Introduction
Light is the ultimate powerful agent for the
maintenance of circadian clocks. However, prolonged exposure to
artificial light disturbs circadian rhythms (1,2) and
leads to retinal degeneration, such as macular degeneration and
retinitis pigmentosa, which is associated with photoreceptor
degeneration (3). Prolonged
exposure to bright light mediates photoreceptor cell death, which
is an irrevocable retinal disorder and causes impaired vision
function, vision loss or blindness (4,5).
However, to the best of our knowledge, the pathogenesis of
light-induced damage to the retina is unclear.
Excessive light exposure promotes a cascade of
oxidative stress-associated events involved in retinal degeneration
(6,7), which serves a pivotal role in
accelerating several degenerative diseases such as age-related
macular degeneration (AMD) and retinitis pigmentosa (RP) (3). Overproduction of reactive oxygen
species (ROS) can lead to retinal functional and morphological
changes that result in vision impairment (8,9).
Nuclear factor erythroid 2-related factor 2 (Nrf-2) is a redox
regulator (10) that belongs to the
leucine zipper transcription factor family, which normally binds to
keap1 in order to form a complex. In response to oxidative stress,
keap1 activity is reduced, which increases Nrf-2 levels to maintain
cellular homeostasis (11).
Thioredoxin (Trx) is an antioxidant, which contains an active site
Cys-Gly-Pro-Cys domain (12). The
latter serves an important role in cellular oxidative stress
(13,14). Trx is a potent regulator of Nrf-2
that serves an important role in redox signaling (15). Additionally, it regulates the
antiapoptotic signaling pathway, which can protect photoreceptor
cells from retinal degeneration (15,16).
Ginkgo biloba (EGb-761) is a traditional
Chinese medicine, which contains a naturally occurring compound
extracted from the green dried leaves of the Ginkgo biloba
tree (17,18). EGb-761 has been used to treat
chronic inflammatory and autoimmune disease in patients for several
decades (18). Due to its
antioxidants and cytoprotective properties, EGb-761 has received
considerable attention regarding the prevention of
neurodegenerative diseases (19).
It can be used to prevent oxidative stress by regulating
antioxidant proteins, such as Nrf-2, which is a redox regulator
that stabilizes oxidative stress (20,21) by
activating the Erk signaling pathway (22). A light damage mouse model has been
previously used to explore the protective effect of Ginkgo
biloba in the development of chronic diseases (23).
Apoptosis, also referred to as programmed cell
death, is the main cause of photoreceptor cell death involved in
retinal degeneration (5). Apoptosis
occurs via the activation of the Bax-dependent mitochondrial
cascade, which releases cytochrome c (Cyc) into the
cytoplasm and consequently initiates the activation of cleaved
caspase-3 (15,24,25).
This suppresses Bcl2, induces apoptosis via the Bax/Bcl2 and
caspase-mediated signaling pathways, and leads to retinal
degeneration (25,26). As discussed in previous studies,
pretreatment with EGb-761 decreases cell death via the Bax/Bcl2
signaling pathway and exerts a protective effect against apoptosis
that delays photoreceptor degeneration (27,28).
However, the protective effect of EGb-761 on light-induced
photoreceptor degeneration and the associated mechanism of action
are not fully understood.
In the present study, a light damage mouse model was
used to evaluate the effects of EGb 761 on light-induced
photoreceptor degeneration and to explore the associated mechanism
of action. The present study aimed to provide evidence for the
treatment or prevention of light-induced photoreceptor
degeneration.
Materials and methods
Animal model
In total, 20 male and 20 female BALB/cJ mice (age,
8-12 weeks), weighing 18-20 g (Dalian Medical University Laboratory
Animal Center) were housed for 1 week for acclimization in the lab
environment. All mice were maintained at 22±2˚C and 30-70%
humidity, under 12-h dark/light cycle with free access of food and
water supply. All procedures were approved by the Institutional
Animal Care and Use Committee of the Dalian Medical University
(Dalian, China). The extract of Ginkgo biloba leaves
(EGb-761) was provided by Sigma-Aldrich; Merck KGaA.
Drug administration
Mice were randomly divided into four groups as
follows: No treatment (Control); Light (L); Light + Low Ginkgo (L +
LG); and Light + High Ginkgo (L + HG). A total of 10 mice were used
in each group. The mice were pretreated with EGb-761 for 7 days and
received an intraperitoneal injection once per day, at a dose of 50
mg/kg (L + LG) or 100 mg/kg (L + HG). The drug was dissolved in
saline solution. Mice in the control and light groups received
intraperitoneal injections of normal saline solution.
Light exposure
The mice were adapted in the dark for 24 h prior to
the experiments and exposed to 5,000 lux (diffuse horizontal
illuminance) of white fluorescent light (400-750 nm) for 24 h. The
procedure of light exposure was the same as that described in a
previous study (29). During light
exposure, one mouse was maintained per cage with a sufficient
supply of food and water. The eyes of four mice were enucleated at
2 weeks after light exposure for outer nuclear layer (ONL)
thickness analysis and electroretinography assessment. The eyes of
six mice were enucleated immediately after light exposure to detect
possible rapid changes by western blotting and reverse
transcription-quantitative PCR (RT-qPCR). After electroretinogram
(ERG) detection, the mice were sacrificed using an overdose of
carbon dioxide (30% volume/min) at the end of the experiments
before enucleation.
ERG
Prior to light exposure, the mice were adapted to
the dark overnight and anesthetized with an intraperitoneal
injection of pentobarbital-sodium (60 mg/kg) 30 min before the
experiment. The pupils were dilated by applying one drop 0.5%
tropicamide prior to performing ERG analysis. ERG was performed
using an LKC ERG system (GT-2008v-3; Gotec, Inc.; http://www.gotechina.com/product/?110_471.html).
