Introduction
Sentinel lymph node (SLN) biopsy constitutes a key
diagnostic tool in the treatment and staging of melanoma (1,2). By
virtue of being the first lymph node to drain a cutaneous lesion,
the SLN is predictive of the metastatic status of a regional lymph
node group (3,4). The presence of metastatic disease in a
SLN remains an important predictor of survival (4). It has previously been shown that
drainage patterns can be altered in patients with melanoma and
previous axillary dissection, such as truncal melanoma mapping to
cervical lymph nodes (5). Recent
studies have demonstrated that there in fact exists a great
variation in the lymphatic drainage in patients with malignant
melanoma as some patients have demonstrated drainage to lymph nodes
outside of conventional nodal basins. This concept referred to as
‘interval nodes’, highlights the link between a primary melanoma
and its regional nodal basin (1,6).
We describe an unusual localization of a limb
malignant melanoma to a neck basin in a patient with no prior node
axillary node dissection.
Case report
The presented patient is followed in our Melanoma
clinic at the authors' institution. All surgical procedures were
also performed at the authors' institution. The patient was
consented separately for her surgical care and for being included
in this research manuscript.
The patient is a 71-year-old Caucasian female who
presented to our clinic in May of 2023 as a referral from a
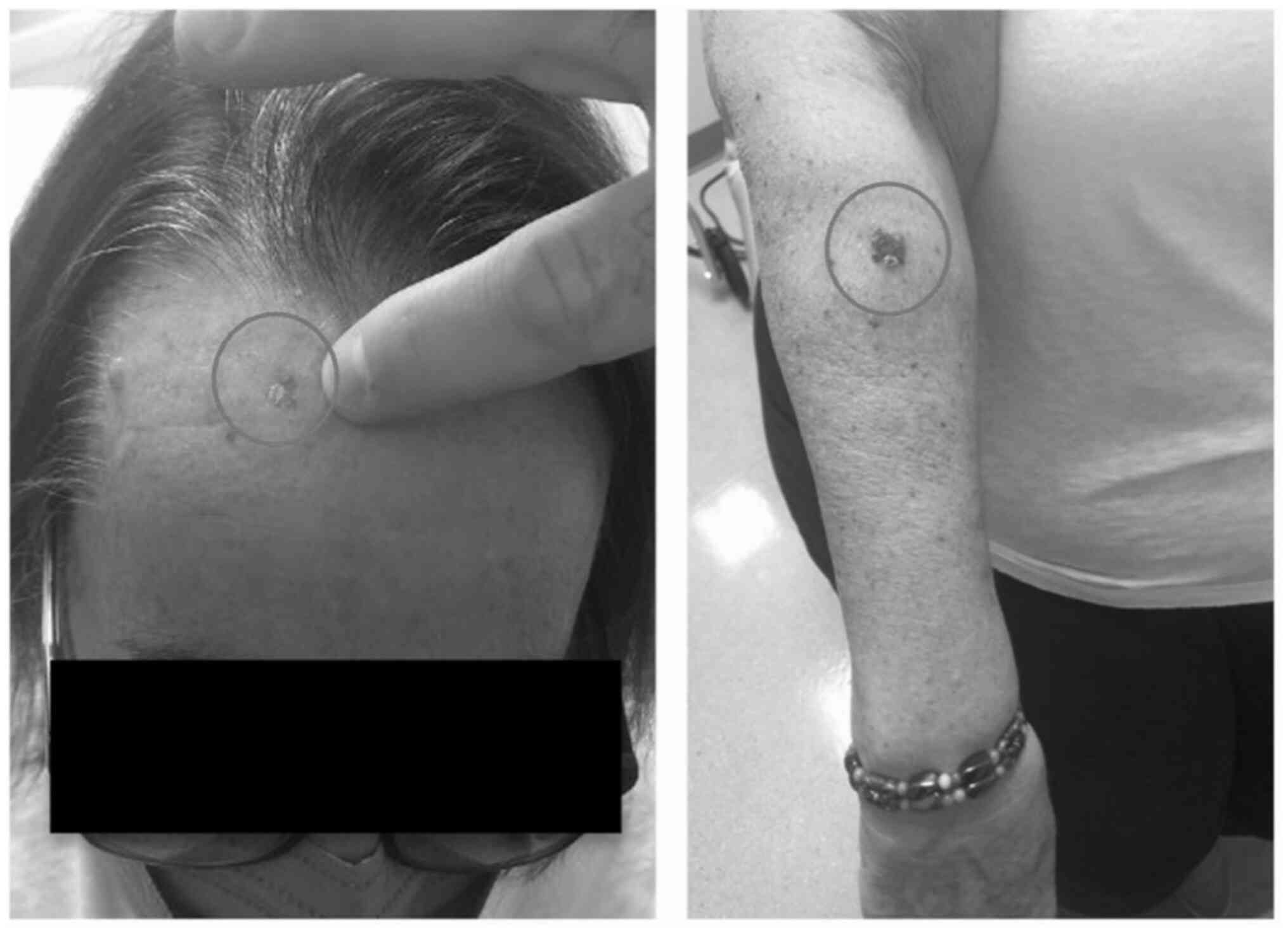
community dermatologist with malignant melanoma lesions on her
forehead measuring 0.4 mm in depth and on her right forearm
measuring 1mm in depth (Fig. 1).
