Introduction
Atrial fibrillation is the most common type of
persistent and rapid arrhythmia, with a prevalence of 0.4–1% among
the total population (1). The
prevalence of atrial fibrillation increases with age, likely due to
structural and electrophysiological changes associated with aging
and age-associated atrial remodeling. Aging may increase the
dispersion of the atrial effective refractory period (2). In addition, L-type Ca channel
expression in the right atrium decreases significantly with age,
even following the substitution of Ba2+ for
Ca2+ (3). By contrast,
the transient outward potassium current (Ito) and persistent
potassium current (Isus) increase with age (3). A recent study (4) reported that the funny current (If),
mediated by hyperpolarization-activated non-specific cation
channels (HCNs), was also elevated in the right atrium of patients
with chronic atrial fibrillation.
To the best of our knowledge, to date, no study has
investigated whether HCN channels and post-transcription regulators
miR-1 and miR-133 contribute to age-associated atrial fibrillation.
In the present study, right atrial appendage samples were collected
from patients with atrial fibrillation during coronary artery
bypass grafting. The expression levels of HCN2 and HCN4 mRNA and
proteins, as well as miR-1 and miR-133, among adult and aged
patients with sinus rhythm or atrial fibrillation were compared in
order to determine whether age-associated changes in expression may
contribute to the pathogenesis of age-associated atrial
fibrillation.
Materials and methods
Patient data
The present study was approved by the ethics
committee of the First Affiliated Hospital of Xinjiang Medical
University (Urumqui, China). Sixty patients undergoing coronary
artery bypass grafting between 2008 and 2013 were enrolled in the
study and all provided informed consent. The study population
comprised 32 males and 28 females (mean age, 55.12±28.23 years).
Patients were divided into three groups according to age and heart
rhythm: The aged chronic atrial fibrillation, aged sinus rhythm and
adult sinus rhythm groups. Patients in the aged chronic atrial
fibrillation group were defined as those ≥65 years, with atrial
fibrillation lasting longer than six months as revealed by
electrocardiography. Patients with liver and kidney function
defects, electrolyte disorders, infections, hyperthyroidism or
diabetes were excluded.
Tissue sample collection and
treatment
Baseline clinical data were recorded preoperatively
(Table I). In vitro
circulation was established during surgery, and once the heartbeat
stopped, a section of free right atrial appendage, ~1.0×0.5×1.0 cm
and weighing ~200 mg, was resected. Following the removal of blood
and fat tissue, atrial tissue samples were flash-frozen in liquid
nitrogen and stored at −80°C for analysis of target RNA and protein
expression.
 | Table IBaseline patient data. |
Table I
Baseline patient data.
| Characteristic | Adult SN (n=20) | Aged SN (n=22) | Aged AF (n=18) |
|---|
| Gender (n) |
| Male | 14 (70%) | 16 (73%) | 13 (72%) |
| Female | 6 (30%) | 6 (27%) | 5 (28%) |
| Age (years) | 40.14±8.73a | 68.34±5.14 | 69.13±4.16 |
| LA (mm) | 35.03±8.23 | 36.11±4.22 | 41.43±6.25 |
| RA (mm) | 33.14±3.32 | 35.32±6.54 | 36.23±8.33 |
| EF (%) | 60.61±3.19a | 41.27±10.75 | 41.34±7.29 |
RNA extraction and cDNA synthesis
Total RNA was extracted using RNAprep pure tissue
kit (Tiangen Biotech Co., Ltd, Beijing, China) according to the
manufacturer's instructions. Optical density (OD) values of the RNA
extracts were measured at 260 and 280 nm on a UV-spectrophotometer
(Thermo Fisher Scientific, San Jose, CA, USA) in order to calculate
the purity and concentration. The OD260/OD280 ranged from 1.8–2.0.
cDNA was reverse transcribed from mRNA templates using Fermentas
reverse transcription kits (Fermentas; Thermo Fisher Scientific,
Pittsburgh, PA, USA). Briefly, a 0.1 ng-5 µg total RNA
sample and 1 µl oligo (dT) 18 primer were mixed and the
volume adjusted to 12 µl with double distilled water
(ddH20). The mixture was centrifuged, placed in a 65°C
water bath for 5 min and then immediately placed on ice.
Subsequently, 4 µl buffer solution, 1 µl RNase
inhibitor, 2 µl 10 mM deoxyribonucleotide triphosphate
(dNTP) mix 2 and 1 µl M-MLV reverse transcriptase (200
U/µl) were added and this mixture was centrifuged and
incubated at 42°C in a water bath for 60 min. The reaction was
stopped by heating to 70°C for 5 min. The obtained cDNA was stored
at −20°C for subsequent reverse transcription-quantitative PCR
(qPCR) analyses.