Phototopic ERGs were generated at 3 cd.sec/m2 flash
intensity. Each flash lasted for 5 msec and the interval was 2 sec.
The amplitudes of the a-wave and b-wave were recorded. The ERG wave
from both eyes of the same animal was recorded simultaneously.
Histological analysis
H&E staining was performed for morphological
analysis as described in our previous study (30). The eyeball was removed and fixed for
24 h at room temperature in Bouin's solution (glacial acetic acid:
Formaldehyde: Saturated picric acid=1:5:15; freshly prepared).
Subsequently, it was placed in 70% ethanol for 48 h at room
temperature, or until the yellow color of Bouin's solution was
faint or disappeared. For dehydration, each eyeball was passed
through a series of alcohol solutions as follows: 80 and 90%
ethanol for 15 min each, 95% ethanol (twice for 10 min each) and
100% ethanol (twice for 10 min each). The eyeballs were
subsequently passed through xylene for 10 min and then 5 min,
respectively, before the tissues were soaked in paraffin wax for 10
min at 58˚C. The paraffin-embedded tissues were sectioned using a
thickness margin of 5 µm. The sections were deparaffinized first
using xylene followed by a descending ethanol gradient. The slices
were stained with H&E each for 5 min at room temperature. The
ONL thickness of the retinal optic nerve was measured at eight
different positions at a distance of 0.22 mm from the inferior
hemisphere to the superior hemisphere using light microscope with
Element BR software (magnification, x40; Ver5.30.00; Nikon
Corporation).
RT-qPCR
The total RNA was extracted using RNAiso Plus
(Takara Bio, Inc.) from the retina tissue according to the
manufacturer's protocols. The concentration of RNA was measured
using a NanoDrop spectrometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.). cDNA was synthesized from 1 µg total RNA using
the PrimeScript™ RT reagent Kit (Perfect Real Time; Takara Bio,
Inc.) according to the manufacturer's protocol. qPCR was performed
using TB Green® Premix Ex Taq™ (Takara Bio, Inc.) as
follows: Initial denaturation at 95˚C for 30 sec; followed by 40
cycles of amplification (95˚C for 30 sec, 95˚C for 30 sec, 55˚C for
30 sec and 72˚C for 30 sec), 95˚C for 1 min, 55˚C for 30 sec, 95˚C
for 30 sec. GAPDH was used as an internal control. The
2-ΔΔCq method (31) was
used to analyze the data. The primer sequences used were: GAPDH
forward, 5'-TGTGATGGGTGTGAACCACGAGAA-3' and reverse,
5'-GAGCCCTTCCACAATGCCAAAGTT-3'; BCL2 antagonist/killer 1 (Bak-1)
forward, 5'-ACGAACTCTTCACCAAGATCGCCT-3' and reverse,
5'-TCAAACCACGCTGGTAGACGTACA-3'; and Cyc forward,
5'-AGATGTTGTTGATGATGGGCCTGC-3' and reverse,
5'-AAGCCAGCTTTCGACTCTTCAGGA-3'.
Western blotting
Total protein was extracted from retinal tissues for
30 min using freshly prepared ice-cold lysis buffer (cat. no.
KGP2100; Nanjing Keygen Biotech Co., Ltd.). The lysate was
homogenized and centrifuged at 1,000 x g for 10 min at 4˚C before
the supernatant was collected and centrifuged again at 12,000 x g
for 20 min at 4˚C. The supernatant was separated and preserved at
-80˚C for subsequent use. The protein concentration was measured
using a bicinchoninic acid protein assay kit (Nanjing KeyGen
Biotech Co., Ltd.). Equal amount (20 µg) of proteins were separated
by SDS-PAGE (12-15%) and transferred onto polyvinylidene difluoride
membranes, which were blocked using 5% nonfat milk at room
temperature for 1 h. Subsequently, the membranes were incubated
with primary antibodies against GAPDH (dilution, 1:1,000;
ProteinTech Group, Inc.), Bcl2 (dilution, 1:1,000; ABclonal Biotech
Co., Ltd.), Bax (dilution, 1:1,000; ABclonal Biotech Co., Ltd.),
cleaved caspase-3 (dilution, 1:1,000; Nanjing KeyGen Biotech Co.,
Ltd.), ERK (dilution, 1:500; Beyotime Institute of Biotechnology),
phosphorylated (p-)Erk (dilution, 1:500; Beyotime Institute of
Biotechnology), Trx (dilution, 1:200; Santa Cruz Biotechnology,
Inc.) and Nrf-2 (dilution, 1:500; cat. no. 163961-1-AP; ProteinTech
Group, Inc.) overnight at 4˚C. The membranes were washed with 1X
TBS with 0.1% Tween 20 (TBST) three times for 15 min (5 min/wash).
Subsequently, the membranes were probed with goat anti-rabbit IgG
for 1 h at room temperature, and washed with 1X TBST three times
for 45 min (15 min/wash). The protein bands were visualized using
enhanced chemiluminescence agent (Beijing Solarbio Science &
Technology Co., Ltd.), and Image lab (version: 4. 1.0.2177; Bio-Rad
Laboratoties, Inc.) software was used to analyze the data.
