Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignant tumors worldwide and also demonstrates an
increasing incidence and mortality, with a poor prognosis that
limits the long-term survival rate of patients (1). In addition, the majority of patients are
in the middle to late stage of disease at the time of diagnosis and
have lost the opportunity for successful treatment (2). Although various treatments are
improving, atypical clinical features and an intrinsic
chemoresistance mechanism continue to limit the long-term survival
of patients with HCC (3). Numerous
efforts have been made to improve the sensitivity of HCC to
chemotherapy. However, few attempts are successful (4) and the resistance mechanism requires
additional study.
The optimal microRNA (miRNA) for clinical
application is required to be involved in multiple behaviors in HCC
development, such as cell proliferation, apoptosis and
chemoresistance. The mature miRNA-34a-5p, which is well known as a
tumor suppressor in hepatitis virus-associated HCC, plays an
important role in cell processes like proliferation and apoptosis,
which made it an optimal biomarker for future clinical usage
(5–8).
Low-expression miRNA-34a-5p was reported to be associated with
certain characteristics and an unfavorable clinical outcome in a
small number of studies (9,10). In addition, miRNA-34a demonstrated an
enhanced anti-proliferative ability by targeting c-met or B-cell
lymphoma-2 (Bcl-2) in HCC cells (9,10).
However, the clinical importance of miRNA-34a-5p in processes such
as chemoresistance has yet to be identified and the specific map of
the mechanism associated with chemoresistance requires
elucidation.
Therefore, the present study aimed to evaluate the
significance of miRNA-34a-5p in patients with HCC that received
radical surgery, and also aimed to identify the mechanism behind
these effects, which include alterations in cell proliferation,
apoptosis and chemoresistance. In addition, the current study
attempted to identify the critical pathway and target gene of
miRNA-34a-5p in patients with HCC.
Materials and methods
Patients
Between September 2009 and September 2012, the
present study investigated a consecutive series of 114 HCC patients
receiving radical surgery in the Cancer Center of Sun Yat-sen
University (Guangzhou, Guangdong, China). The diagnosis of HCC was
universally confirmed by pathology. In total, 114 patients were
followed-up prior to mortality or to the most recent follow-up on
October 30, 2013. The overall survival (OS) time was defined as the
time from diagnosis to the date of death, or to the most recent
follow-up if the patient remained alive. The progression-free
survival (PFS) time was defined as the time between diagnosis and
the date of local failure or distant metastasis, or the time
between diagnosis and mortality or date of the most recent
follow-up if progression did not occur. The present study was
approved by the Clinical Ethics Review Boards of the Third
Affiliated Hospital and Cancer Center of Sun Yat-sen University.
Written informed consent was obtained from all patients at the time
of admission.
Chemicals and reagents
Primary antibodies against AXL (rabbit anti-human
monoclonal; cat. no. 4939; 1:1,000), c-Jun N-terminal protein
kinase (JNK; Rabbit anti-human polyclonal; cat. no. 9252; 1:1,000),
phosphorylated JNK (p-JNK; rabbit anti-human polyclonal; cat. no.
9251; 1:1,000), Bcl-2 (mouse anti-human monoclonal; cat. no. 15071;
1:1,000), phosphatidylinositol 3-kinase (PI3K; rabbit anti-human
monoclonal; cat. no. 11889; 1:1,000), AKT, phosphorylated AKT
(p-AKT; rabbit anti-human polyclonal; cat. no. 9272; 1:1,000),
caspase-3 (rabbit anti-human monoclonal; cat. no. 9665; 1:1,000)
and cleaved caspase-3 (rabbit anti-human monoclonal; cat. no. 9661;
1:1,000) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), and the β-actin antibody (mouse anti-human
polyclonal; cat. no. sc-44478; 1:1,000) was purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). The HRP-conjugated
anti-rabbit IgG (cat. no. 7054, 1:5,000) secondary antibodies were
purchased from Cell Signaling Technology, Inc. The cell-culture
reagent Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine
serum (FBS) were obtained from (Thermo Fisher Scientific Inc.,
Waltham, MA, USA).
Cell lines and cell culture
The human HCC MHCC-97L cell line used in the present
study was purchased from the Cancer Research Institute of Zhongshan
Hospital of Fudan University (Shanghai, China). The cells were
cultured in high-glucose DMEM supplemented with heat-inactivated
10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator containing a 5%
CO2 atmosphere.
Tissue array by in situ hybridization
(ISH)
The expression profile of miRNA-34a-5p was examined.
A total of 180 oligonucleotide DNA probes were used for the
detection of human miRNA-34a-5p. Each sample was independently
hybridized to one array. Hybridization signals were detected using
a DNA chip image analyzer (dChip software, http://www.hsph.harvard.edu/cli/complab/dchip/).
The hybridized signal intensities were normalized to the
intensities of synthetic oligonucleotide DNA probes. Finally, the
normalized data from each sample were graded by expression levels
relative to the median.
Semi-quantitative assessment
The expression level of miRNA-34a-5p was tested by
integrating the percentage of stained cancer cells and the
intensity of the staining. The intensity of staining was scored as
follows: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. The extent of the staining was scored
according to the percentage of stained cells in the field of view,
as follows: 0, no staining; 1, 0–25% stained cells; 2, 26–50%
stained cells; 3, 51–75% stained cells; and 4, 76–100% stained
cells. The intensity score multiplied by the extent score was
considered to be the overall ISH score. The ISH staining level was
assessed and scored by two independent pathologists that were
blinded to the clinicopathological and follow-up data of the
samples.
