Mechanism of isoflurane‑mediated breast cancer growth in vivo
- Authors:
- Published online on: April 30, 2024 https://doi.org/10.3892/ol.2024.14420
- Article Number: 287
Abstract
Introduction
The effect of anesthetics on cancer has been a topic of clinical interest. A number of retrospective studies reported that the use of volatile anesthetic-based general anesthesia was associated with higher incidence of cancer recurrence and worse survival compared to the use of intravenous anesthetic-based anesthesia (1–3). These observations triggered researchers to investigate the underlying mechanism of how anesthetics affect cancer. So far clear volatile anesthetic targets have not been shown in vivo.
Breast cancer is the most frequently diagnosed cancer and the cause of mortality among all cancers in females (4). Although spontaneous tumor growth models or xenogeneic tumor implantation models have been used to study breast cancer in mice (5), they already have immunologically altered background. Instead, EO771 tumor cell implantation congenic model allows us to study tumor growth in fully immunocompetent mice. EO771 cells are breast adenocarcinoma cells derived from a spontaneous mammary tumor from a female C57BL/6 mouse (6) and have been used for congenic breast cancer model (7). Now it is well recognized that anesthetics can affect leukocyte functions (8,9). Thus, using congenic model to study the effect of anesthetics on tumor growth would be logical.
In this study, we examined the impact of commonly used volatile anesthetic isoflurane on tumor growth and its underlying mechanism in vivo. We previously reported that volatile anesthetic isoflurane directly binds to and inhibits critical adhesion molecules leukocyte function-associated antigen-1 (LFA-1) (10–12) and macrophage-1 antigen (Mac-1) (13). LFA-1 and Mac-1 are members of β2 integrins, which belong to a heterodimeric adhesion molecule family consisting of α- and β2-subunits. LFA-1 is also called αLβ2 or CD11a/CD18 and ubiquitously expressed on leukocytes (14). Mac-1 is also called αMβ2, CD11b/CD18 or complement receptor 3 (CR3) and expressed primarily on myeloid cells (15). Thus, we also examined the role of LFA-1 and Mac-1 in tumor growth.
Materials and methods
Mice
Wild type, CD11a (αL) knockout (KO) mice (16) and CD11b (αM) KO mice (17) on the C57BL6 background were obtained from Jackson Laboratory (Bar Harbor, Maine, USA). They were housed under specific pathogen-free conditions, with 12-h light and dark cycles. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Boston Children's Hospital (Protocols 16-03-3120 and 00001574 ‘Anesthetics and tumor recurrence or metastasis’).
EO771 tumor implantation model
The experiments were performed between August 2016 and July 2018. EO771 cells were cultured in RPMI1640/10% FBS. On the day of tumor implantation, mCherry-EO771 cells (7) (mCherry-EO771 cells were kindly given by Dr. Johnstone at University of Melbourne) were collected and suspended in Matrigel matrix (Corning, Inc., Corning, NY). 1×105 of EO771 cells per mouse suspended in 50 ul of Matrigel matrix were implanted at the 4th nipple fat pad in the morning of the experimental days (7). Given subcutaneous tumor injection is minimally invasive (18), for the injection, mice were placed in a quiet room and held in researcher's hand for injection with a 30G needle. No anesthetics were used as approved by the IACUC. Then, tumor size was monitored every other day. Mice behaved actively during our observation. Tumor volume was calculated ½(length × width2), as previously described (19). For IVIS (in vivo imaging system) based tumor imaging, mice were implanted with cells labeled with firefly luciferase and subjected to intraperitoneal injection of Luciferin (15–150 mg/kg) 10 min before the measurement. During the imaging, mice were anesthetized with isoflurane (4% induction, 2–3% maintenance).