Statistical analysis
The experimental data were obtained from at least
three independent experiments. The data are presented as the mean ±
SD. One-way ANOVA was used to analyze the data. All data were
analyzed using GraphPad Prism 8 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protective effect of EGb-761 against
light-induced retinal degeneration
To evaluate the protective effect of EGb-761 on
light-induced retinal damage, mice were exposed to 5,000 lux of
white light for 24 h and treated with EGb-761 for 7 days prior to
exposure to light. To analyze the visual function of the retina, an
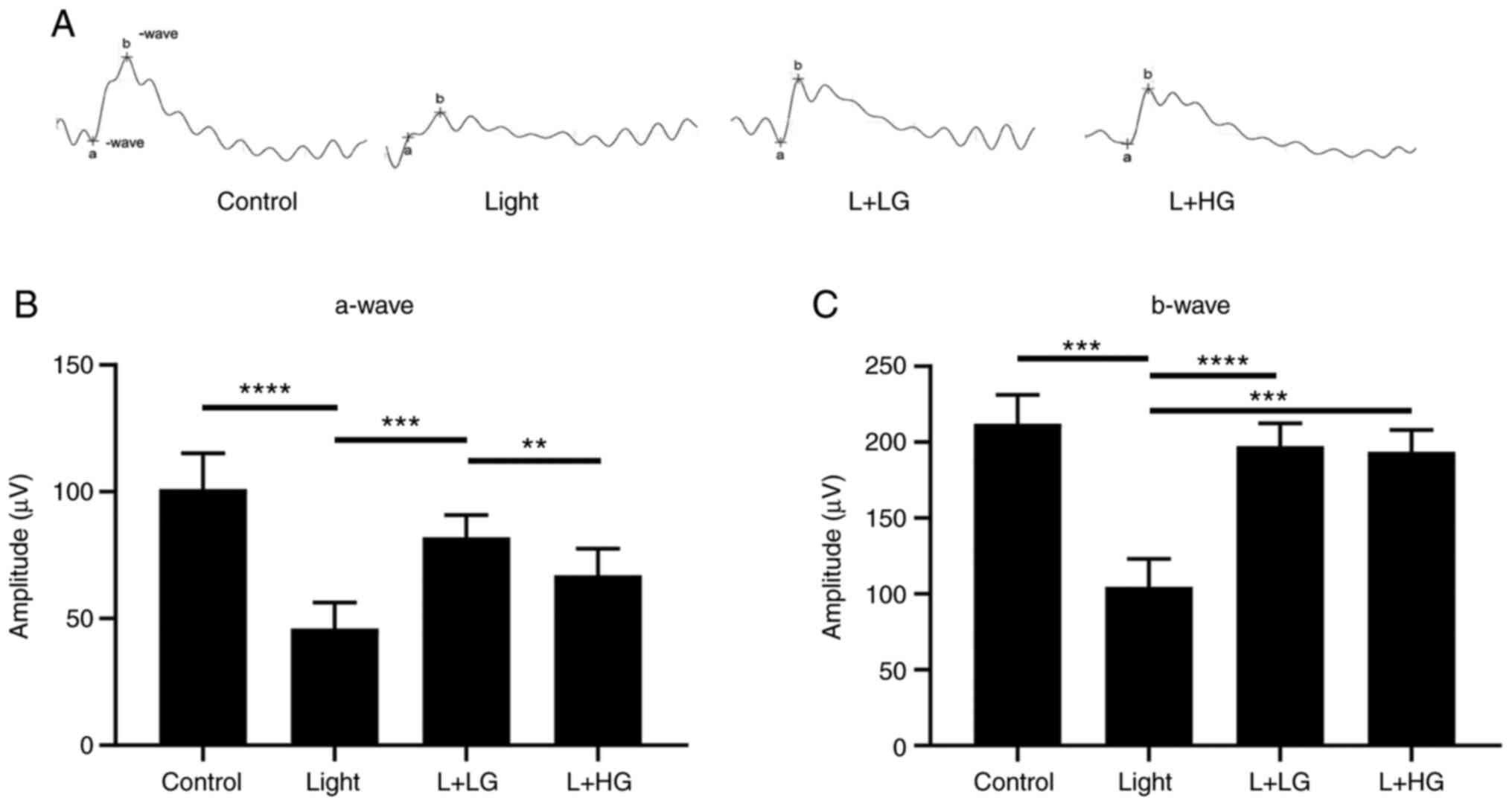
ERG was performed (Fig. 1). The
results suggested that the amplitudes of the a-wave and b-wave were
significantly decreased in mice exposed to 5,000 lux of bright
light for 24 h compared with the levels noted in the control group
(Fig. 1). However, a fractional
protective effect could be observed when mice were treated with
EGb-761 (L + LG and L + HG) for 7 days prior to exposure to white
light. As shown in Fig. 1, the
amplitude of the a-wave was increased (L + LG) and the amplitudes
of the b-wave were significantly increased in the EGb-761 (L + LG
and L + HG) groups compared with that in the light-damage group
(Fig. 1A-C).
EGb-761 delays light-induced
photoreceptor degeneration
To evaluate the protective effect of EGb-761 on
photoreceptor degeneration, H&E staining was performed. The
morphological evaluation using H&E staining indicated a massive
loss in photoreceptor cells, which reduced the ONL thickness of the
retina in the light-induced retinal degeneration group compared
with the control group (Fig. 2).
However, the process could be prevented by pre-treatment with
EGb-761 (LG and HG; Fig. 2). These
findings suggested that EGb-761 could delay photoreceptor
degeneration and improve the visual and morphological function of
the retina during light-induced damage.