Cell transfection
The miRNA-34a-5p mimics
(5′-UGGCAGUGUCUUAGCUGGUUGU-3′), miRNA-34a-5p inhibitor
(5′-ACAACCAGCUAAGACACUGCCA-3′) and negative control miRNA (NC;
5′-CAGUACUUUUGUGUAGUACAA-3′) were synthesized by Guangzhou RiboBio,
Co., Ltd. (Guangzhou, Guangdong, China). miRNA-34a-5p was
overexpressed in MHCC-97L cells using miRNA-34a-5p mimics. The
expression of miRNA-34a-5p was knocked down in MHCC-97L cells using
miRNA-34a-5p inhibitors. The cells were seeded onto a six-well
plate at a density of 2.5×105 cells/well 24 h prior to
transfection. The cells were then transfected with 20 nM
miRNA-34a-5p mimics or 40 nM inhibitors using Lipofectamine RNAiMax
(Invitrogen) according to the manufacturer's instructions. The
cells were harvested for further analysis 48 h subsequent to
transfection. The small interfering (si)RNA for AXL (siAXL) was
synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China),
according to the two designed pairs of siAXL1 and siAXL2 siRNA, and
the transfection was performed according to the manufacturer's
instructions. Three independent experiments were performed.
Cell proliferation assay
Cell proliferation assays were performed using Cell
Counting Assay. The MHCC-97L cells were seeded onto six-well plates
in triplicate at a density of ~3×105 cells/well and
cultured in the growth medium. Subsequent to 24, 48 and 72 h of
culturing, the numbers of cells per well were measured by counting
the total cells using a hemocytometer and a microscope (DMI 4000B;
Leica Microsystems GmbH, Wetzlar, Germany).
Apoptosis assay
The cells were plated into six-well plates at a
density of 3×105 cells/well, and were transfected with
miRNA-34a-5p mimics or miRNA-34a-5p inhibitors using Lipofectamine
RNAiMax. The MHCC-97L cells were treated with cisplatin at a final
concentration of 5 µg/ml, and 12 h after the administration of
cisplatin the cells were collected and stained with Annexin V-FITC
and PI (cat. no. KGA108; Nanjing KeyGen Biotech Co., Ltd., Nanjing,
Jiangsu, China). Flow cytometry was then performed to detect the
apoptosis level of the transfected cells.
Luciferase reporter assay
For assays involving the full-length AXL
3′-untranslated region, the MHCC-97L cells were seeded at a density
of 10,000 cells per well in a 96-well culture plate (Corning, Inc.,
Corning, NY, USA) and incubated for 24 h prior to transfection. In
total, 1 µg of the expression vector was cotransfected with
negative control siRNA (siNC) or the human (h-)miRNA-34a-5p mimic
(5′-UGGCAGUGUCUUAGCUGGUUGU-3′) at a final concentration of 10 nM
using Lipofectamine RNAiMax. The expression levels of firefly and
Renilla luciferase were each measured 24-h post-transfection
on a modulus microplate reader using the Luciferase Assay system
(GeneCopoeia, Rockville, MD, USA). To generate the wild-type and
mutant seed sequence expression constructs, the expression of
Renilla and firefly luciferase was measured 48 h
post-transfection in the MHCC-97L cells, with 25,000 cells per
well, using the Dual-Luciferase Reporter Assay System (Promega,
Madison, WI, USA). The mean ± standard error of the mean is
reported for each transfection condition (n=3). The statistical
significance of differences between the groups was determined by a
two-tailed unpaired t-test for the means of the cells
transfected with siNC, wild-type mimic and mutant mimic
constructs.
Western blot analysis
Western blot analysis was performed to assess AXL
and β-actin expression. Protein extracts from cultured cells were
prepared by suspending cells in lysis buffer containing 0.01% EDTA,
0.1% Triton X-100 and 10% proteinase inhibitor. Protein
concentrations were quantified using a protein assay kit (KGPBCA;
Nanjing KeyGen Biotech Co., Ltd.). Briefly, 50 mg of lysates were
separated on 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes. The membranes were probed overnight at 4°C
with primary antibodies against human AXL (dilution, 1:1,000) and
β-actin (dilution, 1:1,000), followed by incubation with secondary
antibodies (Cell Signaling Technology, Inc.) at a 1:5,000 dilution
for 1 h. The signal was visualized using enhanced
chemiluminescence.
Statistical analysis
All assays were performed in triplicate. The data
are expressed as the mean ± standard deviation. The statistical
analyses were performed using Student's t-test. P<0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS software, version
20.0 (IBM, Armonk, NY, USA).