Her past medical history is significant for benign essential
hypertension, hyperlipidemia, obesity, urinary retention, and UTI.
Her surgical history is significant for a cesarean section. She has
no known allergies. Her family history is significant for heart
disease in her father.
The lesions were present since January of 2023 and
had increased in size over time. No nodes were palpable in either
the neck, parotid or axilla. Both the history, clinical and
dermatoscopic characteristics of the lesions provided the basis for
the diagnosis of melanoma. Subsequent histological examination
(dermatopathology) confirmed the diagnosis of malignant melanoma:
forehead lesion Breslow thickness was 0.4 mm, mitotic rate was less
than 1 per square mm Clark's, with no ulcerations present; and
right forearm lesion Breslow thickness was 1 mm, mitotic rate was 1
per square mm Clark's, with ulceration present.
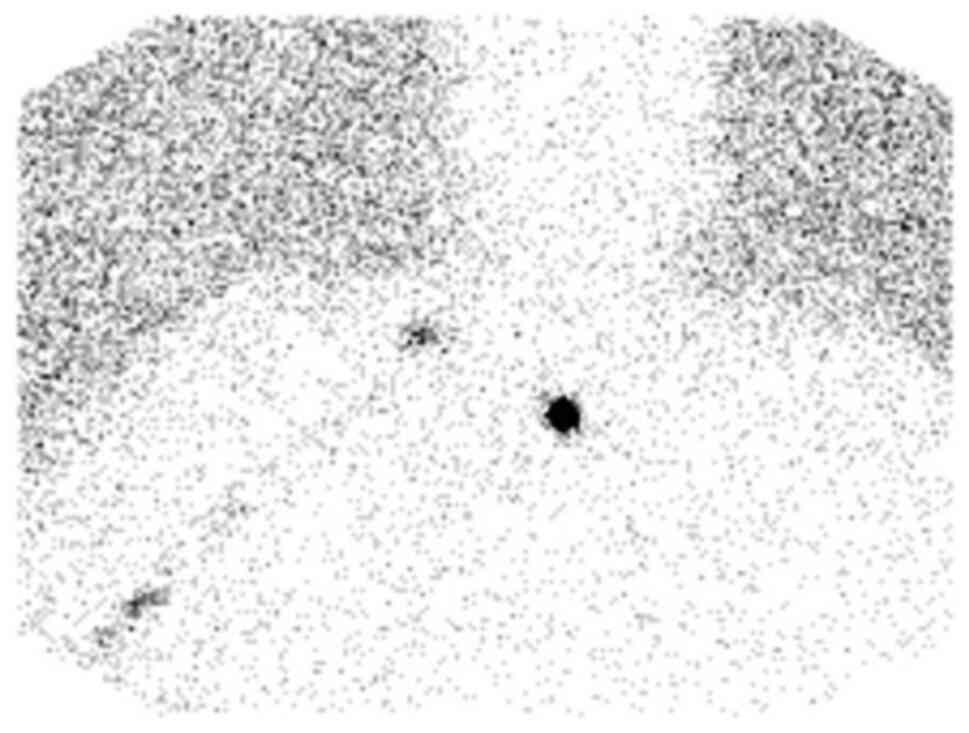
A preoperative lymphoscintigram was performed with
approximately 0.5 mCi of Tc-99m labeled LYMPHOSEEK® (technetium Tc
99m tilmanocept, Cardinal Health, OH, USA) administered as four
intradermal injections around the site of the right forearm lesion.
Subsequently, multiple spot views of the head, neck, and chest were
obtained for lymph node localization. The preoperative
lymphoscintigram demonstrated tracking of radiotracer to the right
supraclavicular region and no uptake in the right axilla (Fig. 2).
