Introduction
MicroRNAs (miRNAs) are well-conserved non-coding
RNAs that regulate gene expression by base-pairing to the
3′-untranslated regions of mRNA targets, thus resulting in
post-transcriptional gene repression or target degradation
(1,2).
Emerging evidence indicates that miRNAs can act as tumor
suppressors or oncogenes and participate in a wide variety of
cellular processes, such as cell growth, differentiation,
inflammation and apoptosis (3,4). It has
also been observed that miRNAs could have played important roles in
tumor development, diagnosis and prognosis (5). Studies have demonstrated that the
single-nucleotide polymorphisms (SNPs) or mutations in miRNA-coding
genes functionally affected the expression and biogenesis of mature
miRNAs and their target genes, consequently contributing to cancer
susceptibility (6–8).
Among the variant SNPs, an important G/C
polymorphism, designated as rs531564, was identified in
pri-miR-124-1 and the G allele was reported to alter the
process and expression of mature miR-124 in the nervous
system (9). Previously, Yang et
al (10) and Ye et al
(11) studied the role of this
polymorphism in the cancer risk among Caucasian populations.
Additionally, several studies suggested that there was no evident
association between the variant genotype of the
pri-miR-124-1 rs531564 polymorphism and cancer risk in
Chinese populations (12–14). However, Xiong et al (15) identified that the G allele of rs531564
was less frequent in the cervical cancer patients when compared
with healthy subjects (P=0.014) and the polymorphism was clearly
associated with a reduced risk of cervical cancer in Chinese women.
In addition, You (16) and Zhang
et al (17) reported that the
rs531564 genotype was significantly associated with the risk of
esophageal squamous cell carcinoma (ESCC) in Chinese populations.
The results were contradictory and inconclusive, therefore a
meta-analysis was performed in the present study to evaluate the
association between the rs531564 polymorphism and cancer
susceptibility in the Chinese population.
Materials and methods
Literature search
A literature search for eligible studies that
explored the association between the pri-miR-124-1 rs531564
polymorphism and cancer risk was carried out using PubMed, EMBASE,
Web of Science and CNKI databases (until October 1, 2014). Keywords
used in the searches included: (‘miR-124’ or
‘miRNA-124’ or ‘rs531564’ or ‘pri-miR-124-1’) and
(‘tumor’ or ‘cancer’ or ‘carcinoma’) and (‘polymorphism’ or ‘SNP’
or ‘allele’ or ‘variation’). The search was performed without
restriction on language or publication years. The reference lists
of retrieved studies were also checked for additional
literature.
Selection criteria
Studies were identified as eligible if they met the
following criteria: i) Evaluated the association of the
pri-miR-124-1 rs531564 polymorphism and cancer risks; ii)
case-control studies; and iii) detailed genotype data for
estimating odds ratios (ORs) and 95% confidence intervals (CIs).
Studies were excluded based on the following criteria: i) Reviews
or animal studies; ii) lack of sufficient data for meta-analysis;
and iii) repeated outcomes from the same samples. When overlapping
data of the same case series were included in more than one study,
only the most recent or complete study was used in the
meta-analysis.
Data extraction
The information extracted from each study was as
follows: First author, publication year, country origin, ethnicity,
cancer types, diagnostic methods for cancer, source of controls,
genotyping methods, the numbers of genotyped cases and controls and
Hardy-Weinberg equilibrium (HWE) for control groups. Quality
assessment of the included studies was evaluated according to the
Newcastle-Ottawa Quality Assessment Scale (NOS) (18). The information was extracted by two
investigators (Fang and Zeng) in duplicate and any disagreements
were discussed by group discussions to achieve a consensus.