Results
The expression of miRNA-34a-5p in HCC
tissues was significantly decreased compared with the
pericarcinomatous areas of the tissue chips
To investigate the expression of miRNA-34a-5p in
hepatocellular carcinoma, the tissue chips consisted of pairs of
malignant and pericarcinomatous tissues that were derived from
tumor samples of 114 patients with HCC that underwent radical
surgery. An ISH assay was conducted to test the levels of
miRNA-34a-5p in the tissue chip. The expression of miRNA-34a-5p in
hepatocellular carcinoma tissues was reduced compared with
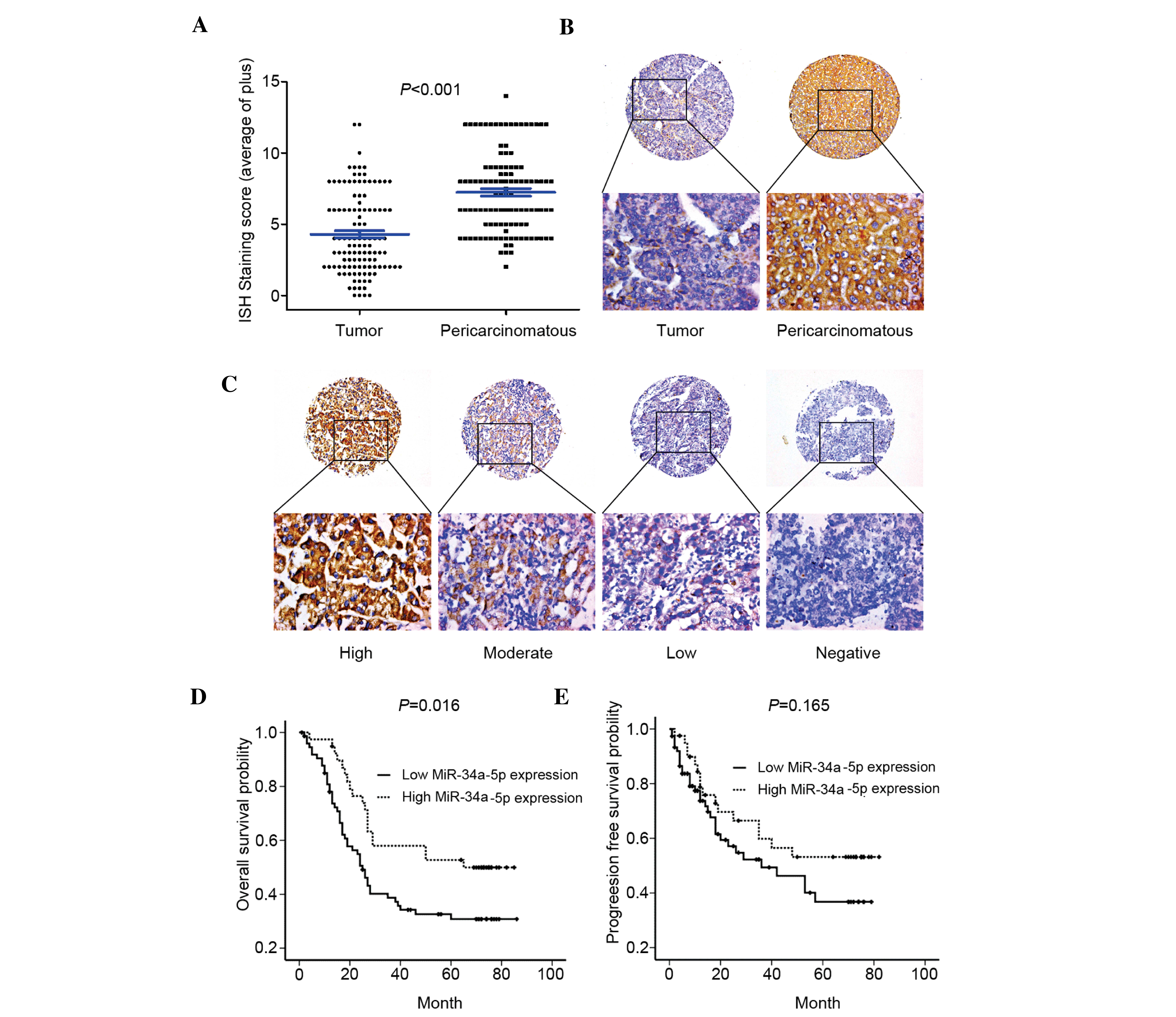
peritumorial tissues (P<0.01; Fig. 1A
and B), which was illustrated by the finding that the average
staining scores for tumor and pericarcinomatous tissues were 4.28
and 7.23, respectively (P<0.001; Table
I).
 | Table I.Expression level of miRNA-34a-5p in
human Hepatocellular carcinoma tissue chip. |
Table I.
Expression level of miRNA-34a-5p in
human Hepatocellular carcinoma tissue chip.
| Expression of
miRNA-34a-5p | Staining score | P-value |
|---|
| Tumor | 4.28 (1.46–7.10) | <0.001 |
|
Pericarcinomatous | 7.23
(4.40–10.06) |
|
High expression of miRNA-34a-5p was
associated with a favorable OS time in patients with HCC
miRNA-34a-5p demonstrated a heterogeneous expression
level in HCC tissues (Fig. 1C). In
order to identify the association between miRNA-34a-5p and the
clinical outcome, the expression level of miRNA-34a-5p was
dichotomized into two categories: the cutoff value of the receiver
operating characteristic curve were used to separate the relative
expression levels of miRNA-34a-5p into low expresshigher and lower
expression groups. Subsequently, Kaplan-Meier analysis revealed
that lower expression of miRNA-34a-5p significantly decreased the
OS time (P=0.016; Fig. 1D) without
influencing the PFS time (P=0.165; Fig.
1F) in HCC patients.
Furthermore, multivariate Cox regression analysis
demonstrated that the expression levels of serum α-fetoprotein
[P=0.01; hazard ratio (HR), 2.841] and miRNA-34a-5p (P=0.038; HR,
0.551) were independent prognostic biomarkers for the prediction of
OS time. Other characteristics, consisting of age, gender, tumor
size, tumor multiplicity, histological grades, stage, liver
cirrhosis, HBV infection and vascular invasion, were not
significantly associated with OS time (Table II).
 | Table II.Multivariate Cox proportional-hazards
analysis in the overall HCC patients. |
Table II.