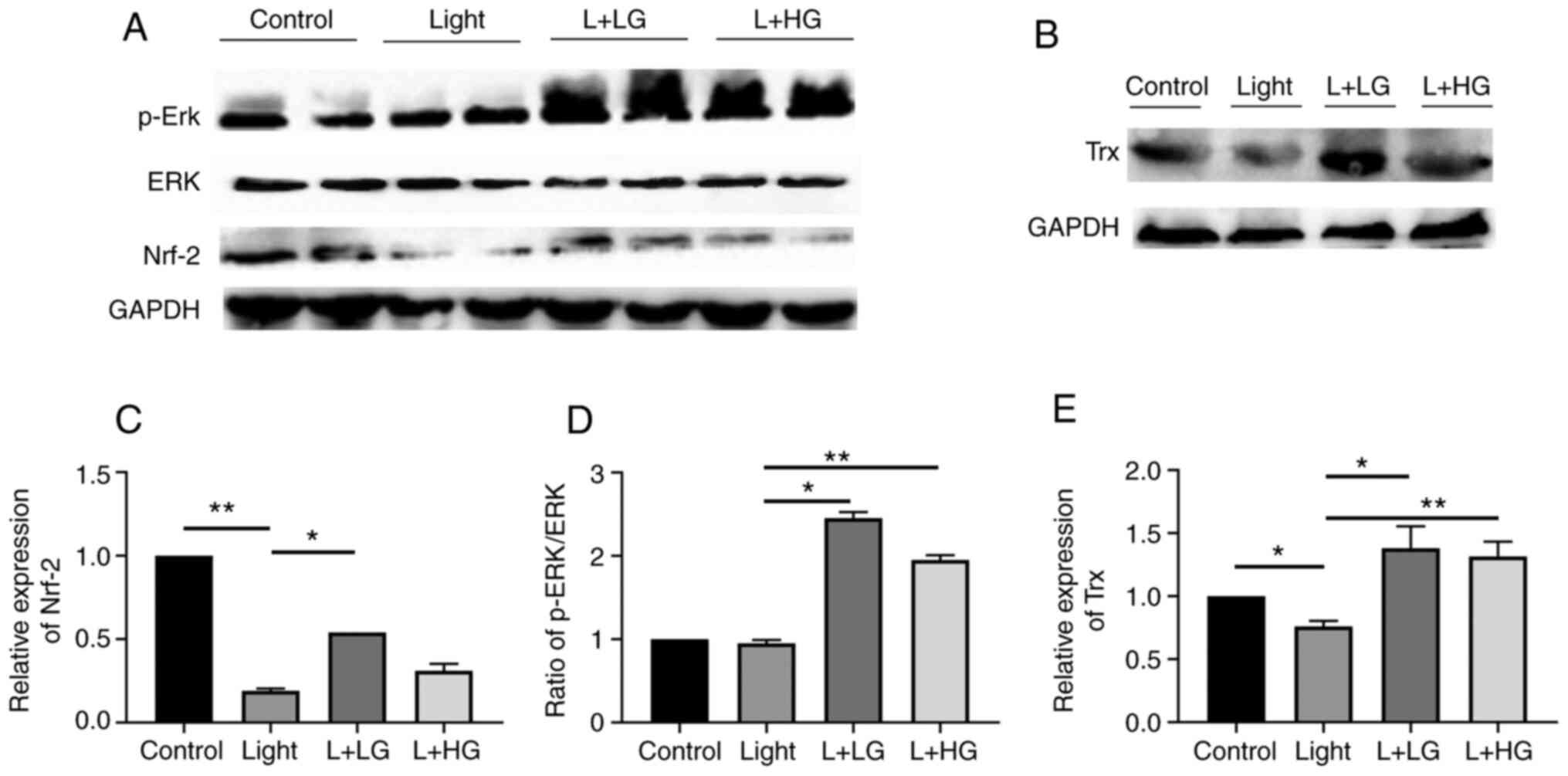
Effect of EGb-761 treatment on the
p-Erk/Nrf-2/Trx signaling pathway in mice exposed to light
The in vivo findings suggested that the
protein levels of Nrf-2/Trx were significantly decreased in the
light-induced retinal degeneration group compared with the control
group. However, pre-treatment with EGb-761 significantly increased
the p-Erk/Nrf-2/Trx levels compared with those in the light-induced
retinal degeneration group. However, there is no significant
difference between that in the Light and L + HG groups for Nrf-2
(Fig. 3). These results indicated
that treatment with EGb-761 reduced oxidative stress-induced by
light damage by activating the p-Erk/Nrf-2/Trx signaling
pathway.
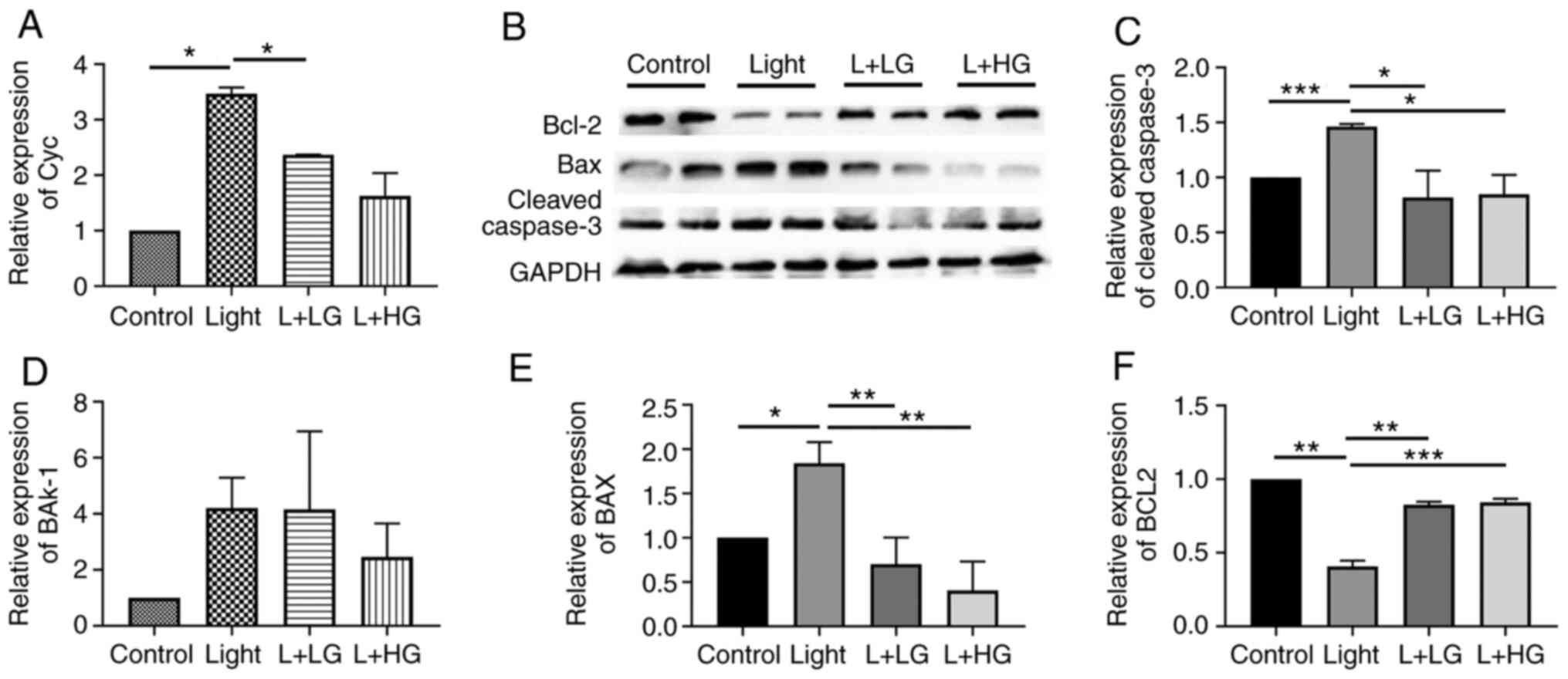
EGb-761 treatment decreases the
expression levels of proteins of the cleaved
caspase-3/Bax/Bak-1/Cyc signaling pathway and increases Bcl-2
expression
In order to investigate the effects of EGb-761 on
light-induced photoreceptor degeneration, RT-qPCR and western
blotting were performed. Quantitative analysis of mRNA (Fig. 4A and B) indicated that the expression levels of
Cyc and Bak-1 were increased in the light-induced retinal
degeneration group compared with the control group. However, there
is no significance in the difference among the groups for Bak-1.
However, the expression levels of Cyc and Bak-1 were decreased
following EGb-761 treatment compared with those in the
light-induced retinal degeneration group. In addition, the protein
expression levels of cleaved caspase-3, Bax and Bcl-2 (Fig. 4C) were detected by western blotting.
The expression levels of cleaved caspase-3 and Bax were increased
in the light-induced retinal degeneration group compare with those
in the control group, which were in turn downregulated following
EGb-761 pre-treatment compared with those in the light damage
group, (Fig. 4D and E). Furthermore, the expression levels of
Bcl-2 were decreased in the light-induced retinal degeneration
group, and they were significantly increased following treatment
with EGb-761 (Fig. 4F). The results
indicated that the expression levels of cleaved caspase-3/Bax/Cyc
were significantly increased in the light-induced retinal
degeneration group compared with the control group, whereas this
effect was inhibited following treatment of the mice with EGb-761.
This suggests that the cleaved caspase-3/Bax/Cyc pathway is the
mechanism through which light induces photoreceptor degeneration
and also showed that EGb 761 inhibits the effects of light on
retinal degeneration through activating the p-ERK/Nrf2/Trx pathway
(Fig. 5).
Discussion
In the present study, the protective effects of