Primer sequences
Primers for qPCR anlysis of HCN2 and HCN4 (Table II), and miR-1 and miR-133
(Table III) were designed using
Primer Premier 5.0™ software (Premier Biosoft, Palo Alto, CA, USA)
based on sequences in GenBank (www.ncbi.nlm.nih.gov/genbank).
 | Table IISequence of HCN2 and HCN4
primers. |
Table II
Sequence of HCN2 and HCN4
primers.
| Gene | Sequence | Length (bp) |
|---|
| GAPDH |
5′-TGCACCACCAACTGCTTAGC-3′ | 87 |
|
5′-GGCATGGACTGTGGTCATGAG-3′ |
| HCN2 |
5′-CCAGCTGTAAGACAGGGACG-3′ | 130 |
|
5′-GCGGGCCAAGTATTGCACTT-3′ |
| HCN4 |
5′-GGGGAATTCGCAACTGAAGC-3′ | 83 |
|
5′-TGCTGCGCCCTTAAATCTCT-3′ |
 | Table IIISequence of the microRNA-1 and -133
primers. |
Table III
Sequence of the microRNA-1 and -133
primers.
| Gene | Sequence |
|---|
| U6 | All-in-one™ miRNA
qPCR (internal primer) reverse primer provided by the reagent
kits |
| microRNA-1 | All-in-one™ miRNA
qPCR primer reverse primer provided by the reagent kits |
| microRNA-133 | All-in-one™ miRNA
qPCR primer reverse primer provided by the reagent kits |
PCR
The PCR reaction system comprised 1 µl sample
cDNA, 10 µM forward and reverse primers (0.5 µl for
each), 0.15 µl Taq enzyme, 2.5 µl buffer and
0.5 µl dNTP, adjusted to a total volume of 25 µl with
ddH20. The reaction conditions were as follows:
Pre-denaturation at 95°C for 3 min, 30 cycles of denaturation at
94°C for 30 sec, annealing at 56°C for 30 sec, extension at 72°C
and a final extension at 72°C for 10 min. PCR products were
detected by 1% agarose gel electrophoresis (5 V/m). A Bio-Rad gel
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
employed for band visualization and gel photography.
qPCR
qPCR analysis was performed at a series of cDNA
dilutions, including 10, 102, 103,
104, 105 and 106. The primers (10
µM) were used at gradient concentrations of 0.25, 0.5, 0.75,
1, 1.25 and 1.5 µl. The amplification efficiency of each
primer pair ranged from 0.9–1.1. The primer concentration with the
highest amplification efficiency was selected for subsequent
quantitative analysis. The qPCR reaction system included 2X of 10
µl SYBR Premix (Tiangen Biotech Co., Ltd), 10 µM
forward and reverse primers (0.6 µl of each) and 1 µl
cDNA, with total volume adjusted to 20 µl using
ddH20. The prepared reaction solution was analyzed using
Bio-Rad fluorescence PCR (Bio-Rad Laboratories, Inc.). Reaction
conditions were as follows: Pre-denaturation at 95°C for 15 min and
40 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for
10 sec, and extension at 72°C for 20 sec. The fluorescent signal
was recorded during the extension phase of each cycle using the
CFX96 real-time PCR detection system (Bio-Rad PCR; Bio-Rad
Laboratories, Inc.). Melting curve analysis (95–65°C) was performed
following the reaction.
Western blot analysis
Total protein was extracted from tissue lysates for
western blot analysis (Beyotime Institute of Biotechnology, Haimen,
China). Total protein concentration was determined using
bicinchoninic acid reagent kits (Beyotime Institue of
Biotechnology). For electrophoretic separation, 20 µg
protein per gel lane was boiled in loading buffer and loaded onto
12% polyacrylamide gels. Separated proteins were transferred
electrophoretically onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc.) and blocked in 5% fat-free powdered milk at 4°C
overnight. Following blocking, primary polyclonal rabbit antibodies
against HCN2, (cat. no. BS3372; 1:500, Bioworld Technology, Inc.,
St. Louis Park,. MN, USA) and HCN4 (cat. no. BS3687; 1:250,
Bioworld Technology, Inc.), and primary mouse monoclonal antibody
against GAPDH (cat. no. sc-365062; 1:500, Santa Cruz Biotechnology
Inc., Dallas, TX, USA) were added drop-wise and the membranes were
incubated for 2 h at room temperature. Following three washes in
Tris-buffered saline and Tween-20 (TBST; 10 min/wash), the
corresponding secondary antibody solution was added and membranes
were incubated for 1 h at room temperature. Following three
additional washes in TBST (Beyotime Institue of Biotechnology),
immunolabeling was visualized by electrochemiluminescence (ECL; EMD
Millipore, Billerica, MA, USA) and the chemiluminescence signal
captured by an imaging system (ChemiDoc®-It HR 410
imaging system; UVP, LLC, Upland, CA, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation or as rate and percentage. All data were analyzed using
SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Group means of continuous data were compared by one-way analysis of
variance and categorical rates and percentages by χ2
tests. P<0.05 was considered to indicate a statistically
significant difference between values.