Multivariate Cox proportional-hazards
analysis in the overall HCC patients.
|
| Overall survival |
|---|
|
|
|
|---|
| Variable | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
| <50
years vs. ≥50 years | 1.355 | 0.810–2.266 | 0.248 |
| Gender |
|
|
|
| Male vs.
female | 0.914 | 0.351–2.380 | 0.853 |
| Tumor size |
|
|
|
| <5 cm
vs. ≥5 cm | 1.342 | 0.767–2.349 | 0.302 |
| Tumor
multiplicity |
|
|
|
| Single
vs. mutiple | 2.514 | 0.981–6.443 | 0.055 |
| Histological
grade |
|
|
|
| Poor
vs. moderate and well | 1.029 | 0.798–1.328 | 0.824 |
| Stage |
|
|
|
| I + II
vs. III + IV | 0.990 | 0.566–1.730 | 0.971 |
| HBV infection |
|
|
|
|
Negative vs. positive | 1.013 | 0.400–2.567 | 0.979 |
| Liver
cirrhosis |
|
|
|
|
Negative vs. positive | 1.070 | 0.588–1.949 | 0.824 |
| Vascular
invasion |
|
|
|
|
Negative vs. positive | 1.556 | 0.685–3.536 | 0.291 |
| Serum AFP |
|
|
|
| <20
µg/l vs. ≥20 µg/l | 2.841 | 1.517–5.322 | 0.001 |
| miRNA-34a-5p
expression |
|
|
|
| Low vs.
high | 0.551 | 0.314–0.968 | 0.038 |
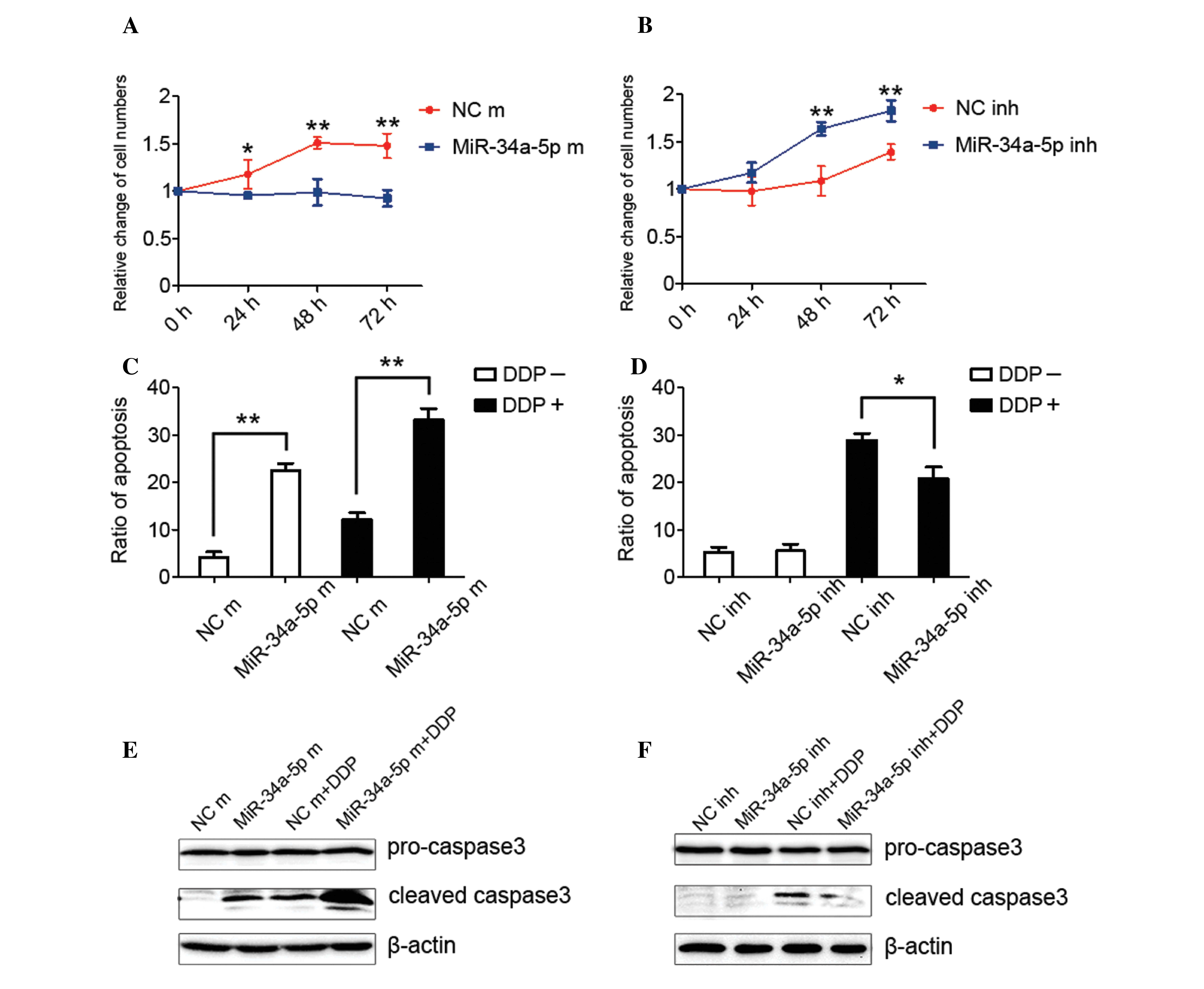
miRNA-34a-5p inhibited proliferation
of the HCC cell line
In order to identify the role of miRNA-34a-5p in the
proliferation of HCC cells, miRNA-34a-5p mimics were transfected
into in the human MHCC-97L cell line. Cell counting illustrated
that cell proliferation was inhibited by forced expression of
miRNA-34a-5p, with the best inhibition effect being demonstrated at
48 h subsequent to transfection (P<0.05; Fig. 2A). In addition, the miRNA-34a-5p
inhibitor was transfected into in the human MHCC-97L cell line and
it was found that cell proliferation was significantly promoted
(P<0.05; Fig. 2B).
miRNA-34a-5p elevated cell apoptosis
and decreased the chemoresistance of HCC cells to cisplatin
Flow cytometry was utilized to assess the effects of
miRNA-34a-5p on cell apoptosis in the MHCC-97L cell line.
