, steroid-free chemotherapy may
also promote viral replication (5). In certain cases, HBV reactivation
occurred within the first two weeks of chemotherapy (6), which was likely to be prior to immune
reconstitution following withdrawal of the anticancer agents. One
possible mechanism for the early reactivation of HBV is that
cytotoxic agents may have a direct stimulatory effect on HBV
replication.
In the present study, the aim was to investigate
whether epirubicin stimulates HBV replication directly. If this
hypothesis is valid, it may be a novel mechanism of the HBV
reactivation caused by cytotoxic chemotherapy.
Materials and methods
Construction of the stable HBV-expressing
cell line, HepG2-HBV1.1
HepG2 cells (American Type Culture Collection,
Rockefeller, MD, USA) were transfected with pneo-CH9/HBV1.1 (Center
for Molecular Biology, Heidelberg University, Heidelberg, Germany;
recipient, Dr Lanlin, Southwest Hospital affiliated to Third
Military Medical University, Chongqing, China) (7), which contains the cytomegalovirus
promoter and 1.1 copies of the HBV genome, and then selected with
G418 antibiotic (1,200 μg/ml; Merck and Co., Inc., Rahway, NJ,
USA). Isolated cell colonies with the HBV genome integrated were
selected subsequent to confirmation by Southern blotting and
western blot analysis.
Cell culture and treatment
HepG2, HepG2-HBV1.1 and HepG2.2.15 (Xiangya School
of Medicine, Central South University, Changsha, China) cells were
cultured in minimum essential medium (MEM; Gibco-BRL, Grand Island,
NY, USA) with 10% fetal bovine serum (FBS; Hyclone, Shanghai,
China) and maintained in a humidified incubator with 5%
CO2 at 37°C. For cytotoxic chemotherapy treatment, the
cells were incubated with epirubicin (0–1 μM, Hisun Chemical Co.,
Ltd., Zhejiang, China) for 24 h. The medium was then replaced with
fresh serum-free MEM medium for a further 48 h incubation and the
cells were then harvested.
Effects of epirubicin on the viability of
HepG2.2.15 and HepG2-HBV1.1 cells by trypan blue exclusion
assay
HepG2.2.15 (4×105 cells/well) and
HepG2-HBV1.1 (6×105 cells/well) cells were seeded in
six-well plates. Following adherence of the cells, epirubicin was
added to the medium at various concentrations (0–1 μM) for 24 h,
followed by 48 h incubation in a drug-free culture medium. The
cells in each well were washed with phosphate-buffered saline twice
following the addtion of 200 μl trypsin, and then placed in a 37°C
5% CO2 incubator for 1–2 min. Subsequently 800 μl 10%
FBS MEM was added to each well to terminate the reaction, and the
cell suspension by pipetting several times to disperse the cells
evenly and count the cell number. The viability of cells = live
cells/total cells.
MTT assay
HepG2.2.15 (1×105 cells/well) and
HepG2-HBV1.1 (1×105 cells/well) cells were inoculated in
96-well plates. Following adherence of the cells, epirubicin was
added to the medium at various concentrations (0–1 μM) for 24 h,
and then 20 μl 0.5% MTT solution was added to each well for 4 h.
Subsequently, the medium in each well was discarded and 150 μl DMSO
was added. The plate was agitated for 10 min in order to dissolve
the crystals and the absorbance of each well was measured at an
optical denisty of 490 nm.