In some experiment, either 100 µg of isotype control or CD11a monoclonal antibody (mAb) (clone M17/4) was given on day 7 and day 10 after tumor implantation. Some mice were also exposed to 1% isoflurane (induction and maintenance) or 2.1% sevoflurane (induction and maintenance) for 4 h on day 7 after tumor implantation. Because the minimum alveolar concentration (MAC; the concentration at which 50% of mice do not respond to tail clamping) is 1.3% for isoflurane (20) and 1 MAC is 2.8% for sevoflurane (21), 2.1% sevoflurane matches the potency of 1% isoflurane. We intended to provide them to mice at clinically relevant doses, not for full general anesthesia. The total number of mice used was described in each Figure legend. We observed tumor growth for 2 weeks expect the experiment using CD11b KO mice. For CD11b KO mice experiment, we observed up to 3 weeks due to slower tumor growth. If tumors exhibited abrasion and fluid leakage, we euthanized and excluded mice from the study. In this study, we euthanized one mouse due to the leakage from the tumor bed. We observed redness (abrasion) at the leakage site but did not measure the size of the abrasion. At the end of observation, all mice were euthanized with CO2 (30–70% of the chamber volume per minute, approximately 4–5 min). Euthanasia was confirmed by the lack of movement including respiration and heartbeat.
Tumor bed histology analysis
Tumor tissue beds were fixed using 4% paraformaldehyde. Hematoxylin & Eosin (H&E) staining was done using the Leica ST5020 Multi-staining machine in Boston Children's Hospital pathology core.
Eicosanoid measurements of mass at tumor bed
Tumor beds were removed and kept in −80°C freezer until use. Then, tumor mass was subjected to mechanical disruption for lipid extraction. The lipids were extracted with methanol and diluted with water containing 0.1% formic acid to yield a final methanol concentration of 20%. Reverse-phase mass spectrometry (MS)-based quantitation technique for eicosanoids was previously described (22). After addition of deuterium-labeled internal standards, the samples were loaded on Oasis HLB cartridge (Waters, Milford, MA). The column was washed with 1 ml of water, 1 ml of 15% methanol, and 1 ml of petroleum ether and then eluted with 0.2 ml of methanol containing 0.1% formic acid. Eicosanoids were quantified by reverse-phase HPLC-electrospray ionization-tandem MS method.
Reverse transcription-quantitative PCR (RT-qPCR)
Tumor bed tissues were collected and kept in −80°C until use. Tissues were suspended in Trizol (Thermofischer, Waltham, MA) and homogenized. Then, samples were subjected to RNA purification per the company's protocol. A total of 1 µg RNA was then converted to first-strand cDNA. RT-qPCR was performed using SYBR Green PCR Master Mix (Thermo Fisher Scientific) on StepOnePlus System (Applied Biosystems, Waltham, MA). For data normalization, GAPDH was used as an internal reference, and the fold change in gene expression was calculated using the comparative Ct method (2-ddCt) (23). Primers used for RT-qPCR were TNF-α Forward CCCTCACACTCAGATCATCTTCT,ReverseGCTACGACGTGGGCTACAG; IL-1β forward GCAACTGTTCCTGAACTCAACT, reverse ATCTTTTGGGGTCCGTCAACT; IL-6 forward GCTACCAAACTGGATATAATCAGG A reverse CCAGGTAGCTATGGTACTCCAGAA; CXCR1 forward TCTGGACTAATCCTGAGGGTG, reverse GCCTGTTGGTTATTGGAACTCTC; G-CSF forward ATGGCTCAACTTTCTGCCCAG, reverse CTGACAGTGACCAGGGGAAC; GAPDH forward GCACAGTCAAGGCCGAGAAT, GAPDH reverse GCCTTCTCCATGGTGGTGAA.
In vitro EO771 cell growth assessment
We examined the growth of EO771 cells with or without isoflurane exposure. Isoflurane exposure was done in an airtight chamber as we previously performed (24,25). Isoflurane concentration was measured by infrared spectroscopy (Ultima, Datex Instrument Corp., Helsinki, Finland). Cells were detached by trypsin and the number of live cells was counted following trypan blue staining using a hemocytometer.