Transfection of miRNA-34a-5p mimics into the MHCC-97L cell line
significantly increased cell apoptosis by at least two-fold
(P<0.05; Fig. 2C). However,
transfection of miRNA-34a-5p inhibitors did not reduce the cell
apoptosis ratio, which was quite low. To further validate the
aforementioned results, cleaved caspase-3 was tested by western
blot analysis in the same setting. It was found that the expression
of cleaved caspase-3 was increased subsequent to transfection with
miRNA-34a-5p mimics (P<0.05; Fig.
2E). Consistent with the results of flow cytometry, inhibition
of miRNA-34a-5p in the MHCC-97L cells did not reduce the expression
level of cleaved caspase-3, which was evidently low (P<0.05;
Fig. 2F).
Cisplatin/DPP treatment enhanced apoptosis in the
MHCC-97L cells. Therefore, cisplatin-treated MHCC-97L cells were
used to determine the influence of miRNA-34a-5p on the
chemoresistance of HCC cells. Firstly, transfection of miRNA-34a-5p
mimics into the cisplatin-treated MHCC-97L cells significantly
increased the level of apoptosis (P<0.05; Fig. 2C). The apoptosis ratio of MHCC-97L
cells administered with cisplatin was consistently reduced through
inhibition of miRNA-34a-5p (P<0.05; Fig. 2D). The findings of the western blot
analysis further confirmed these results. The levels of the
apoptosis protein activated caspase-3 were elevated by treatment
with cisplatin. This elevation was enhanced by transfecting cells
with miRNA-34a-5p mimics. By contrast, the levels of cleaved
caspase-3 were decreased by transfection with the miRNA-34a-5p
inhibitor (P<0.05; Fig. 2D and
F).
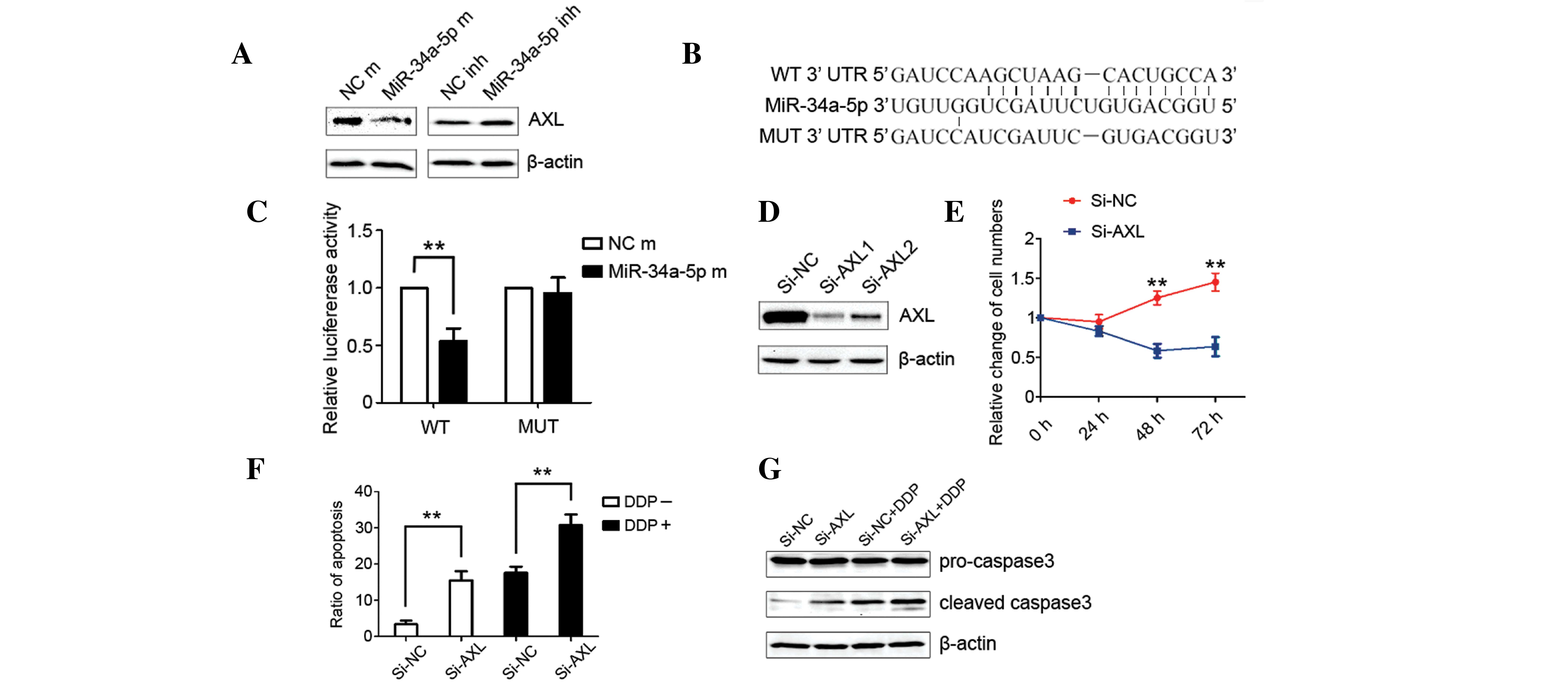
AXL was the direct target of
miRNA-34a-5p
In order to identify the specific target gene of
miRNA-34a-5p that mediates the promotion of the apoptosis ratio,
the Targetscan (Whitehead Institute for Biomedical Research,
Cambridge, MA, USA) and MiRbase (Griffiths-Jones Lab, University of
Manchester, Manchester, UK) databases were consulted and searched
for the relative gene of proliferation. Among the associated target
genes from the initial search were the AXL, PTPN18, CDKN1C and BAK
genes, which were considered to be the most likely target genes
involved in the miRNA-34a-5p-induced decrease in cell proliferation
and increase in apoptosis. Western blot analysis revealed that the
expression levels of PTPN18, CDKN1C and BAK were not downregulated
by transfection with miRNA-34a-5p mimics.