Statistical analysis
The pooled ORs and 95% CIs were summarized to assess
the strength of the association between the pri-miR-124-1
rs531564 polymorphism and cancer susceptibility with five genetic
models: Allele contrasts (G vs. C), and homozygote (GG vs. CC),
heterozygote (CG vs. CC), dominant (GG/CG vs. CC) and recessive
models (GG vs. CG/CC), separately. Heterogeneity was examined with
the χ2 and I2 test. When heterogeneity was
absent (P>0.10, I2<50), a fixed effect model was
used for secondary analysis. Otherwise, a random effect model was
employed (19,20). HWE was measured with the Pearson's
goodness-of-fit test in control groups and P<0.05 was deemed not
to conform to HWE. Sensitivity analysis was conducted to assess the
stability of the combined results by the omission of every single
study each time or excluding studies with disrupted HWE. Funnel
plots were also used to estimate the possible publication bias. All
the analyses were operated using Cochrane Collaboration's Review
Manager Software 5.2 and P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of studies
Based on the selection criteria above, five
case-control studies (13–17) were finally enrolled in the
meta-analysis with publication years that ranged from 2011 to 2014.
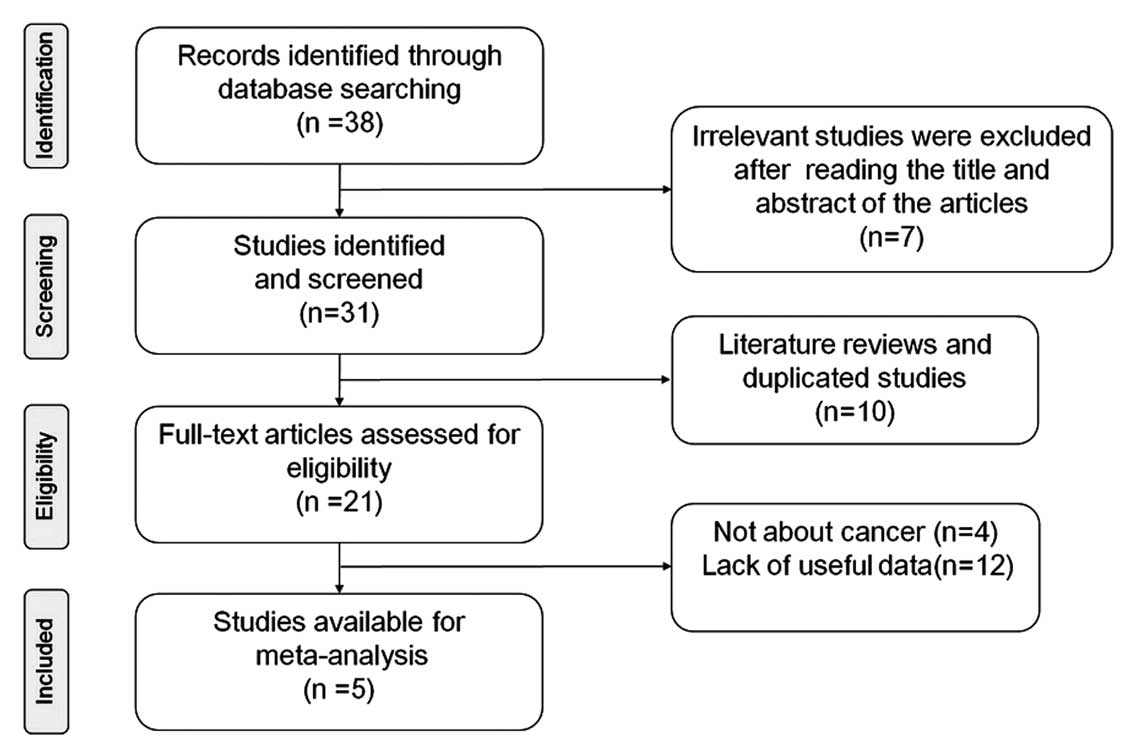
The detailed literature retrieval process is presented in Fig. 1. A total of 4,763 subjects were
involved in the meta-analysis, including 2,253 cancer cases and
2,510 healthy controls. Multiple cancer types were analyzed in the
studies, including triple-negative breast cancer (13), cervical cancer (15) and ESCC (14,16–17). The
diagnoses of these tumors were confirmed by histological or
histopathological examinations. In addition, three studies used
hospital-based controls (13–15), one used population-based controls
(17) and one used population- and
hospital-based controls (16).