Statistical analysis
Data are presented as the mean ± SD. Unpaired Student's t-test and two-way mixed ANOVA with Bonferroni post hoc analysis were used. Statistical significance was defined as P< 0.05. All the statistical calculations were performed using PRISM5 software (GraphPad Software, La Jolla, CA).
Results
Isoflurane exposure facilitated breast cancer growth
We examined the effect of commonly used volatile anesthetic isoflurane on breast cancer growth (Fig. 1A). We administered 1% isoflurane for 4 h to mice at 7 days after EO771 implantation, mimicking the duration for patients receiving breast cancer resection. As expected, isoflurane significantly facilitated breast cancer growth (tumor size at day 13, 343.3 +/- 132.9 mm3, maximum 683 mm3 for no exposure and 686.7 +/- 265.8 mm3, maximum 1,366 mm3 for isoflurane exposure) (Fig. 1B). We also tested another volatile anesthetic sevoflurane. Sevoflurane also significantly facilitated breast cancer growth (tumor size at day 13, 331.0 +/- 122.0 mm3, maximum 614 mm3 for no exposure and 731.4 +/- 292.6 mm3, maximum 1,503 mm3 for sevoflurane exposure) (Fig. 1C).
LFA-1 deficiency was associated with faster tumor growth, but Mac-1 deficiency was not
We previously showed that isoflurane directly bound to and inhibited adhesion molecules LFA-1 and Mac-1. Thus, we first examined the role of LFA-1 and Mac-1 in breast cancer growth. The deficiency of LFA-1 significantly facilitated the growth of EO771 cells as the tumor size at day 13 was 369.4 +/- 146.4 mm3 (maximum 685 mm3) for WT mice and 1,393.8 +/- 134.6 mm3 (maximum 1,639 mm3) for CD11a KO mice (Fig. 2A). Because KO mice could have compensatory changes, we also examined the effect of LFA-1 using CD11a monoclonal blocking antibody in both WT and CD11a KO mice. In line with the finding in Fig. 2A, CD11a mAb administration facilitated the growth of EO771 cells in WT mice (tumor size at day 13 1,467.2 +/- 372.6 mm3, maximum 1,725 mm3 for isotype antibody group and 2,697.0 +/- 109.2 mm3, maximum 2,725 mm3 for CD11a mAb group) (Fig. 2B). No difference was observed in CD11a KO mice (CD11a KO with isotype group, 2,510.9+/-350.0 mm3, maximum 2,758 mm3, and CD11a KO with CD11a mAb group, 3,088.0 +/- 405.4 mm3, maximum 3,374 mm3). In contrast, Mac-1 deficiency did not affect tumor growth (tumor size at day 21 1,769.3 +/- 545.4 mm3, maximum 2,798 mm3 for WT and 1,521.9 +/- 689.6 mm3, maximum 2,343 mm3 for CD11b KO mice) (Fig. 2C). Taken together, we found that both LFA-1 deficiency and inhibition significantly enhanced the growth of EO771 cells.