The expression of AXL was found to be significantly
reduced in the cells that were transfected with miRNA-34a-5p
mimics, while enhanced AXL expression was observed in the cells
that were treated with miRNA-34a-5p inhibitors (P<0.05; Fig. 3A). This potential direct interaction
between miRNA-34a-5p and AXL was further investigated by the
binding in situ analysis in the Targetscan, which revealed
that miRNA-34a-5p directly bound with wild-type AXL and lost this
capability when AXL was mutated (Fig.
3B). The luciferase reporter assay was then utilized to confirm
this direct interaction. The primers for mutant and wild-type AXL
were designed and 1 µg each of the expression vector and
h-miRNA-34a-5p mimic were co-transfected into the MHCC-97L cells.
The luciferase reporter assay illustrated that the luciferase
activity of wild-type AXL was decreased significantly when
co-transfected with h-miRNA-34a-5p mimics. The luciferase activity
of mutant AXL was also similar to that of the control group
(P<0.01; Fig. 3C).
miRNA-34a-5p modulates cell
proliferation, apoptosis and chemoresistance by targeting AXL
The influence of AXL on the proliferation and
apoptosis of MHCC-97L cells was evaluated to determine the
mediating role of AXL in the decreased cell proliferation and
increased apoptosis induced by miRNA-34a-5p. siAXL was transfected
into the MHCC-97L cell line and significantly reduced the
expression of AXL (P<0.05; Fig.
3D). Cell counting revealed that silencing AXL completely
suppressed cell proliferation (P<0.05; Fig. 3E). In addition, transfection with
siAXL increased the apoptosis ratio of MHCC-97L cells (P<0.05;
Fig. 3F). Notably, transfection of
cells with siAXL enhances the apoptosis induced by cisplatin. The
results of flow cytometry on cell apoptosis were further confirmed
by western blot analysis of the expression of cleaved caspase-3
(P<0.05; Fig. 3G). These results
were in accordance with the aforementioned biological behaviors
induced by the ectopic expression of miRNA-34a-5p.
miRNA-34a-5p may act through the
JNK-Bcl-2 signaling pathway
In order to reveal the signaling pathway by which
miRNA-34a-5p exerted anticancer effects in HCC cells, western blot
analysis was used to assess the alteration of several secondary
messengers in various signaling pathways. The present results
revealed that transfection with miRNA-34a-5p mimics significantly
increased the phosphorylation of JNK and decreased the levels of
Bcl-2. In addition, transfection with miRNA-34a-5p mimics
demonstrated little effect on the expression levels of PI3K and
p-AKT (P<0.05; Fig. 4). In
addition, silencing of AXL by transfection of cells with siAXL also
increased the levels of p-JNK and decreased the expression of
Bcl-2, without influencing the level of PI3K and p-AKT. All these
data indicate that miRNA-34a-5p may exert its anticancer function
by targeting AXL through the p-JNK-Bcl-2 signaling pathway.
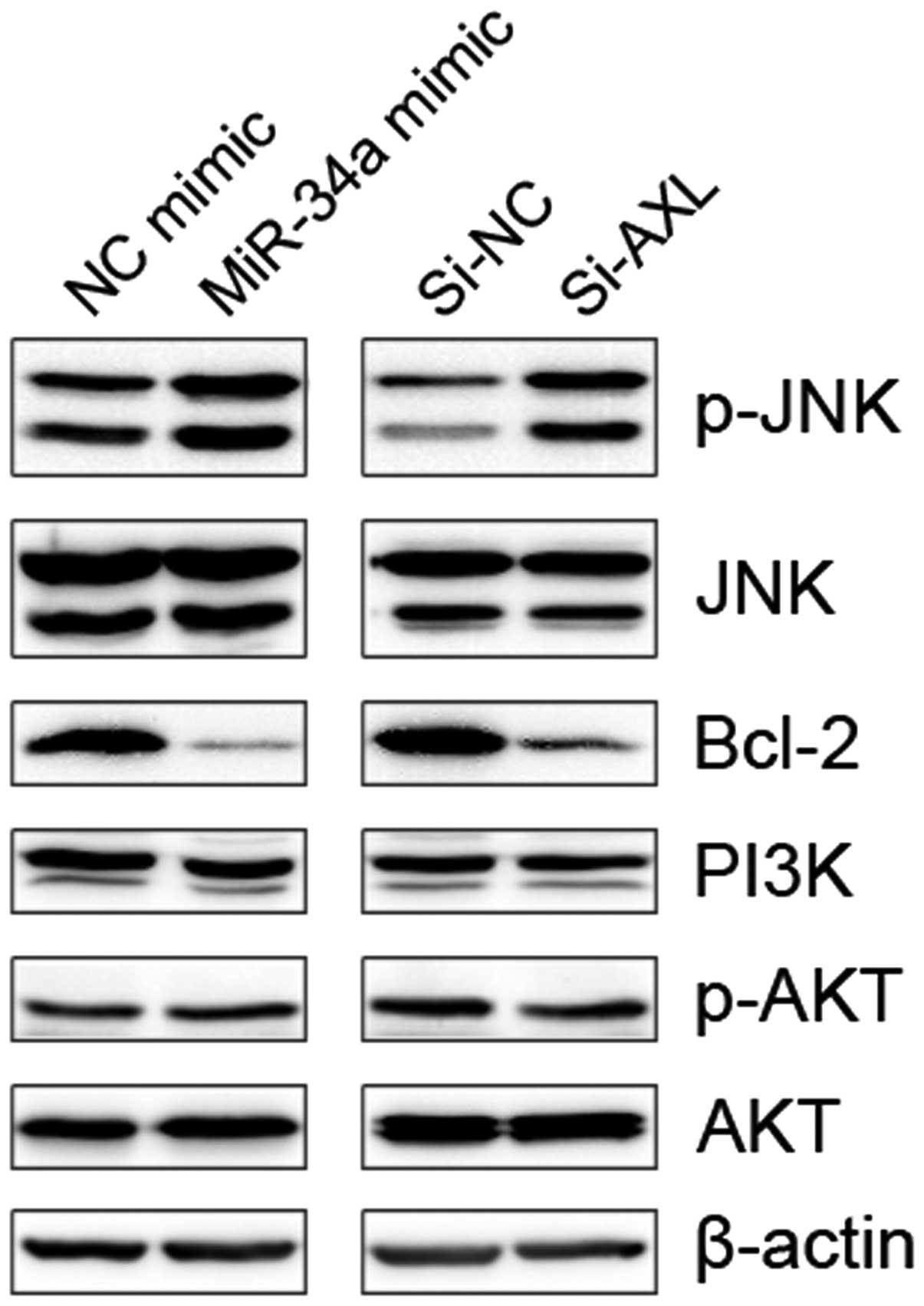 | Figure 4.miRNA-34a-5p may modulate the
chemosensitivity of HCC cells through the JNK-Bcl-2 signaling