Polymerase chain reaction-based ligase detection reaction was
performed in two studies (14,15) and
three used other methods. The genotype frequencies of the controls
were all fitted in HWE, except for one study by You (16). The NOS scores of the selected studies
were >5 (moderate-to-high quality). The main characteristics and
methodological quality of the included studies are summarized in
Table I.
 | Table I.Characteristics of the included
studies in the meta-analysis. |
Table I.
Characteristics of the included
studies in the meta-analysis.
|
| Case genotypes | Control
genotypes |
|
|---|
|
|
|
|
|
|---|
| First author,
year | Country | Ethnicity | Cancer type | Diagnostic
method | Source of
controls | Genotyping | CC | CG | GG | CC | CG | GG | PHWE | NOS score | (Refs.) |
|---|
| Ma, 2013 | China | Asian | TNBC |
Histopathological | HB | MassARRAY | 126 | 52 | 4 | 136 | 45 | 8 | 0.098 | 7 | (13) |
| Xiong, 2014 | China | Asian | Cervical |
Histopathological | HB | PCR-LDR | 91 | 15 | 1 | 151 | 51 | 6 | 0.507 | 6 | (15) |
| You, 2011 | China | Asian | ESCC |
Histopathological | PB/HB | MALDI-TOF-MS | 166 | 69 | 9 | 105 | 72 | 4 | 0.037 | 6 | (16) |
| Yin, 2013 | China | Asian | ESCC |
Histopathological | HB | PCR-LDR | 454 | 146 | 11 | 470 | 168 | 19 | 0.400 | 7 | (14) |
| Zhang, 2014 | China | Asian | ESCC | Histological | PB | SNaPshot | 803 | 295 | 11 | 910 | 331 | 34 | 0.555 | 8 | (17) |
Quantitative synthesis
In the present analysis, data from five case-control
studies were collected and analyzed to investigate the association
between the pri-miR-124-1 rs531564 polymorphism and cancer
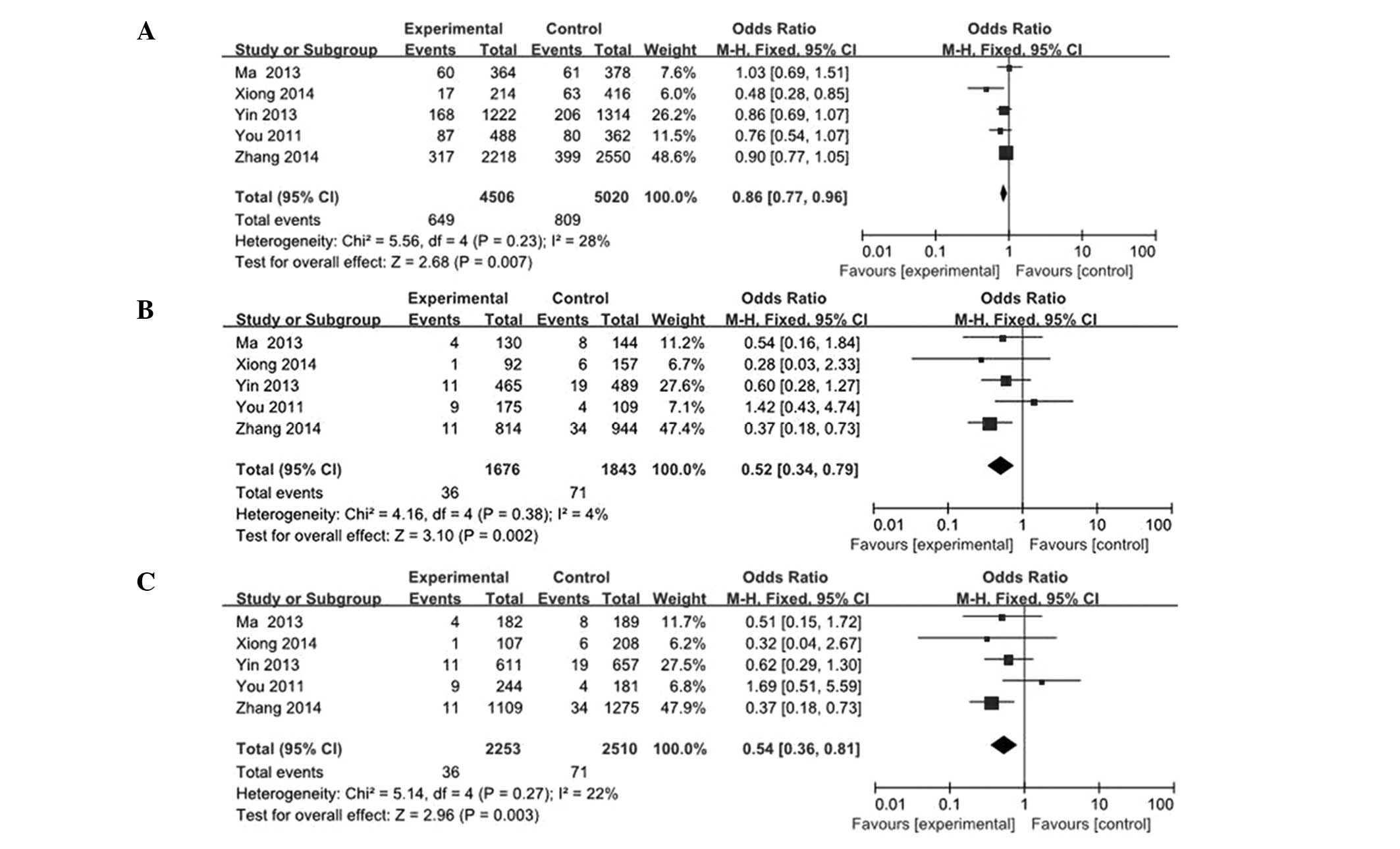
risk. As a result, the meta-analysis of overall studies revealed
that a significantly decreased cancer risk was observed in the G
vs. C, GG vs. CC and GG vs. CG/CC models tested (G vs. C: OR, 0.86;
95% CI, 0.77–0.96; GG vs. CC: OR, 0.52; 95% CI, 0.34–0.79; GG vs.
CG/CC: OR, 0.54; 95% CI, 0.36–0.81; Fig.
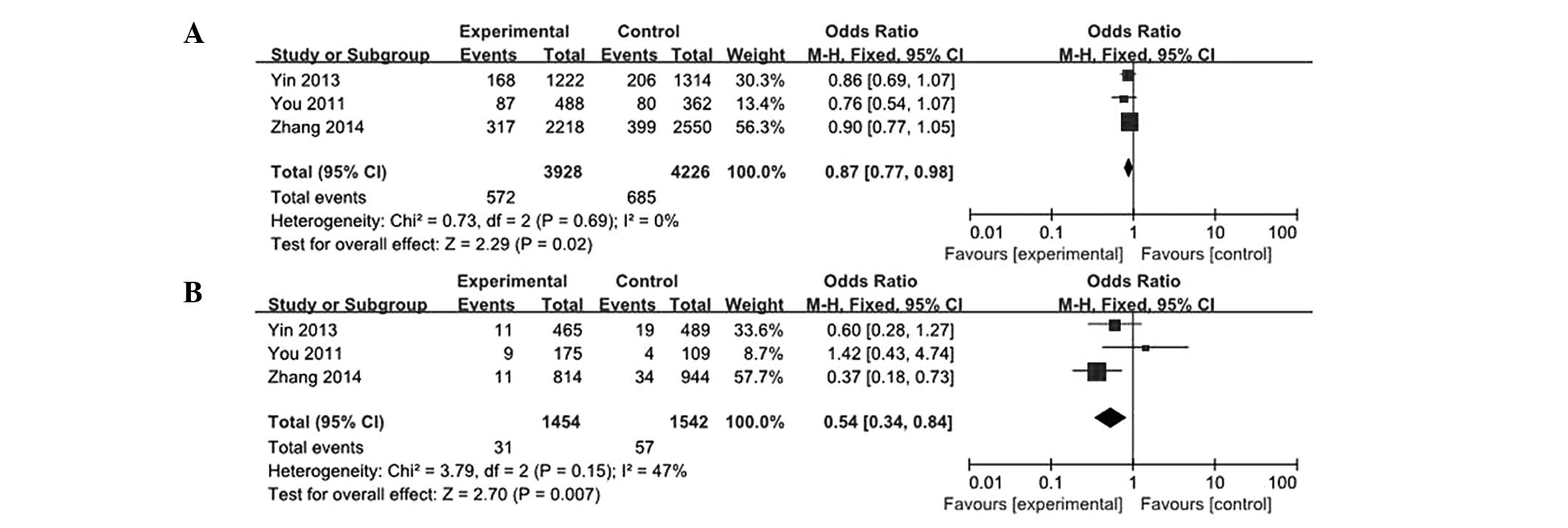
2). In the subgroup analysis by cancer types, the results
showed that the rs531564 genotype was markedly associated with a
decreased risk of ESCC (G vs. C: OR, 0.87; 95% CI, 0.77–0.98; GG vs
CC: OR, 0.54; 95% CI, 0.34–0.84; Fig.