EGb 761 were assessed in retinal degeneration. In addition,
the molecular mechanisms involved in the regulation of oxidative
stress and the cell death signaling pathway induced by white light
(5,000 lux) were explored. The results suggested that prolonged
exposure to white light led to photoreceptor cell degeneration,
characterized by the activation of the corresponding apoptotic
signaling pathway. However, little is known regarding the signaling
pathway associated with these processes. Treatment of animals with
EGb-761 markedly reduced oxidative stress via activation of the
Nrf-2/Trx/Erk signaling pathway. By contrast to this effect,
treatment of the animals with EGb 761 reduced apoptosis via
the regulation of the Bax/Bcl2 and caspase signaling pathways.
Therefore, the observations suggested that EGb 761 may serve
a crucial role in the clinical treatment of retinal degenerative
diseases.
The severity of light-induced retinal impairment is
commonly associated with the induction of oxidative stress, which
is dependent on the light intensity and exposure time (32). In various studies (23,29,33),
the rat/mouse model has been exposed to a variety of white light
sources, with different intensities that ranged between 1,000 and
20,000 lux (6,7,8,12). In
addition, different irradiation rhythms, different light sources
and different spectra have different effects on the retina
(3). It has been reported in
previous studies, that the decrease of light intensity and
alteration in the light constituents can reduce the effect of
retinal degeneration (3,6,34).
These conditions are known to cause retinal histopathological and
functional changes in animals (6,35).
Therefore, the present study evaluated the effects of exposure to
5,000 lux of light for 24 h in a mouse model by focusing on the
assessment of different retinal functions. The selected range was
based on previous study (6).
Furthermore, our research group has used the same range in previous
study (3). Therefore, in the
present study, this range was selected. In the present study,
prolonged exposure to white light triggered a large burst in
photoreceptor cell death, which resembled the pathogenesis of
retinal degeneration. The results suggested that the induction of
morphological and functional changes following white light (5,000
lux) exposure contributed to the development of retinal diseases.
The amplitudes of the a-wave and b-wave determined the induction of
specific disorders associated with retinal function, and the
amplitudes were decreased in the light damage group and were
accompanied by loss of photoreceptor cells, reducing ONL thickness
in the outer segment of the retina. These results were similar to
those reported previously (29).