pathway. Western blot analysis was performed to detect the
expression of JNK, AKT, PI3K and Bcl-2, as well as the
phosphorylation of JNK and AKT in HCC cells. NC, negative control;
miRNA-34a-5p, microRNA-34a-5p; miR-34a, miRNA-34a; Si-NC, small
interfering NC RNA; Si-AXL, small interfering RNA for AXL; JNK.
c-Jun N-terminal kinase; p-JNK, phosphorylated JNK; Bcl-2; B-cell
lymphoma 2; PI3K, phosphoinositide 3-kinase; p-AKT, phosphorylated
AKT; HCC, hepatocellular carcinoma. |
Discussion
An increasing quantity of evidence suggests that
miRNA-34a is a key mediator of p53 tumor suppression (11) that plays an important role in the
development of HCC. miRNA-34a demonstrates anticancer properties
through its action on the proliferation, apoptosis, invasion and
metastasis of HCC cells (5–8). However, no studies have investigated the
role of miRNA-34a in chemoresistance, which is a critical feature
of HCC and has led to the limited survival of patients with
advanced disease. The present study initially identified the role
of miRNA-34a-5p in the chemoresistance of HCC cells. miRNA-34a-5p
was found to significantly reduce the chemoresistance of HCC cells,
as apoptosis induced by cisplatin was increased by transfection of
cells with miRNA-34a-5p mimics. In addition, miRNA-34a has been
reported to sensitize HCC cells to sorafenib (10). The function of miRNA-34a-5p in
facilitating systematic therapy was confirmed at a clinical level
in the present study. Patients with various miRNA-34a expression
levels demonstrated similar PFS times, but different OS times,
which indicated that the impact of miRNA-34a on the prognosis of
HCC patients was mainly exerted subsequent to the recurrence of the
malignancy. Transcatheter arterial chemoembolization (TACE) and
systematic therapy, which was mainly chemotherapy using sorafenib,
were the main strategies for the treatment of advanced HCC
(3). Thus, sensitivity to
chemotherapy and sorafenib became a critical factor for the
prognosis of patients with HCC, in which miRNA-34a evidently played
an important role, as indicated by the present study and a previous
study by Yang et al (10).
The present results were in accordance with the
majority of the findings from previous studies. Previous studies
did not report consistent findings on the function of miRNA-34a.
Dang et al (9) reported
results that were consistent with the present findings that
miRNA-34a-5p decreased cell proliferation and increased apoptosis
in three HCC cell lines, which differed from the present cell lines
and consisted of the HepG2, HepB3 and SNU449 cell lines. However,
Li et al (6) did not identify
this function of miRNA-34a in HepG2 cells. In addition, Li et
al (6), Dang et al
(9) and the present study concluded
that downregulation of the expression of miRNA-34a was
significantly increased in HCC tissues. However, Pineau et
al (12) observed contrasting
results. The different sources of the samples and the various
assays performed may partly explain the discordance of the varying
results.