Quantification of HBV DNA copies by
fluorescent quantitative polymerase chain reaction (qPCR)
HBV replicative intermediates in the cells were
obtained as follows. Cells from one 35-mm diameter dish were lysed
with 0.5 ml lysis buffer containing 10 mM Tris-HCl (pH 8.0), 1 mM
EDTA, 1% NP-40 and 2% sucrose at 37°C for 10 min. The cell debris
and nuclei were removed by centrifugation at 13,000 × g and the
supernatant was mixed with 200 μl 35% polyethylene glycol (PEG)
8000 (Beyotime Institute of Biotechnology, Shanghai, China)
containing 1.5 M NaCl. Subsequent to incubation on ice for 2 h, the
viral nucleocapsids were pelleted by centrifugation at 12,000 xg
for 10 min at 4°C, followed by 24 h digestion at 37°C in 400 μl
digestion buffer containing 0.5 mg/ml pronase (Takara Bio, Inc.,
Shiga, Japan), 0.5% sodium dodecyl sulfate (SDS), 10 mM Tris-HCl
(pH 8.0) and 10 mM EDTA. The digestion mixture was extracted twice
with phenol, and the DNA was precipitated with ethanol and
dissolved in Tris-EDTA (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) buffer.
In order to collect viral particles instead of free viral DNA in
the culture medium, the supernatant was subjected to 35% PEG 8000
precipitation overnight according to the methods of a previous
study (8) and then the
precipitates were digested as previously described.
The quantification of HBV copies was performed by
SYBR-Green assays using FastStart Universal SYBR-Green Master mix
(Roche Diagnostics GmbH, Mannheim, Germany). Primers for
amplification of the HBV DNA fragments were designed specifically
for the conserved region of the HBV gene by Huada Gene Sci-Tech
Company (Shenzhen, China) and the sequences were as follows:
Forward primer (F2150), 5′-CCTAGTAGTCAGTTATGTCAAC-3′; and reverse
primer (R2300), 5′-TCTATAAGCTGGAGGAGTGCGA-3′. The plasmid
pneo-CH9/HBV1.1 at different concentrations (5×102,
5×103, 5×104, 5×105,
5×106 and 5×107 copies/μl) served as a
template to make the standard curve.
Quantification of HBV pregenomic (pg)RNA
by fluorescent qPCR
Total RNA was extracted by a DNA-free RNA Mini
Extraction kit (Watson, Shanghai, China) and 1 μg total RNA was
used for cDNA synthesis, which was conducted by reverse
transcription using the PrimeScript RT reagent kit (Perfect Real
Time; Takara Bio, Inc.). Relative quantification of the target
genes (HBV 3.5 kb mRNA) was performed using SYBR Green assays, with
β-actin mRNA as an endogenous control. The expression values of the
target genes were calculated using the 2−ΔΔCt
method.
Southern blot analysis
HBV replicative intermediates were extracted from
the cells or the supernatant of the culture medium according to the
methods previously described and then separated on 0.8% agarose
gels. The DNA samples were transferred to nylon membranes (Roche
Diagnostics GmbH). Subsequent to ultraviolet crosslinking and
prehybridization, the membranes were hybridized with a
digoxigenin-labeled HBV-specific probe using a Random-Primed DNA
Labeling kit (Roche Diagnostics GmbH). The signal was detected by
exposure on an X-ray film and scanning using the Versa Doc Imaging
system (Bio-Rad, Hercules, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
The levels of HBsAg and HBV e antigen (HBeAg) in the
culture medium and cell extracts were assessed by ELISA using
Antibody to Hepatitis B Surface Antigen and Antibody to Hepatitis B
Virus E Antigen ELISA kits, according to the manufacturer’s
instructions (Kehua Biotech Co, Ltd, Shanghai, China). The levels
of HBV core antigen (HBcAg) in the culture medium were assessed
using an ELISA kit (Disease Diagnosis Reagent and Vaccine
Engineering Technology Research Center of China Infectious State,
Xiamen University, Xiamen, China). This kit contained two types of
96-well plate. One was coated with HBsAb, to capture only the Dane
particle. The other was coated with HBcAb, therefore it detected
the total HBcAg from the Dane particles and the HBV nucleocapsids.
Each experiment was performed in triplicate and independently
repeated three times.