EGb-761 is used for the treatment of different
diseases, such as neurodegenerative and retinal disorders (19). Functional and morphological analysis
demonstrated that EGb-761 could inhibit light-induced retinal
damage. These results support previously reported data (23). However, little is known regarding
the molecular mechanism of EGb 761. Therefore, the present study
investigated the mechanism by which EGb 761exerted a protective
effect against light-induced retinal degeneration. Previous
literature reviews have reported that the effect of EGb 761 is
dose-dependent (36,37). A dose range of 40-300 mg/kg has been
found to be more effective in the treatment of specific diseases
(38,39). The present study demonstrated that
treatment with a low dose (50 mg/kg) of EGb 761 was more effective
in preventing retinal degeneration than high-dose treatment (100
mg/kg).
A previous study has reported that prolonged and
intense exposure to light promotes the induction of oxidative
stress, which is involved in the pathogenesis of various retinal
diseases (40). The Erk/Trx/Nrf-2
signaling pathway serves a pivotal role in redox balance. When the
levels of cellular oxidative stress are increased, the p-Erk axis
of unfolded protein response mediates nuclear translocation of
antioxidant Nrf-2 leading to increased expression of Trx (41), reduced photooxidative stress and
retinal degeneration (42).
Overall, the p-Erk/Nrf-2/Trx cascade is tightly involved in the
regulation of the redox signaling pathway (43). Previous studies have reported that
the use of antioxidants is considered the chief regulator of the
cellular redox mechanism mediated via the p-Erk/Nrf-2/Trx axis
(3,44). This signaling pathway
(p-Erk/Nrf-2/Trx) can maintain the biological and physiological
function of the retina (45). In
the present study, EGb 761 treatment increased the levels of Trx,
Nrf-2 and p-Erk in a light damage mouse model. Therefore, the
results suggested that EGb 761 acted as an antioxidant that could
serve a vital role in retinal degeneration.
The excess levels of ROS and oxidative stress
initiate apoptosis or programmed cell death (46), which causes photoreceptor cell death
leading to various retinal diseases and blindness (47). The Bak-1/Cyc/Bax/cleaved caspase-3
signaling pathway has attracted considerable attention and is
considered the major cause of photoreceptor cell death noted in
retinal diseases (47-49).
The cell death mechanism is initiated by the translocation of Bax
and Bak to the mitochondrial membrane, and Cyc release into the
cytosol (50,51). This initiates the activation of
cleaved caspase-3, suppressing Bcl2, and induces morphological and
biochemical changes in the retina (25,26).
The results of the present study indicated that exposure to light
upregulated the levels of Cyc, Bak-1, Bax and cleaved caspase-3 in
the retina, while this process was inhibited following pretreatment
of the mice with EGb 761. This effect could protect photoreceptor
cells from apoptosis.
Overall, the data demonstrated that EGb 761
treatment delayed photoreceptor degeneration in light-exposed mice.
The mechanism involved activation of the p-Erk/Nrf-2/Trx axis and
downregulation of the apoptotic Bax/Bak-1/Cyc/cleaved caspase-3
signaling pathway, which was concomitant with the upregulation of
Bcl2 expression. These results may suggest a putative role of EGb
761 in the treatment of retinal degeneration. The lack of
measurements of Cyc release into the cytosol and measurements of
mitochondrial membrane potential was a limitation of the present
study.
In conclusion, the present study suggested that EGb
761 treatment delayed photoreceptor degeneration induced by light.
The underlying mechanism was associated with inhibition of
apoptosis via downregulation of the Bax/Cyc/cleaved caspase-3
signaling pathway and upregulation of Bcl2, which led to inhibition
of oxidative stress via the activation of the p-Erk/Nrf-2/Trx
signaling pathway.
Acknowledgements
The authors would like to thank Miss Jinjuan Lv,
Miss Limin Wei and Miss Jiao Li, Department of Histology and
Embryology, Dalian Medical University (Dalian, China) for their
technical assistance during the experiments.
Funding
Funding: The present study was supported from the National
Natural Science Foundation of China (grant no. 31371218) and the
Basic Scientific Research Projects of the Liaoning Provincial
Education Department (grant no. LQ2017005). This work was also
supported by the Natural Science Foundation of Liaoning Province
(grant no. 2020-BS-189). The Liaoning Provincial Program supported
this work for the Top Discipline of Basic Medical Sciences.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC and CZ designed the study, carried out
experiments, analyzed the data, and wrote and revised the
manuscript. SS contributed to the experiments and revised the
manuscript. XR and LK designed, supervised and provided funding for
the experiments. XR and LK also checked the analyzed data and
revised the manuscript critically for important intellectual
content revised. XR and LK are responsible for confirming the
authenticity of the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Animal Care and Use Committee of the Dalian Medical University
(Dalian, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nash TR, Chow ES, Law AD, Fu SD, Fuszara
E, Bilska A, Bebas P, Kretzschmar D and Giebultowicz JM: Daily
blue-light exposure shortens lifespan and causes brain
neurodegeneration in Drosophila. NPJ Aging Mech Dis.
5(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Contín M, Benedetto M, Quinteros-Quintana
M and Guido M: Light pollution: The possible consequences of
excessive illumination on retina. Eye (Lond). 30:255–263.
2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kong L, Liu B, Zhang C, Wang B, Wang H,
Song X, Yang Y, Ren X, Yin L, Kong H and Ma H: The therapeutic
potential of sulforaphane on light-induced photoreceptor
degeneration through antiapoptosis and antioxidant protection.