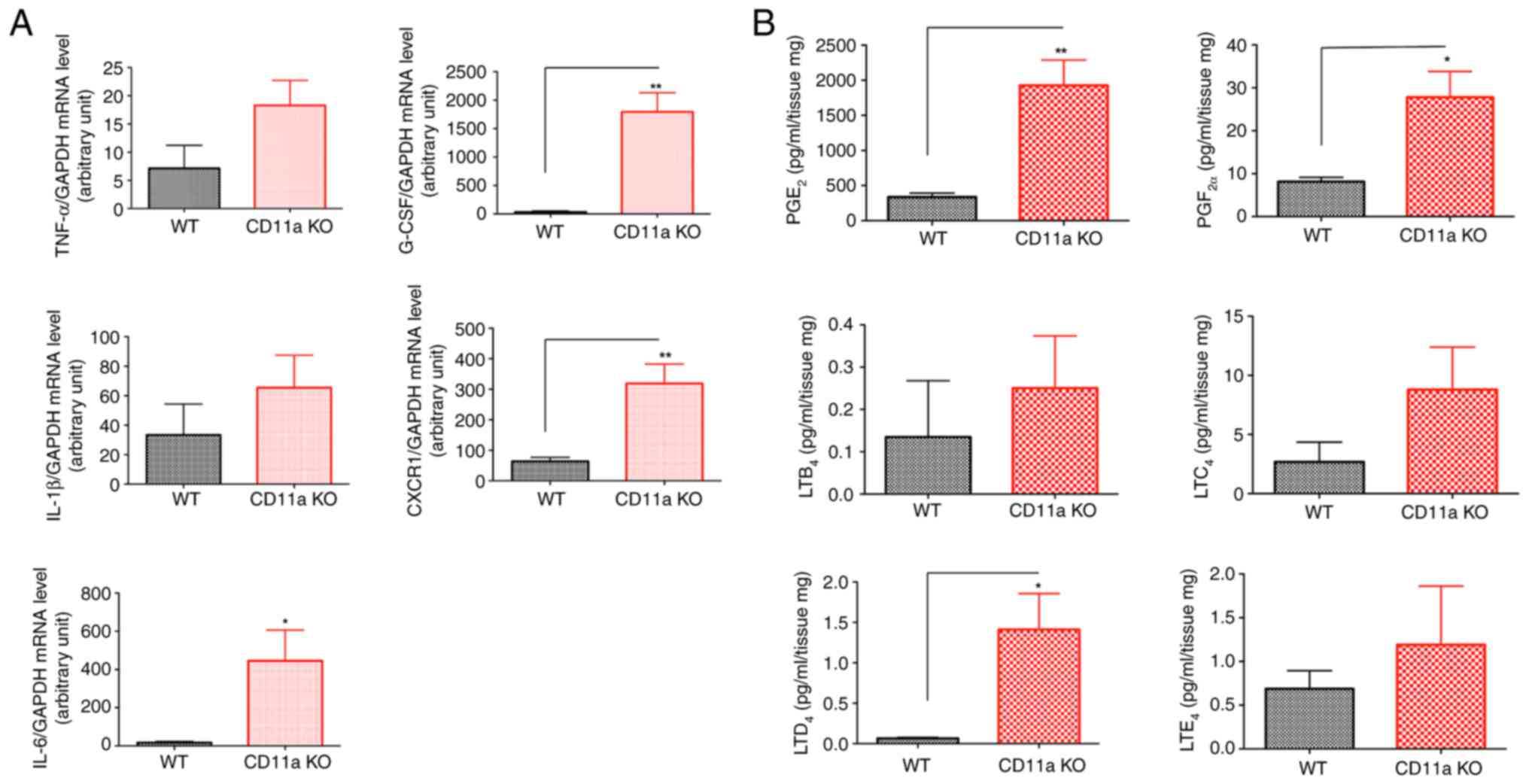
Breast cancer bed showed higher pro-tumor cytokine levels and PGE2/LTD2 levels
Because proinflammatory cytokines and a subset of lipid mediators have been shown associated with the growth of tumor, we examined their levels in CD11a KO mice. Pro-tumor cytokines IL-6, CXCR1 and G-CSF levels were significantly elevated in the tumor bed of CD11a KO mice (Fig. 3A). We also examined prostaglandin (PG) and leukotriene (LT) levels. We found that PGE2 and LTD4 levels were significantly elevated in CD11a KO mice (Fig. 3B). These data are in line with clinical data as PGE2 mediated signal is important in propagating breast cancer (26) and LTD4 level was elevated in patients with breast cancer (27).
Isoflurane did not further increase breast cancer size
The data so far indicated that LFA-1 would be isoflurane target to modulate tumor size. To test this hypothesis, we examined the effect of isoflurane in CD11a KO mice. Supportive of our hypothesis, isoflurane did not significantly affect the size of tumor in CD11a KO mice (tumor size at day 3, 1,115.0 +/- 1,07.7 mm3, maximum 1,304 mm3 for CD11a KO mice, and 1,210.6 +/- 115.0 mm3, maximum 1,326 mm3 for CD11a KO mice with isoflurane exposure) (Fig. 4A). LFA-1 is exclusively expressed on leukocytes. Thus, we also tested if isoflurane directly affected tumor size in vitro. As expected, isoflurane did not increase the EO771 cell number (Fig. 4B). This suggests that LFA-1 would be a major isoflurane target to modulate breast cancer growth in vivo.