Western blot analysis of HBcAg
expression
Cellular proteins were extracted using radio
immunoprecipitation buffer supplemented with phenylmethanesulfonyl
fluoride. Protein concentrations were determined using a
bicinchoninic acid assay protein concentration determination kit
(Beyotime Institute of Biotechnology, Shanghai, China). Equal
quantities of sample were separated using 10% SDS-polyacrylamide
gel electrophoresis and transferred to polyvinylidene fluoride
membranes. The membranes were incubated with monoclonal mouse
anti-HBcAg (6D1D10E4; Huazhong University of Science and
Technology, Wuhan, China; diluted 1:150) or monoclonal rabbit
anti-β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA;
diluted 1:2,500). Following incubation, the membranes were washed
three times, and goat anti-rabbit or goat anti-mouse secondary
antibody (Santa Cruz Biotechnology, Inc.; diluted 1:2,500) was
added for 1 h. The membranes were washed three times with
Tris-buffered saline and Tween 20 buffer, and signals were detected
using the Enhanced Chemiluminescence Detection system (Pierce
Biotechnology, Inc., Rockford, IL, USA).
Statistical analysis
The data are presented as the mean ± standard
deviation of at least three independent experiments. Statistical
significance was determined by Student’s t-test. The differences
between groups were assessed by one-way analysis of variance using
the Bonferroni post hoc test and P≤0.05 was considered to indicate
a statistically significant difference. All analyses were performed
using the Statistical Package for the Social Sciences statistical
software for Windows, version 10.1.4 (SPSS, Inc., Chicago, IL,
USA).
Results
Effect of epirubicin on the viability of
stable HBV-expressing cell lines
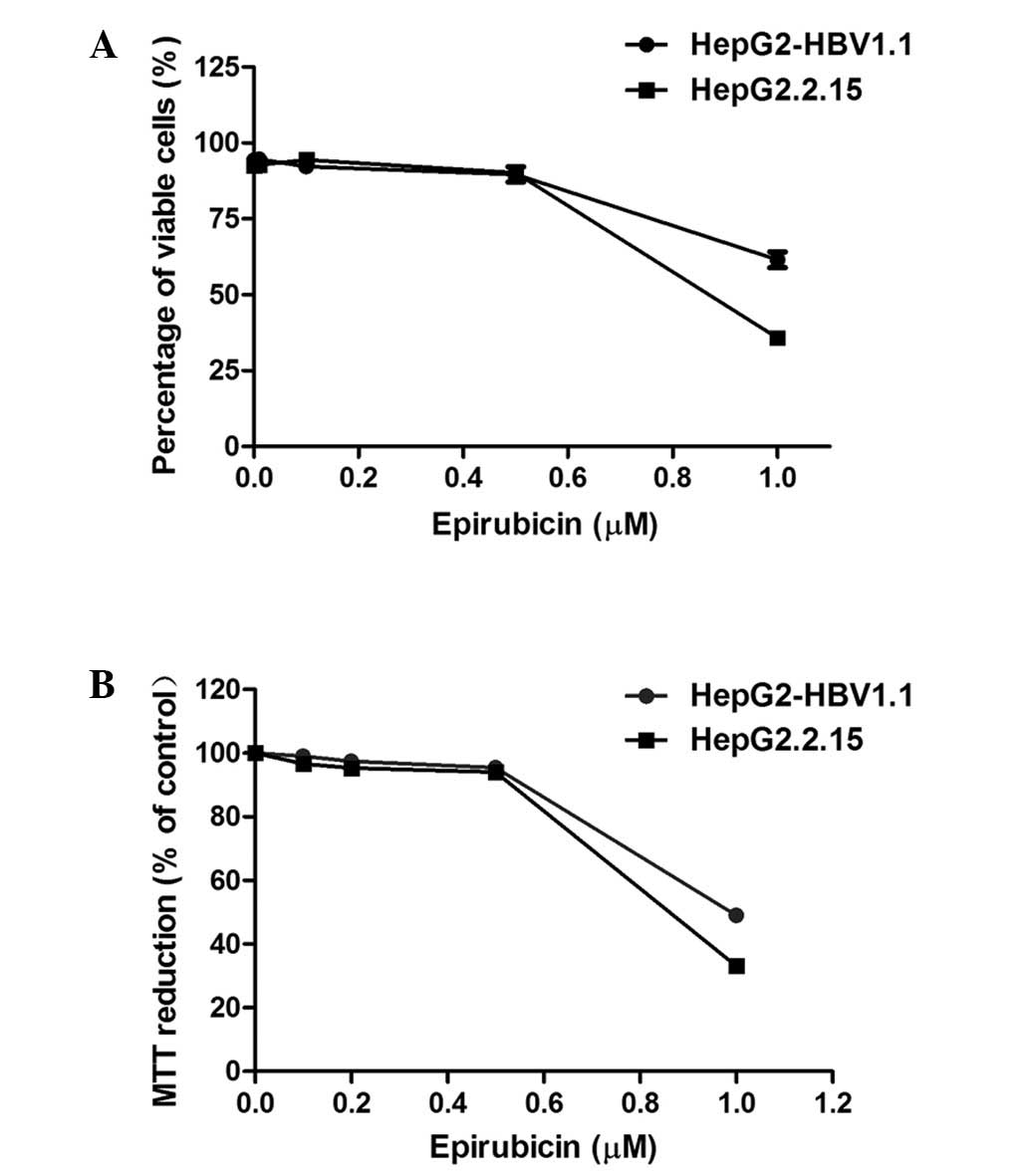
Epirubicin, as a cytotoxic chemotherapy agent,
induces cell apoptosis or necrosis at high concentrations (8,9),
which may be associated with HBV replication. The aim of the
present study was to investigate whether epirubicin promotes HBV