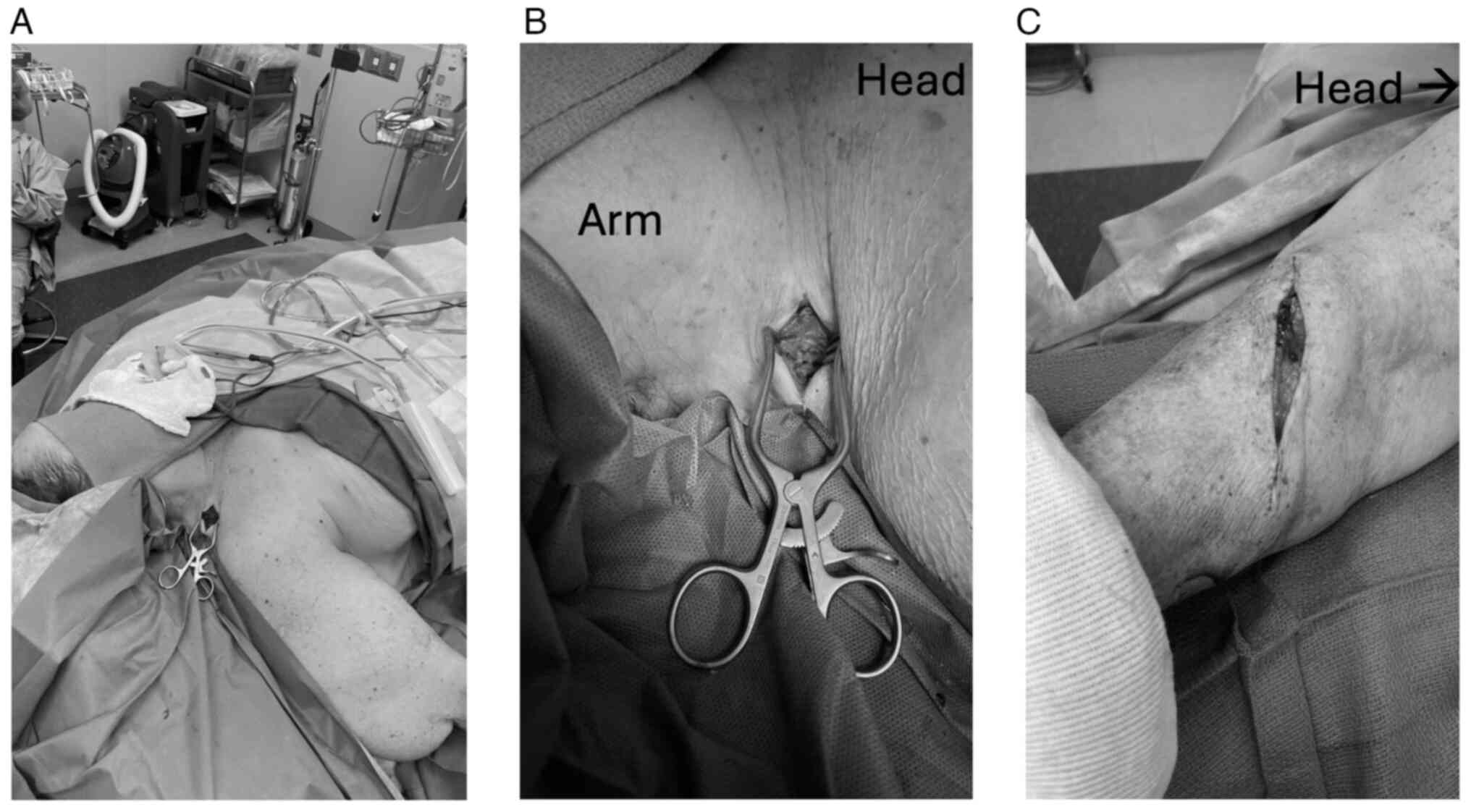
In conformity with the AJCC Classification, given
the report that the forehead lesion is 0.4 mm in depth with no
ulcerations present, we performed a wide excision only to the level
of frontalis muscle fascia (Fig.