Neurochem Int. 100:52–61. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hu Z, Zhang Y, Wang J, Mao P, Lv X, Yuan
S, Huang Z, Ding Y, Xie P and Liu Q: Knockout of Ccr2 alleviates
photoreceptor cell death in rodent retina exposed to chronic blue
light. Cell Death Dis. 7(e2468)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Murakami Y, Notomi S, Hisatomi T, Nakazawa
T, Ishibashi T, Miller JW and Vavvas DG: Photoreceptor cell death
and rescue in retinal detachment and degenerations. Prog Retin Eye
Res. 37:114–140. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schäfer N, Grosche A, Schmitt SI, Braunger
BM and Pauly D: Complement components showed a time-dependent local
expression pattern in constant and acute white light-induced
photoreceptor damage. Front Mol Neurosci. 10(197)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Maeda A, Palczewska G, Golczak M, Kohno H,
Dong Z, Maeda T and Palczewski K: Two-photon microscopy reveals
early rod photoreceptor cell damage in light-exposed mutant mice.
Proc Natl Acad Sci USA. 111:E1428–E1437. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bian M, Du X, Cui J, Wang P, Wang W, Zhu
W, Zhang T and Chen Y: Celastrol protects mouse retinas from bright
light-induced degeneration through inhibition of oxidative stress
and inflammation. J Neuroinflammation. 13(50)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Masuda T, Shimazawa M and Hara H: Retinal
diseases associated with oxidative stress and the effects of a free
radical scavenger (Edaravone). Oxid Med Cell Longev.
2017(9208489)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smolková K, Mikó E, Kovács T,
Leguina-Ruzzi A, Sipos A and Bai P: Nuclear factor erythroid
2-related factor 2 in regulating cancer metabolism. Antioxid Redox
Signal. 33:966–997. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Canning P, Sorrell FJ and Bullock AN:
Structural basis of Keap1 interactions with Nrf-2. Free Radic Biol
Med. 88:101–107. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tanito M, Kwon YW, Kondo N, Bai J,
Masutani H, Nakamura H, Fujii J, Ohira A and Yodoi J:
Cytoprotective effects of geranylgeranylacetone against retinal
photooxidative damage. J Neurosci. 25:2396–2404. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lv J, Bao S, Liu T, Wei L, Wang D, Ye W,
Wang N, Song S, Li J, Chudhary M, et al: Sulforaphane delays
diabetes-induced retinal photoreceptor cell degeneration. Cell
Tissue Res. 382:477–486. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishinaka Y, Masutani H, Nakamura H and
Yodoi J: Regulatory roles of thioredoxin in oxidative
stress-induced cellular responses. Redox Rep. 6:289–295.
2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cebula M, Schmidt EE and Arnér ESJ: TrxR1
as a potent regulator of the Nrf2-Keap1 response system. ARS.
23:823–853. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ren X, Sun H, Zhang C, Li C, Wang J, Shen
J, Yu D and Kong L: Protective function of pyridoxamine on retinal
photoreceptor cells via activation of the p-Erk1/2/Nrf-2/Trx/ASK1
signalling pathway in diabetic mice. Mol Med Rep. 14:420–424.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Isah T: Rethinking Ginkgo biloba L.:
Medicinal uses and conservation. Pharmacogn Rev. 9:140–148.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zuo W, Yan F, Zhang B, Li J and Mei D:
Advances in the studies of Ginkgo biloba leaves extract on
aging-related diseases. Aging Dis. 8:812–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zaghlool SS, Hanaf LK, Afifi NM and
Ibrahim ER: Histological and immunohistochemical study on the
protective effect of Ginkgo biloba extract against
glutamate-induced neurotoxicity in male albino rat retinal cells.
Egypt J Histol. 35:176–188. 2012.
|
|
20
|
Chen XJ, Ren SM, Dong JZ, Qiu CG, Chen YW
and Tao HL: Ginkgo biloba extract-761 protects myocardium by
regulating Akt/Nrf-2 signal pathway. Drug Des Devel Ther.
13:647–655. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu SQ, Xu CY, Qin MB, Tan L, Zhuge CF,
Mao YB, Lai MY and Huang JA: Ginkgo biloba extract enhances
chemotherapy sensitivity and reverses chemoresistance through
suppression of the KSR1-mediated ERK1/2 pathway in gastric cancer
cells. Oncol Rep. 33:2871–2882. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Czauderna C, Palestino-Dominguez M,
Castven D, Becker D, Zanon-Rodriguez L, Hajduk J, Mahn FL, Herr M,
Strand D, Strand S, et al: Ginkgo biloba induces different gene
expression signatures and oncogenic pathways in malignant and
non-malignant cells of the liver. PLoS One.
13(e0209067)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ranchon I, Gorrand JM, Cluzel J,
Droy-Lefaix MT and Doly M: Functional protection of photoreceptors
from light-induced damage by dimethylthiourea and Ginkgo biloba
extract. Invest Ophth Vis. 40:1191–1199. 1999.PubMed/NCBI
|
|
24
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochromec release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Arango-Gonzalez B, Trifunović D, Sahaboglu
A, Kranz K, Michalakis S, Farinelli P, Koch S, Koch F, Cottet S,
Janssen-Bienhold U, et al: Identification of a common non-apoptotic
cell death mechanism in hereditary retinal degeneration. PLoS One.
9(e112142)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ali D, Tripathi A, Al Ali H, Shahi Y,
Mishra KK, Alarifi S, Alkahtane AA and Manohardas S: ROS-dependent
Bax/Bcl2 and caspase 3 pathway-mediated apoptosis induced by zineb
in human keratinocyte cells. Onco Targets Ther.
11(489)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang A, Yang Q, Li Q, Wang X, Hao S, Wang
J and Ren M: Ginkgo Biloba L. extract reduces H2O2-induced bone
marrow mesenchymal stem cells cytotoxicity by regulating
mitogen-activated protein kinase (MAPK) signaling pathways and
oxidative stress. Med Sci Mon Int Med J Exp Clin Res. 24:3159–3167.