replication at lower concentrations. The cytotoxicity of epirubicin
on HepG2.2.15 and HepG2-HBV1.1 cells was examined using trypan blue
exclusion and MTT assays. From the results of the two assays
(Fig. 1), 0.5 μM epirubicin was
revealed to exhibit no significant cytotoxicity to either
HepG2.2.15 or HepG2-HBV1.1 cells. Therefore, in subsequent
experiments, 0.5 μM served as the working concentration of
epirubicin.
HBV DNA and pgRNA levels increase
concentration-dependently in stable HBV-expressing cell lines
following epirubicin treatment
HepG2.2.15 (10)
and HepG2-HBV1.1 (11) cells were
employed as stable HBV-expressing cell lines. HepG2.2.15
(4×105 cells/well) and HepG2-HBV1.1 (6×105
cells/well) cells were treated with epirubicin at various
concentrations (0–0.5 μM) for 24 h, followed by 48 h incubation in
drug-free culture medium. Intracellular HBV nucleocapsids were
extracted at 72 h after drug treatment, and viral replication
intermediates were subsequently measured by fluorescent qPCR and
Southern blot analysis. The number of HBV DNA copies was
concentration-dependently upregulated upon epirubicin stimulation
(Fig. 2A). The number of
intracellular HBV DNA copies increased 6.35±0.49- and
11.26±1.31-fold in the HepG2.2.15 and HepG2-HBV1.1 cells,
respectively, with 0.5 μM epirubicin treatment. Similarly, the
concentration-dependent effect of epirubicin on HBV replication in
the cells was confirmed by Southern blot analysis (Fig. 2B). The HBV DNA was extracted from
the supernatant of the culture medium following precipitation by
PEG 8000 and analyzed using qPCR (Fig.
2C) and Southern blotting (Fig.
2D). Epirubicin upregulated the levels of HBV DNA in the
supernatant in a concentration-dependent manner. Additionally, 0.5
μM epirubicin increased the secretion of HBV DNA copies by
6.30±0.57- and 6.31±1.17-fold in the HepG2.2.15 and HepG2-HBV1.1
cells, respectively. To further determine the effects of epirubicin
on the levels of HBV RNA, the levels of HBV pgRNA were measured by
qPCR using primers targeting the 3.5 kb mRNA of the viral genome.