3). The main results are summarized in Table II.
 | Table II.Meta-analysis of the rs531564
polymorphism with the cancer risk. |
Table II.
Meta-analysis of the rs531564
polymorphism with the cancer risk.
|
| Test of
association | Test of
heterogeneity |
|---|
|
|
|
|
|---|
| Study groups | OR (95% CI) | Z | P-value | Model | Ph | I2,
% |
|---|
| Overall |
|
|
|
|
|
|
| G vs.
C | 0.86
(0.77–0.96) | 2.68 | 0.007 | F | 0.23 | 28 |
| GG vs.
CC | 0.52
(0.34–0.79) | 3.10 | 0.002 | F | 0.38 | 4 |
| CG vs.
CC | 0.86
(0.67–1.10) | 1.24 | 0.220 | R | 0.03 | 62 |
| GG/CG
vs. CC | 0.84
(0.67–1.03) | 1.65 | 0.100 | R | 0.08 | 52 |
| GG vs.
CG/CC | 0.54
(0.36–0.81) | 2.96 | 0.003 | F | 0.27 | 22 |
| ESCC |
|
|
|
|
|
|
| G vs.
C | 0.87
(0.77–0.98) | 2.29 | 0.020 | F | 0.69 | 0 |
| GG vs.
CC | 0.54
(0.34–0.84) | 2.70 | 0.007 | F | 0.15 | 47 |
| CG vs.
CC | 0.87
(0.68–1.11) | 1.14 | 0.260 | R | 0.08 | 60 |
| GG/CG
vs. CC | 0.88
(0.77–1.01) | 1.76 | 0.080 | F | 0.23 | 32 |
| GG vs.
CG/CC | 0.64
(0.30–1.37) | 1.15 | 0.250 | R | 0.09 | 59 |
Moderate heterogeneity was observed in the CG vs. CC
and GG/CG vs. CC models tested [CG vs. CC: P=0.22, P-value of Q
test for heterogeneity test (Ph)=0.03, I2=62%;
GG/CG vs. CC: P=0.10, Ph=0.08, I2=52%; Table II]. Therefore, the random effect
models were used and sensitivity analyses were performed. When the
study by Xiong et al (15) was
removed, the heterogeneity in the GG/CG vs. CC model was largely
reduced, but the result remained insignificant (P=0.13,
Ph=0.25, I2=26%). When the study with disrupted
HWE by You (16) was removed, the
heterogeneity in the GG/CG vs. CC model was reduced and the result
was consistently insignificant (P=0.15, Ph=0.12,
I2=49%). Sensitivity analysis indicated that the pooled
ORs were not altered by deleting one study at a time (data not
shown).