Discussion
In this study, we showed that volatile anesthetic isoflurane and sevoflurane exposure significantly enhanced breast cancer growth. We also suggested that LFA-1 facilitate breast cancer growth via affecting LFA-1.
The role of anesthetic selection in cancer resection surgery has been a hot topic. Wigmore et al (1) reported that the use of intravenous anesthetics was significantly associated with better overall survival and less cancer recurrence than the use of volatile anesthetics. This landmark paper ignited the discussion of whether or not intravenous or volatile anesthetics should be used for general anesthesia for cancer resection surgery. A number of retrospective studies examined various type of cancer surgeries, largely favoring the use of intravenous anesthetics (28). In parallel, many investigators examined the effect of anesthetics using in vitro cell culture system and in vivo animal models. However, the mechanism of anesthetic-mediated tumor growth has been less studied in vivo. We previously demonstrated that commonly used volatile anesthetics isoflurane and sevoflurane directly bind to and inhibit LFA-1 (10–12), while an intravenous anesthetic propofol did not affect LFA-1 function at clinically relevant doses (29). We previously demonstrated the importance of LFA-1 as a volatile anesthetic target relevance in K562 cells, leukemia cells by showing that the inhibition of LFA-1 by isoflurane and sevoflurane attenuated natural killer (NK) cell- mediated cytotoxicity (30). In our data, we showed both isoflurane and sevoflurane facilitated breast cancer growth. While isoflurane also bound to and blocked Mac-1 (11,12), sevoflurane did not bind to and inhibit Mac-1 (10,13). Taken together, our data suggested that LFA-1 served as a target for both isoflurane and sevoflurane to facilitate breast cancer growth.
As LFA-1 is exclusively expressed on leukocytes, we expect that isoflurane significantly enhanced tumor growth via altering the phenotype of leukocytes. The analysis of tumor beds showed an increase in PGE2 and LTD4 levels in CD11a KO mice compared to WT mice. However, we still do not know what triggered this change. As LFA-1 is ubiquitously expressed on leukocytes, it is imperative to examine the role of LFA-1 in all leukocyte types. For example, LFA-1 is involved in NK cell-mediated tumor cytotoxicity (31,32). LFA-1 activation enriches tumor-specific T cells to improve anti-tumor responses (33). Both neutrophils and macrophages play a significant role in cancer immunity (34,35). However, how LFA-1 on neutrophils and macrophages affect cancer growth is not known. In the future, it would be critical to determine how LFA-1 affects cancer growth via leukocytes in vivo. In line with an increase in PGE2 in CD11a KO mice, we previously showed that PGE2 levels were significantly increased by isoflurane (21).
We need to note a few issues. While we measured the size of tumor to calculate the volume in the same way throughout the study, we did not use IVIG for additional confirmation. Although it is very common to study the effect of anesthetics on tumor growth as in our case, it is important to point out that anesthetics are usually given for tumor resection. To be completely in line with this scenario, it would be important to examine the effect of anesthetics using tumor resection model. However, a simple tumor resection and recurrence model has not been reported in breast cancer yet. In the model using 4T1 breast cancer cells, a nephrectomy has also been done to see tumor recurrence (36). In this study, we used the EO771 cell model, but it would be important to examine different types of cancer given each cancer is very different. LFA-1 binds to its ligand intercellular adhesion molecule-1 (ICAM-1). The expression of ICAM-1 can vary. For example, ICAM-1 is expressed more in triple negative breast cancer cells compared with other types (37). Although our data highly supported that both isoflurane and sevoflurane affected LFA-1 and facilitated tumor growth based on our previous structural studies (10–12), we did not show the direct binding of volatile anesthetics in vivo. Therefore, confirmatory experiment is needed to explicitly support the direct interaction between LFA-1 and isoflurane (sevoflurane) in vivo.