The levels of HBV pgRNA increased markedly with epirubicin
treatment (Fig. 3).
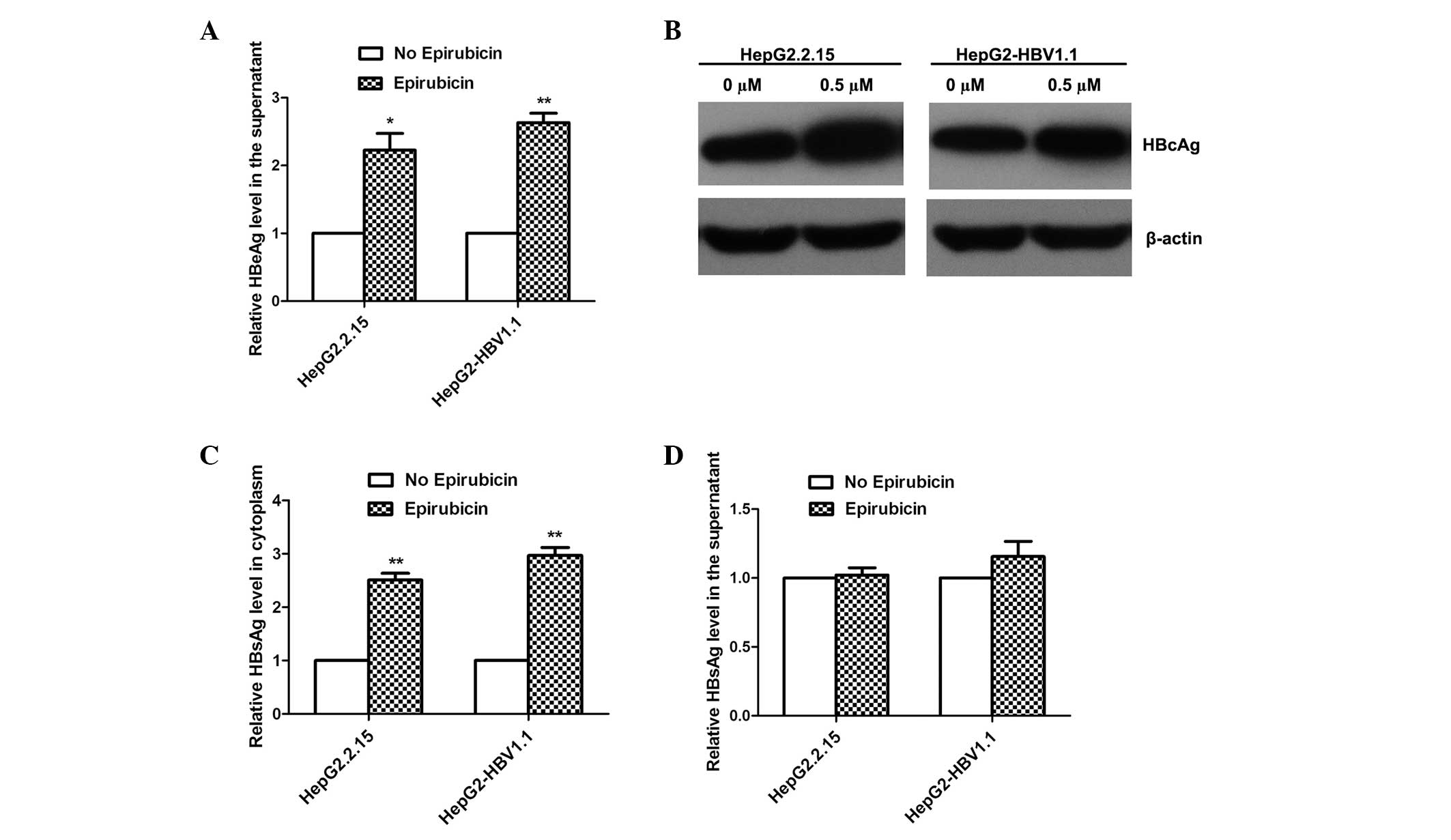
Epirubicin promotes HBV protein
expression
To elucidate the effects of epirubicin on the HBV
proteins expressed, the levels of HBeAg, HBcAg and HBsAg were
detected by ELISA and western blot analysis. With 0.5 μM epirubicin
treatment, the levels of HBeAg in the supernatant were upregulated,
as detected by ELISA (Fig. 4A),
and the levels of HBcAg expression in the cytoplasm increased
markedly, as detected by western blot analysis (Fig. 4B). Notably, epirubicin increased
the levels of HBsAg expression in the cytoplasm (Fig. 4C), while the levels of HBsAg
secretion were not markedly increased (Fig. 4D).