Assessment of publication bias
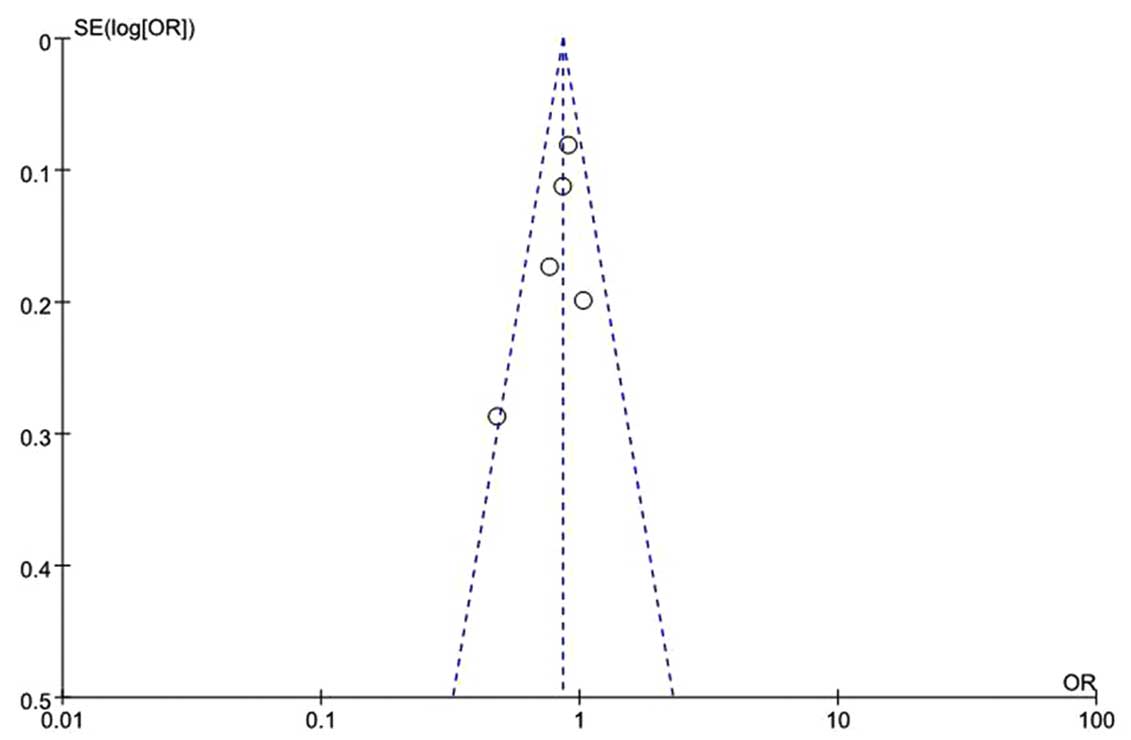
Funnel plots were performed to access the
publication bias of eligible studies, in which the standard error
of log (OR) for each study was plotted against its log (OR). The
graphical funnel plots (Fig. 4)
appeared symmetrical and no clear evidence of publication bias was
observed among all the genetic models.
Discussion
miRNAs are emerging as crucial regulators of diverse
biological pathways and have been indicated in the etiology of
human tumors through modulating approximately one third of the
human genome (21,22). Disruption of miRNA-dependent
regulation by SNPs in either miRNAs genes or miRNA-binding sites
resulted in a myriad of diseases including human malignancy
(23,24). However, the effects of genetic
variants in miRNA-related genes on cancer susceptibility remain
largely unknown.
To the best of our knowledge, this is the first
meta-analysis studied on the association between the
pri-miR-124-1 rs531564 polymorphism and cancer risk in
Chinese populations. According to the study, the rs531564
polymorphism was significantly correlated with a reduced cancer
risk in the G vs. C, GG vs. CC and GG vs. CG/CC models tested when
all the studies were pooled into analysis. However, heterogeneity
existed in the heterozygous (CG vs. CC: Ph=0.03,
I2=62%) and dominant models (GG/CG vs. CC:
Ph=0.08, I2=52%) (Table II). Following the removal of the
study by Xiong et al (15),
the heterogeneity in the dominant model was largely reduced,
suggesting that the rs531564 polymorphism may have the opposite
effects in different tumors and causing the heterogeneity, yet the
limitation of cancer types inhibited further verification. In the
stratified analysis by cancer sites, the results confirmed that the
rs531564 variant carriers had decreased risks for ESCC in the G vs.