In summary, we showed that isoflurane significantly facilitated breast cancer growth via affecting LFA-1.
Acknowledgements
The authors would like to thank Ms. Janice Bautista (Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children's Hospital) for technical support.
Funding
The present study was supported by the National Institute of General Medical Sciences (grant no. K08GM101345).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
SK performed experiments and wrote the manuscript. WW performed experiments. LH performed experiments. TO performed experiments and edited the manuscript. KY designed the study, performed experiments and wrote the manuscript. SK, LH and KY confirmed the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
For animal experiments, Boston Children's Hospital IACUC approval (approved protocol nos. 16-03-3120 and 00001574; Boston, MA, USA) was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Wigmore TJ, Mohammed K and Jhanji S: Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: A retrospective analysis. Anesthesiology. 124:69–79. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jun IJ, Jo JY, Kim JI, Chin JH, Kim WJ, Kim HR, Lee EH and Choi IC: Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: A retrospective observational study. Sci Rep. 7:140202017. View Article : Google Scholar : PubMed/NCBI | |
|
Wu ZF, Lee MS, Wong CS, Lu CH, Huang YS, Lin KT, Lou YS, Lin C, Chang YC and Lai HC: Propofol-based total intravenous anesthesia is associated with better survival than desflurane anesthesia in colon cancer surgery. Anesthesiology. 129:932–941. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sakamoto K, Schmidt JW and Wagner KU: Mouse models of breast cancer. Methods Mol Biol. 1267:47–71. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Casey AE, Laster WR Jr and Ross GL: Sustained enhanced growth of carcinoma EO771 in C57 black mice. Proc Soc Exp Biol Med. 77:358–362. 1951. View Article : Google Scholar : PubMed/NCBI | |
|
Johnstone CN, Smith YE, Cao Y, Burrows AD, Cross RS, Ling X, Redvers RP, Doherty JP, Eckhardt BL, Natoli AL, et al: Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis Model Mech. 8:237–251. 2015.PubMed/NCBI | |
|
Stollings LM, Jia LJ, Tang P, Dou H, Lu B and Xu Y: Immune modulation by volatile anesthetics. Anesthesiology. 125:399–411. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yuki K and Eckenhoff RG: Mechanisms of the immunological effects of volatile anesthetics: A review. Anesth Analg. 123:326–335. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yuki K, Astrof NS, Bracken C, Soriano SG and Shimaoka M: Sevoflurane binds and allosterically blocks integrin lymphocyte function-associated antigen-1. Anesthesiology. 113:600–609. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG and Shimaoka M: The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J. 22:4109–4116. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Yuki K, Bu W, Xi J, Sen M, Shimaoka M and Eckenhoff RG: Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J. 26:4408–4417. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Jung S and Yuki K: Differential effects of volatile anesthetics on leukocyte integrin macrophage-1 antigen. J Immunotoxicol. 13:148–156. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shimaoka M and Springer TA: Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2:703–716. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ho MK and Springer TA: Mac-1 antigen: quantitative expression in macrophage populations and tissues, and immunofluorescent localization in spleen. J Immunol. 128:2281–2286. 1982. View Article : Google Scholar : PubMed/NCBI | |
|
Ding ZM, Babensee JE, Simon SI, Lu H, Perrard JL, Bullard DC, Dai XY, Bromley SK, Dustin ML, Entman ML, et al: Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 163:5029–5038. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, Arnaout MA and Mayadas TN: A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity. 5:653–666. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Berrueta L, Bergholz J, Munoz D, Muskaj I, Badger GJ, Shukla A, Kim HJ, Zhao JJ and Langevin HM: Stretching reduces tumor growth in a mouse breast cancer model. Sci Rep. 8:78642018. View Article : Google Scholar : PubMed/NCBI | |