Epirubicin promotes the excretion of HBV
nucleocapsids in stable HBV-expressing cells
To investigate why the levels of HBsAg secretion did
not increase with epirubicin treatment, the HBcAg levels in the
supernatant were detected by ELISA. As the HBV virus cannot secrete
free HBcAg into the culture medium, HBcAg in the supernatant can
only be from HBV Dane particles or HBV nucleocapsids. Therefore,
the HBcAg in the supernatant represents the levels of HBV Dane
particles or nucleocapsids. However, no significant change in the
levels of Dane particles in the supernatant was identified
following treatment with epirubicin, although the overall levels of
HBcAg from Dane particles and nucleocapsids in the supernatant
markedly increased following 0.5 μM epirubicin treatment (Fig. 5). This result suggests that
epirubicin induced HepG2-HBV1.1 cells to excrete HBV nucleocapsids
instead of HBV Dane particles.
Discussion
HBV reactivation is an emerging clinical challenge
in HBV carriers receiving anticancer chemotherapy. All types of
anticancer drugs used in chemotherapy have been associated with HBV
reactivation, including the classic cytostatics, monoclonal
antibodies and steroids (12). The
rate of HBV reactivation is highest in patients with breast cancer
(41–56%; 2,13) and lymphoma (24–67%; 14,15). This high incidence
may be explained in part by the intensive chemotherapy required to
treat the diseases.
Epirubicin is one of the most commonly used drugs to
treat breast cancer and lymphoma, particularly in patients who have
undergone surgery to remove the tumor. Similar to other cytotoxic
drugs, epirubicin exhibits immunosuppressive activity, which is
considered to be the main mechanism of HBV reactivation. However,
there may be other mechanisms responsible for the HBV reactivation
caused by cytotoxic anticancer drugs in addition to
immunosuppression.
In the present study, the effects of epirubicin on
HBV replication in HepG2.2.15 and HepG2-HBV1.1 cells were observed.
The number of HBV DNA copies was concentration-dependently
increased by epirubicin in the cytoplasm and the supernatant of the
culture medium. Furthermore, the levels of intracellular HBsAg and
HBcAg, and secreted HBeAg also increased following epirubicin
treatment. However, epirubicin did not markedly affect the levels
of HBsAg secretion. This result signified that the abundant
secretion of HBV DNA copies stimulated by epirubicin was not
extracted from HBV Dane particles, as HBsAg is the main component
of the outer layer of the particles. By detection of the levels of
HBcAg in the supernatant of the cell culture, it was demonstrated
that epirubicin promoted the cells to excrete HBV nucleocapsids
instead of Dane particles. This corresponds with the lack of
significant change in the levels of HBsAg secretion following
epirubicin treatment. These results indicate that epirubicin
increased the levels of HBV DNA and RNA production, and protein
expression but did not affect the levels of secreted virus
particles. Similar to this phenomenon, corticosteroids have been
demonstrated to increase the total intracellular levels of HBV DNA,
RNA and HBsAg without affecting the levels of secreted HBsAg in
previous studies (16,17).