C and GG vs. CC models tested, indicating the polymorphism may have
a potentially protective value in ESCC patients (Fig. 3).
Recently, the role of miR-124 in cancer
development has received increasing attention. Shi et al
(25) demonstrated that
miR-124 was commonly reduced in prostate cancer, which
directly targets the androgen receptor and subsequently induces an
increased expression of the tumor suppressive gene, p53.
Furuta et al (26) suggested
cyclin-dependent kinase 6 (CDK6) as a possible target for
miR-124, which was a key modulator of cell cycle and
differentiation. The study also indicated that the restoration of
miR-124 expression impairs the growth of hepatocellular
carcinoma cells. In addition, decreased expression of
miR-124 was also detected in glioblastoma multiforme (GBM)
and transfection of miR-124 induced
G0/G1 cell cycle arrest in GBM cell lines
through inhibition of CDK6 and phosphorylated retinoblastoma
proteins (27). Therefore,
miR-124 may act as a tumor suppressor and have significant
effects on the cancer risk by modulating these targets.
However, several limitations to the present
meta-analysis should be considered. Firstly, the number of studies
for each site-specific cancer was so limited that further stratified
analysis on breast and cervical cancer could not be performed.
Secondly, lack of sufficient data and detailed individual
information restricted further evaluation on the effects of
potential interactions between the genetic variation and
epidemiological factors such as age, gender and lifestyle-related
factors. Thirdly, the cumulative effects of miRNA-related genetic
variants were also not investigated. Fourthly, there was not enough
study on the Chinese population and no study on other populations
in the meta-analysis. Therefore, larger sample sizes with different
ethnic groups are required for further study. Finally, only
published studies in English or Chinese were enrolled in this
analysis, and a potential language bias may exist.
In conclusion, the meta-analysis suggested a
significant association between the pri-miR-124-1 rs531564
polymorphism and decreased cancer susceptibility in Chinese
population, which may be a potential biomarker for the early
diagnosis of ESCC. However, investigations of diverse ethnic
populations and various types of cancer are of great value to
verify these findings.
Acknowledgements
The present study was sponsored by the Scientific
Research Foundation for the Returned Overseas Chinese Scholars,
State Education Ministry, and was supported by grants from the
Natural Science Foundation of China (nos. 30873044 and
81272372).
References
|
1
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Farh KK, Grimson A, Jan C, Lewis BP,
Johnston WK, Lim LP, Burge CB and Bartel DP: The widespread impact
of mammalian microRNAs on mRNA repression and evolution. Science.
310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ruan K, Fang X and Ouyang G: MicroRNAs:
Novel regulators in the hallmarks of human cancer. Cancer Lett.
285:116–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen T, Li Z, Yan J, Yang X and Salminen
W: MicroRNA expression profiles distinguish the carcinogenic
effects of riddelliine in rat liver. Mutagenesis. 27:59–66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barbarotto E, Schmittgen TD and Calin GA:
MicroRNAs and cancer: profile, profile, profile. Int J Cancer.
122:969–977. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu Z, Liang J, Wang Z, Tian T, Zhou X,
Chen J, Miao R, Wang Y, Wang X and Shen H: Common genetic variants
in pre-microRNAs were associated with increased risk of breast
cancer in Chinese women. Hum Mutat. 30:79–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang F, Sun G, Zou Y, Li Y, Hao L and Pan
F: Association of microRNA-499 rs3746444 polymorphism with cancer
risk: Evidence from 7188 cases and 8548 controls. PLoS One.