In the present study, the miRNA-34a/AXL pathway was
identified as a novel pathway leading to the chemoresistance of
HCC. AXL is a member of the Tyro3, AXL and MER receptor tyrosine
kinase family and it plays a diverse role in multiple cellular
processes, including survival, apoptosis and proliferation
(13), with the exception of
chemoresistance. In addition, previous studies on the function of
AXL in HCC were mainly focused on the oncogenesis, metastasis and
proliferation of the cells (14–18). In
the present study, it was found that transfection of HCC cells with
siAXL enhanced the apoptosis induced by cisplatin in HCC cells. The
interaction between miRNA-34a and AXL was initially identified in
breast cancer by Mackiewicz et al (19). Therefore, the miRNA-34a-AXL pathway
may play a significant role in HCC patients with a low expression
of miRNA-34a.
In the current study, it was found that the
miRNA-34a-AXL pathway mediated the phosphorylation of JNK and
expression of Bcl-2, which are each apoptosis-associated messengers
(20). Thus, the apoptosis effect
induced by the miRNA-34a-AXL pathway in HCC may be mediated by JNK
and Bcl-2. However, the roles of JNK and Bcl-2 in this biological
process have yet to be fully identified.
In conclusion, the present study identified that
miRNA-34a-5p enhanced the sensitivity of chemotherapy by targeting
AXL in hepatocellular carcinoma. Low expression of miRNA-34a-5p in
HCC tissues yielded an unfavorable OS time for patients with HCC
that received radical surgery by promoting proliferation and
chemoresistance in HCC cells.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 81372374), the
Combination Project of Production, Education and Research from
Guangdong Province and Ministry of Education (grant no.
2012B091100460), and the Science and Technology Planning Project of
Guangdong Province (grant nos. 2011B031800076, 2011B031800014 and
2012B031800259).
References
|
1
|
Jia CC, Wang TT, Liu W, et al:
Cancer-associated fibroblasts from hepatocellular carcinoma promote
malignant cell proliferation by HGF secretion. PLoS One.
8:e632432013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Dong M, Lin Q, et al: Comparison of
current staging systems for advanced hepatocellular carcinoma not
amendable to locoregional therapy as inclusion criteria for
clinical trials. Asia Pac J Clin Oncol. 9:86–92. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graf D, Vallböhmer D, Knoefel WT, et al:
Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med.
25:430–437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheng L, Xiong M, Li C and Meng X:
Reversing multidrug-resistant by RNA interference through silencing
MDR1 gene in human hepatocellular carcinoma cells subline
Bel-7402/ADM. Pathol Oncol Res. 20:541–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng J, Zhou L, Xie QF, et al: The impact
of miR-34a on protein output in hepatocellular carcinoma HepG2
cells. Proteomics. 10:1557–1572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li N, Fu H, Tie Y, et al: miR-34a inhibits
migration and invasion by down-regulation of c-Met expression in
human hepatocellular carcinoma cells. Cancer Lett. 275:44–53. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tryndyak VP, Ross SA, Beland FA and
Pogribny IP: Down-regulation of the microRNAs miR-34a, miR-127 and
miR-200b in rat liver during hepatocarcinogenesis induced by a
methyl-deficient diet. Mol Carcinog. 48:479–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang P, Li QJ, Feng Y, et al:
TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment
promotes venous metastases of HBV-positive hepatocellular
carcinoma. Cancer Cell. 22:291–303. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dang Y, Luo D, Rong M and Chen G:
Underexpression of miR-34a in hepatocellular carcinoma and its
contribution towards enhancement of proliferating inhibitory
effects of agents targeting c-MET. PLoS One. 8:e610542013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang F, Li QJ, Gong ZB, et al:
MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular
carcinoma cells to sorafenib treatment. Technol Cancer Res Treat.
13:77–86. 2014.PubMed/NCBI
|
|
11
|
Bommer GT, Gerin I, Feng Y, et al:
p53-mediated activation of miRNA34 candidate tumor-suppressor
genes. Curr Biol. 17:1298–1307. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pineau P, Volinia S, McJunkin K, et al:
miR-221 overexpression contributes to liver tumorigenesis. Proc
Natl Acad Sci USA. 107:264–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Linger RM, Keating AK, Earp HS and Graham
DK: TAM receptor tyrosine kinases: biologic functions, signaling,
and potential therapeutic targeting in human cancer. Adv Cancer
Res. 100:35–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He L, Zhang J, Jiang L, et al:
Differential expression of Axl in hepatocellular carcinoma and
correlation with tumor lymphatic metastasis. Mol Carcinog.
49:882–891. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HJ, Jeng YM, Chen YL, Chung L and Yuan
RH: Gas6/Axl pathway promotes tumor invasion through the
transcriptional activation of Slug in hepatocellular carcinoma.
Carcinogenesis. 35:769–775. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Jia L, Ma ZH, Ma QH, Yang XH and
Zhao YF: Axl glycosylation mediates tumor cell proliferation,
invasion and lymphatic metastasis in murine hepatocellular
carcinoma. World J Gastroenterol. 18:5369–5376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Jia L, Ma H, Li Y, Ma Z and Zhao Y:
Axl gene knockdown inhibits the metastasis properties of
hepatocellular carcinoma via PI3K/Akt-PAK1 signal pathway. Tumour
Biol. 35:3809–3817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu MZ, Chan SW, Liu AM, et al: AXL
receptor kinase is a mediator of YAP-dependent oncogenic functions
in hepatocellular carcinoma. Oncogene. 30:1229–1240. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mackiewicz M, Huppi K, Pitt JJ, Dorsey TH,
Ambs S and Caplen NJ: Identification of the receptor tyrosine
kinase AXL in breast cancer as a target for the human miR-34a
microRNA. Breast Cancer Res Treat. 130:663–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J and Lin A: Role of JNK activation in
apoptosis: A double-edged sword. Cell Res. 15:36–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|