HBV nucleocapsids are closely associated with HBV
pathogenesis. The outer layer of HBV nucleocapsids consists of
HBcAg, which is the main factor responsible for HBV-associated
acute liver failure (ALF). Studies have suggested that HBcAg is
able to directly activate B cells to produce specific antibodies
(IgG1 and IgM anti-HBc), without the aid of T lymphocytes. This
implicates the importance of B cell immunity in the pathogenesis of
HBV-associated ALF (18,19). In the present study, epirubicin
stimulated stable HBV-expressing cells to excrete HBV
nucleocapsids, which may be the principle cause of the severe liver
damage induced by cytotoxic anticancer drugs.
It has been confirmed that the recovering immune
system following withdrawal of cytotoxic drugs plays a role in
liver damage. However, the mechanisms for the marked increase in
the levels of HBV replication in the early stages of anticancer
treatment remain unclear. In the current study, epirubicin
increased the levels of HBV DNA, RNA and protein expression, and
directly promoted HBV nucleocapsid secretion under cytotoxic
stress. This may be a novel mechanism of HBV reactivation in HBV
carriers receiving anticancer chemotherapy. One possible link
between cytotoxic stresses and activation of HBV replication is
that cell cycle arrest may be induced by cytotoxic drugs (20–22).
The present study also demonstrated that the cell cycle was
inhibited at the G2/M-phase by epirubicin (data not shown). Further
studies are required to confirm these hypotheses.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81201282 and 30901280),
Chongqing Natural Science Foundation (grant no. cstc2012jjA10047)
and the Ph.D. Programs Foundation of Ministry of Education of China
(grant no. 20125503120004).
References
|
1
|
Liu CJ, Chen PJ, Chen DS and Kao JH:
Hepatitis B virus reactivation in patients receiving cancer
chemotherapy: natural history, pathogenesis, and management.
Hepatol Int. 7:316–326. 2013. View Article : Google Scholar
|
|
2
|
Lubel JS and Angus PW: Hepatitis B
reactivation in patients receiving cytotoxic chemotherapy:
diagnosis and management. J Gastroenterol Hepatol. 25:864–871.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tur-Kaspa R, Burk RD, Shaul Y and Shafritz
DA: Hepatitis B virus DNA contains a glucocorticoid-responsive
element. Proc Natl Acad Sci USA. 83:1627–1631. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liaw YF: Hepatitis viruses under
immunosuppressive agents. J Gastroenterol Hepatol. 13:14–20. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang
MC, Tsao CJ, Kao WY, Uen WC, Hsu CH, Tien HF, et al; Lymphoma
Committee of Taiwan Cooperative Oncology Group. Steroid-free
chemotherapy decreases risk of hepatitis B virus (HBV) reactivation
in HBV-carriers with lymphoma. Hepatology. 37:1320–1328. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang JP, Vierling JM, Zelenetz AD, Lackey
SC and Loomba R: Hepatitis B virus management to prevent
reactivation after chemotherapy: a review. Support Care Cancer.
20:2999–3008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nassal M: The arginine-rich domain of the
hepatitis B virus core protein is required for pregenome
encapsidation and productive viral positive-strand DNA synthesis
but not for virus assembly. J Virol. 66:4107–4116. 1992.PubMed/NCBI
|
|
8
|
Muscarella DE and Bloom SE: The
contribution of c-Jun N-terminal kinase activation and subsequent
Bcl-2 phosphorylation to apoptosis induction in human B-cells is
dependent on the mode of action of specific stresses. Toxicol Appl
Pharmacol. 228:93–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu X, Fu A and Luo KQ: A high-throughput
fluorescence resonance energy transfer (FRET)-based endothelial
cell apoptosis assay and its application for screening vascular
disrupting agents. Biochem Biophys Res Commun. 418:641–646. 2012.