2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Lv J, Cheng Y, Du J, Chen D, Li C
and Zhang J: Apoptosis induced by Ginkgo biloba (EGb761) in
melanoma cells is Mcl-1-dependent. PLoS One.
10(e0124812)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tang L, Bao S, Du Y, Jiang Z, Wuliji A,
Ren X, Zhang C, Chu H, Kong L and Ma H: Antioxidant effects of
Lycium barbarum polysaccharides on photoreceptor degeneration in
the light-exposed mouse retina. Biomed Pharmacother. 103:829–837.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu J, Wei L, Wang Z, Song S, Lin Z, Zhu
J, Ren X and Kong L: Protective effect of Liraglutide on diabetic
retinal neurodegeneration via inhibiting oxidative stress and
endoplasmic reticulum stress. Neurochem Int: 104624, 2019.
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lin CW, Yang CM and Yang CH: Effects of
the emitted light spectrum of liquid crystal displays on
light-induced retinal photoreceptor cell damage. Int J Mol Sci.
20(2318)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Randazzo J, Zhang Z, Hoff M, Kawada H,
Sachs A, Yuan Y, Haider N and Kador P: Orally active
multi-functional antioxidants are neuroprotective in a rat model of
light-induced retinal damage. PLoS One. 6(e21926)2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chu-Tan JA, Rutar M, Saxena K, Wu Y,
Howitt L, Valter K, Provis J and Natoli R: Efficacy of 670 nm light
therapy to protect against photoreceptor cell death is dependent on
the severity of damage. Int J Photoenergy 2016, 2016.
|
|
35
|
Iliescu DA, Ciubotaru A, Ghiţă MA, Dumitru
A and Zăgrean L: Effect of sevoflurane preconditioning on
light-induced retinal damage in diabetic rats. Rom J Ophthalmol.
62:24–33. 2018.PubMed/NCBI
|
|
36
|
Ma K, Xu L, Zhan H, Zhang S, Pu M and
Jonas J: Dosage dependence of the effect of Ginkgo biloba on the
rat retinal ganglion cell survival after optic nerve crush. Eye
(Lond). 23:1598–1604. 2009.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Biddlestone L, Corbett AD and Dolan S:
Oral administration of Ginkgo biloba extract, EGb-761 inhibits
thermal hyperalgesia in rodent models of inflammatory and
post-surgical pain. Br J Pharmacol. 151:285–291. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheng D, Liang B and Li Y:
Antihyperglycemic effect of Ginkgo biloba extract in
streptozotocin-induced diabetes in rats. Biomed Res Int.
2013(162724)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Okumus S, Taysi S, Orkmez M, Saricicek E,
Demir E, Adli M and Al B: The effects of oral Ginkgo biloba
supplementation on radiation-induced oxidative injury in the lens
of rat. Pharmacogn Mag. 7:141–145. 2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kernt M, Walch A, Neubauer AS, Hirneiss C,
Haritoglou C, Ulbig MW and Kampik A: Filtering blue light reduces
light-induced oxidative stress, senescence and accumulation of
extracellular matrix proteins in human retinal pigment epithelium
cells. Clin Exp Ophthalmol. 40:e87–e97. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Aydin Y, Chedid M, Chava S, Williams DD,
Liu S, Hagedorn CH, Sumitran-Holgersson S, Reiss K, Moroz K, Lu H,
et al: Activation of PERK-Nrf-2 oncogenic signaling promotes
Mdm2-mediated Rb degradation in persistently infected HCV culture.
Sci Rep. 7(9223)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Munemasa Y, Ahn J, Kwong J, Caprioli J and
Piri N: Redox proteins thioredoxin 1 and thioredoxin 2 support
retinal ganglion cell survival in experimental glaucoma. Gene Ther.
16:17–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kong L, Tanito M, Huang Z, Li F, Zhou X,
Zaharia A, Yodoi J, McGinnis JF and Cao W: Delay of photoreceptor
degeneration in tubby mouse by sulforaphane. J Neurochem.
101:1041–1052. 2007.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wu L, Gao L, Cao Y, Chen F, Sun T and Liu
Y: Analysis of the protective mechanism of liraglutide on
retinopathy based on diabetic mouse model. Saudi J Biol Sci.
26:2096–2101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ma Q: Role of Nrf-2 in oxidative stress
and toxicity. Annu Rev Pharmacol Toxicol. 53:401–426.
2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ryter SW, Kim HP, Hoetzel A, Park JW,
Nakahira K, Wang X and Choi AM: Mechanisms of cell death in
oxidative stress. Antioxid Redox Signal. 9:49–89. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Lo AC, Woo TT, Wong RL and Wong D:
Apoptosis and other cell death mechanisms after retinal detachment:
Implications for photoreceptor rescue. Ophthalmologica. 226 (Suppl
1):S10–S17. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Peter ME, Heufelder AE and Hengartner MO:
Advances in apoptosis research. Proc Natl Acad Sci USA.
94:12736–12737. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
D'amelio M, Cavallucci V and Cecconi F:
Neuronal caspase-3 signaling: Not only cell death. Cell Death
Differ. 17:1104–1114. 2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Adams JM and Cory S: Bcl-2-regulated
apoptosis: Mechanism and therapeutic potential. Curr Opin Immunol.
19:488–496. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gogada R, Prabhu V, Amadori M, Scott R,
Hashmi S and Chandra D: Resveratrol induces p53-independent,
X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein
oligomerization on mitochondria to initiate cytochrome c release
and caspase activation. J Biol Chem. 286:28749–28760.
2011.PubMed/NCBI View Article : Google Scholar
|