3A) followed by primary closure for the forehead melanoma, as
there was no indication for SLN biopsy. However, given the report
that right forearm melanoma is 1 mm in depth with ulceration
present, we performed wide excision as well as SLN of the right
supraclavicular region (Fig. 3B and
C). We marked out a 1 cm margin around the biopsy site in the
forearm and then injected technetium-99m followed by methylene blue
for intraoperative lymph node identification. We listened with the
Geiger counter, there was no uptake in the axilla, which correlated
with the preoperative lymphoscintigram, and there was uptake in the
right supraclavicular region along the trapezius in level 5 of the
right neck. We then marked out a transverse incision in the right
neck, incised through the skin and subcutaneous tissue, identified
the trapezius muscle, identified some of the cutaneous nerves and
the deeper motor nerves going to the trapezius and retracted those
anteriorly and along the border of sternocleidomastoid and deep
cervical based nodes identified a cluster of lymph nodes which were
carefully dissected out. The count of the lymph node external to
the patient was 606 Sievert. The final count in the right neck was
0 Sievert. No additional nodes were taken. Postoperative analysis
revealed margins to be negative. Lymph node biopsy results were
also negative for melanoma on hematoxylin and eosin (H&E)
staining and SOX10 and HMB45 immunostaining. The patient followed
up 2 weeks postoperatively. Her incisions were healing
appropriately and she is overall doing well. Pathology from her
surgical excisions from both the forehead and forearm demonstrated
a melanoma of final depth of 0.4 and 3 mm respectively with
negative margins. Lymph node biopsy demonstrated lymph node
negative for melanoma. The patient will continue to follow up with
the medical oncology team for routine melanoma surveillance every 3
to 6 months for 5 years.
Discussion
Prior studies have demonstrated great variation in
the lymphatic drainage in patients with malignant melanoma and have
highlighted cases of interval nodes and unusual localization to
regional nodal basins, most common in the head and neck, followed
by the upper extremity (1,2,7). We
presented a rare case of localization of a right forearm malignant
melanoma to the right supraclavicular node, skipping the axillary
basin. Although it is unclear how prior axillary node dissection
may impact localization pattern, our case highlights a patient with
no prior axillary node dissection, other surgeries or radiation in
the area. The presented patient also had the co-existence of a
lesion on the forehead measuring 0.4 mm in depth and
non-ulcerated.
In our patient, preoperative lymphoscintigram
applied to the right forearm lesion demonstrated no uptake in the
axilla but an uptake in the right supraclavicular region. This
correlated with the intraoperative findings using a handheld Geiger
counter, which confirmed a signal only in the right supraclavicular
region along the trapezius and level 5. Knowledge of this ‘skipped
sentinel lymph node’ pattern outlined in this paper will add to our
understanding of disease localization, future metastasis and unique
presentations. This can also potentially set the ground for future
investigation into the mechanism behind this phenomenon.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
OFN and JC contributed to the study design, writing
and revisions of this manuscript. OFN and JC confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by the
patient.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SLN
|
sentinel lymph node
|
|
AJCC
|
American Joint Committee on Cancer
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
McMasters KM, Chao C, Wong SL, Wrightson
WR, Ross MI, Reintgen DS, Noyes RD, Cerrito PB and Edwards MJ;
Sunbelt Melanoma Trial Group, : Interval sentinel lymph nodes in
melanoma. Arch Surg. 137:543–549. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Clary BM, Brady MS, Lewis JJ and Coit DG:
Sentinel lymph node biopsy in the management of patients with
primary cutaneous melanoma: Review of a large single-institutional
experience with an emphasis on recurrence. Ann Surg. 233:250–258.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gershenwald JE, Colome MI, Lee JE,
Mansfield PF, Tseng C, Lee JJ, Balch CM and Ross MI: Patterns of
recurrence following a negative sentinel lymph node biopsy in 243
patients with stage I or II melanoma. J Clin Oncol. 16:2253–2260.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gershenwald JE, Thompson W, Mansfield PF,
Lee JE, Colome MI, Tseng CH, Lee JJ, Balch CM, Reintgen DS and Ross
MI: Multi-institutional melanoma lymphatic mapping experience: The
prognostic value of sentinel lymph node status in 612 stage I or II
melanoma patients. J Clin Oncol. 17:976–983. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson C, Intenzo C, Mastrangelo MJ,
Feeney K and Berger AC: Altered drainage patterns in patients with
melanoma and previous axillary dissection. J Dermatol. 40:564–566.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uren RF, Howman-Giles R, Thompson JF,
McCarthy WH, Quinn MJ, Roberts JM and Shaw HM: Interval nodes: The
forgotten sentinel nodes in patients with melanoma. Arch Surg.
135:1168–1172. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matter M, Nicod Lalonde M, Allaoua M,
Boubaker A, Liénard D, Gugerli O, Cerottini JP, Bouzourene H,
Bischof Delaloye A and Lejeune F: The role of interval nodes in
sentinel lymph node mapping and dissection for melanoma patients. J
Nucl Med. 48:1607–1613. 2007. View Article : Google Scholar : PubMed/NCBI
|