View Article : Google Scholar
|
|
10
|
Sells MA, Chen ML and Acs G: Production of
hepatitis B virus particles in Hep G2 cells transfected with cloned
hepatitis B virus DNA. Proc Natl Acad Sci USA. 84:1005–1009. 1987.
View Article : Google Scholar
|
|
11
|
Zhang Z, Liu X, Chen J, Su H, Luo Q, Ye J,
Tang N, Zhang W, Chen W, Ko BC and Huang A: Heparin sulphate
D-glucosaminyl 3-O-sulfotransferase 3B1 plays a role in HBV
replication. Virology. 406:280–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manzano-Alonso ML and Castellano-Tortajada
G: Reactivation of hepatitis B virus infection after cytotoxic
chemotherapy or immunosuppressive therapy. World J Gastroenterol.
17:1531–1537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai MS, Wu PF, Shyu RY, Lu JJ and Chao TY:
Hepatitis B virus reactivation in breast cancer patients undergoing
cytotoxic chemotherapy and the role of preemptive lamivudine
administration. Liver Int. 24:540–546. 2004. View Article : Google Scholar
|
|
14
|
Yeo W, Zee B, Zhong S, Chan PK, Wong WL,
Ho WM, Lam KC and Johnson PJ: Comprehensive analysis of risk
factors associating with Hepatitis B virus (HBV) reactivation in
cancer patients undergoing cytotoxic chemotherapy. Br J Cancer.
90:1306–1311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeo W, Chan PK, Zhong S, Ho WM, Steinberg
JL, Tam JS, Hui P, Leung NW, Zee B and Johnson PJ: Frequency of
hepatitis B virus reactivation in cancer patients undergoing
cytotoxic chemotherapy: a prospective study of 626 patients with
identification of risk factors. J Med Virol. 62:299–307. 2000.
View Article : Google Scholar
|
|
16
|
Lau JY, Bain VG, Smith HM, Alexander GJ
and Williams R: Modulation of hepatitis B viral antigen expression
by immunosuppressive drugs in primary hepatocyte culture.
Transplantation. 53:894–898. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McMillan JS, Shaw T, Angus PW and
Locarnini SA: Effect of immunosuppressive and antiviral agents on
hepatitis B virus replication in vitro. Hepatology. 22:36–43.
1995.PubMed/NCBI
|
|
18
|
Farci P, Diaz G, Chen Z, Govindarajan S,
Tice A, Agulto L, Pittaluga S, Boon D, Yu C, Engle RE, et al: B
cell gene signature with massive intrahepatic production of
antibodies to hepatitis B core antigen in hepatitis B
virus-associated acute liver failure. Proc Natl Acad Sci USA.
107:8766–8771. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu W, Chen Z, Cheng N, Watts NR, Stahl SJ,
Farci P, Purcell RH, Wingfield PT and Steven AC: Specificity of an
anti-capsid antibody associated with Hepatitis B Virus-related
acute liver failure. J Struct Biol. 181:53–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Casavant NC, Luo MH, Rosenke K,
Winegardner T, Zurawska A and Fortunato EA: Potential role for p53
in the permissive life cycle of human cytomegalovirus. J Virol.
80:8390–8401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang YQ, Wang LW, Yan SN and Gong ZJ:
Effects of cell cycle on telomerase activity and on hepatitis B
virus replication in HepG2 2.2.15 cells. Hepatobiliary Pancreat Dis
Int. 3:543–547. 2004.PubMed/NCBI
|
|
22
|
Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen
L, Wu E, Ye X, Gao GF, Wang F, et al: Loss of microRNA 122
expression in patients with hepatitis B enhances hepatitis B virus
replication through cyclin G(1) -modulated P53 activity.
Hepatology. 55:730–741. 2012. View Article : Google Scholar
|