7:e450422012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roy R, De Sarkar N, Ghose S, Paul RR, Pal
M, Bhattacharya C, Chowdhury SK, Ghosh S and Roy B: Genetic
variations at microRNA and processing genes and risk of oral
cancer. Tumour Biol. 35:3409–3414. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi L, Hu Y, Zhan Y, Wang J, Wang BB, Xia
HF and Ma X: A SNP site in pri-miR-124 changes mature miR-124
expression but no contribution to Alzheimer's disease in a
Mongolian population. Neurosci Lett. 515:1–6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang H, Dinney CP, Ye Y, Zhu Y, Grossman
HB and Wu X: Evaluation of genetic variants in microRNA-related
genes and risk of bladder cancer. Cancer Res. 68:2530–2537. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye Y, Wang KK, Gu J, Yang H, Lin J, Ajani
JA and Wu X: Genetic variations in microRNA-related genes are novel
susceptibility loci for esophageal cancer risk. Cancer Prev Res
(Phila). 1:460–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y, Du WD, Chen G, Ruan J, Xu S, Zhou
FS, Zuo XB, Lv ZJ and Zhang XJ: Association analysis of genetic
variants in microRNA networks and gastric cancer risk in a Chinese
Han population. J Cancer Res Clin Oncol. 138:939–945. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma F, Zhang P, Lin D, Yu D, Yuan P, Wang
J, Fan Y and Xu B: There is no association between microRNA gene
polymorphisms and risk of triple negative breast cancer in a
Chinese Han population. PLoS One. 8:e601952013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin J, Wang X, Zheng L, et al:
Hsa-miR-34b/c rs4938723 T>C and hsa-miR-423 rs6505162 C>A
polymorphisms are associated with the risk of esophageal cancer in
a Chinese population. PLoS One. 8:e805702013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiong X, Cheng J, Liu X, Tang S and Luo X:
Correlation analysis between miR-124 rs531564 polymorphisms and
susceptibility to cervical cancer. Nan Fang Yi Ke Da Xue Xue Bao.
34:2102132014.(In Chinese). PubMed/NCBI
|
|
16
|
You WY: A Case Control study on the
Association between Polymorphisms of microRNA Genes and
Susceptibility for Kazakh's Esophageal Cancer (unpublished PhD
thesis). Shihezi University 2011, (In Chinese).
|
|
17
|
Zhang J, Huang X, Xiao J, et al:
Pri-miR-124 rs531564 and pri-miR-34b/c rs4938723 polymorphisms are
associated with decreased risk of esophageal squamous cell
carcinoma in Chinese populations. PLoS One. 9:e1000552014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
DerSimonian R and Kacker R: Random-effects
model for meta-analysis of clinical trials: An update. Contemp Clin
Trials. 28:105–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu W, Xu J, Liu S, Chen B, Wang X, Li Y,
Qian Y, Zhao W and Wu J: Effects of common polymorphisms rs11614913
in miR-196a2 and rs2910164 in miR-146a on cancer susceptibility: A
meta-analysis. PLoS One. 6:e204712011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radojicic J, Zaravinos A, Vrekoussis T,
Kafousi M, Spandidos DA and Stathopoulos EN: MicroRNA expression
analysis in triple-negative (ER, PR and Her2/neu) breast cancer.
Cell Cycle. 10:507–517. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salzman DW and Weidhaas JB: SNPing cancer
in the bud: microRNA and microRNA-target site polymorphisms as
diagnostic and prognostic biomarkers in cancer. Pharmacol Ther.
137:55–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicoloso MS, Sun H, Spizzo R, et al:
Single-nucleotide polymorphisms inside microRNA target sites
influence tumor susceptibility. Cancer Res. 70:2789–2798. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin Y and Lee CG: Single Nucleotide
Polymorphisms Associated with microRNA Regulation. Biomolecules.
3:287–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi XB, Xue L, Ma AH, Tepper CG,
Gandour-Edwards R, Kung HJ and deVere White RW: Tumor suppressive
miR-124 targets androgen receptor and inhibits proliferation of
prostate cancer cells. Oncogene. 32:4130–4138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furuta M, Kozaki KI, Tanaka S, Arii S,
Imoto I and Inazawa J: miR-124 and miR-203 are epigenetically
silenced tumor-suppressive microRNAs in hepatocellular carcinoma.
Carcinogenesis. 31:766–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Silber J, Lim DA, Petritsch C, et al:
miR-124 and miR-137 inhibit proliferation of glioblastoma
multiforme cells and induce differentiation of brain tumor stem
cells. BMC Med. 6:142008. View Article : Google Scholar : PubMed/NCBI
|