|
Tomayko MM and Reynolds CP: Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Sonner JM, Gong D, Li J, Eger EI II and Laster MJ: Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 89:1030–1034. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Dahan A, Sarton E, Teppema L, Olievier C, Nieuwenhuijs D, Matthes HW and Kieffer BL: Anesthetic potency and influence of morphine and sevoflurane on respiration in mu-opioid receptor knockout mice. Anesthesiology. 94:824–832. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Okuno T, Koutsogiannaki S, Hou L, Bu W, Ohto U, Eckenhoff RG, Yokomizo T and Yuki K: Volatile anesthetics isoflurane and sevoflurane directly target and attenuate Toll-like receptor 4 system. FASEB J. 33:14528–14541. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Zha H, Matsunami E, Blazon-Brown N, Koutsogiannaki S, Hou L, Bu W, Babazada H, Odegard KC, Liu R, Eckenhoff RG and Yuki K: Volatile anesthetics affect macrophage phagocytosis. PLoS One. 14:e02161632019. View Article : Google Scholar : PubMed/NCBI | |
|
Yuki K, Bu W, Shimaoka M and Eckenhoff R: Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin αIIbβ3. PLoS One. 8:e604152013. View Article : Google Scholar : PubMed/NCBI | |
|
Walker OL, Dahn ML, Power Coombs MR and Marcato P: The prostaglandin E2 pathway and breast cancer stem cells: Evidence of increased signaling and potential targeting. Front Oncol. 11:7916962022. View Article : Google Scholar : PubMed/NCBI | |
|
Akaydin S, Ramazanoğlu S, Salihoğlu EM, Karanlik H and Demokan S: Leukotriene D4 levels in patients with breast cancer. FABAD J Pharm Sci. 47:331–338. 2022. | |
|
Yuki K: The role of general anesthetic drug selection in cancer outcome. Biomed Res Int. 2021:25630932021. View Article : Google Scholar : PubMed/NCBI | |
|
Koutsogiannaki S, Schaefers MM, Okuno T, Ohba M, Yokomizo T, Priebe GP, DiNardo JA, Sulpicio SG and Yuki K: From the cover: Prolonged exposure to volatile anesthetic isoflurane worsens the outcome of polymicrobial abdominal sepsis. Toxicol Sci. 156:402–411. 2017.PubMed/NCBI | |
|
Tazawa K, Koutsogiannaki S, Chamberlain M and Yuki K: The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett. 266:23–31. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Barber DF, Faure M and Long EO: LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 173:3653–3659. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Gao N, Wang C, Yu Y, Xie L, Xing Y, Zhang Y, Wang Y, Wu J and Cai Y: LFA-1/ICAM-1 promotes NK cell cytotoxicity associated with the pathogenesis of ocular toxoplasmosis in murine model. PLoS Negl Trop Dis. 16:e00108482022. View Article : Google Scholar : PubMed/NCBI | |
|
Hickman A, Koetsier J, Kurtanich T, Nielsen MC, Winn G, Wang Y, Bentebibel SE, Shi L, Punt S, Williams L, et al: LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. J Clin Invest. 132:e1541522022. View Article : Google Scholar : PubMed/NCBI | |
|
Hedrick CC and Malanchi I: Neutrophils in cancer: Heterogeneous and multifaceted. Nat Rev Immunol. 22:173–187. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
DeNardo DG and Ruffell B: Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 19:369–382. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tai LH, Tanese de Souza C, Sahi S, Zhang J, Alkayyal AA, Ananth AA and Auer RA: A mouse tumor model of surgical stress to explore the mechanisms of postoperative immunosuppression and evaluate novel perioperative immunotherapies. J Vis Exp. 512532014.PubMed/NCBI | |
|
Guo P, Huang J, Wang L, Jia D, Yang J, Dillon DA, Zurakowski D, Mao H, Moses MA and Auguste DT: ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci USA. 111:14710–14715. 2014. View Article : Google Scholar : PubMed/NCBI |