Results
Patient baseline data
No statistically significant differences in gender
ratio or inner diameter of the right atrium were identified amongst
the three groups (P>0.05). The aged atrial fibrillation and aged
sinus rhythm groups did not differ significantly in mean age or
left ventricular ejection fraction (P>0.05), whereas a
significant difference in the inner diameter of the left atrium was
detected between the two groups (P<0.05). Significant
differences in left ventricular ejection fraction (P<0.05), but
not the inner diameter of the left atrium (P>0.05), were
detected between the aged and adult sinus rhythm groups. Baseline
data are summarized in Table
I.
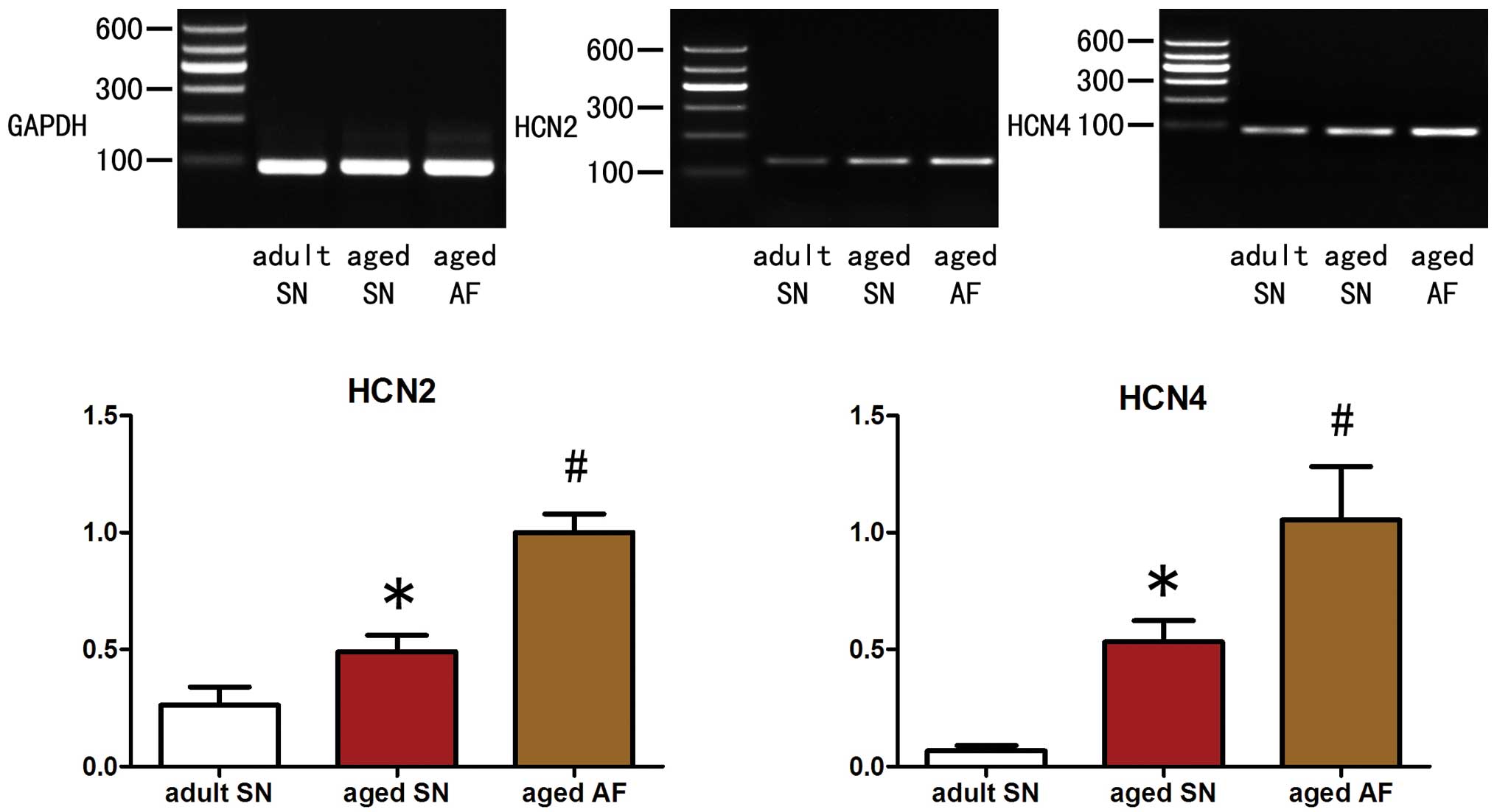
Expression levels of HCN2 and HCN4
channel mRNA in the right atrial appendage vary between groups
Compared with the adult sinus rhythm group, the aged
sinus rhythm group exhibited significantly elevated expression
levels of HCN2 and HCN4 mRNA (P<0.05; HCN2, 0.49±0.07 vs.
0.26±0.08; HCN4, 0.53±0.09 vs. 0.07±0.02; Fig. 1). Compared with the aged sinus
rhythm group, the aged atrial fibrillation group also exhibited
significantly enhanced levels of HCN2 and HCN4 mRNA expression
(P<0.05; HCN2, 1.00±0.08 vs. 0.49±0.07; HCN4, 1.05±0.23 vs.
0.53±0.09; Fig. 1).
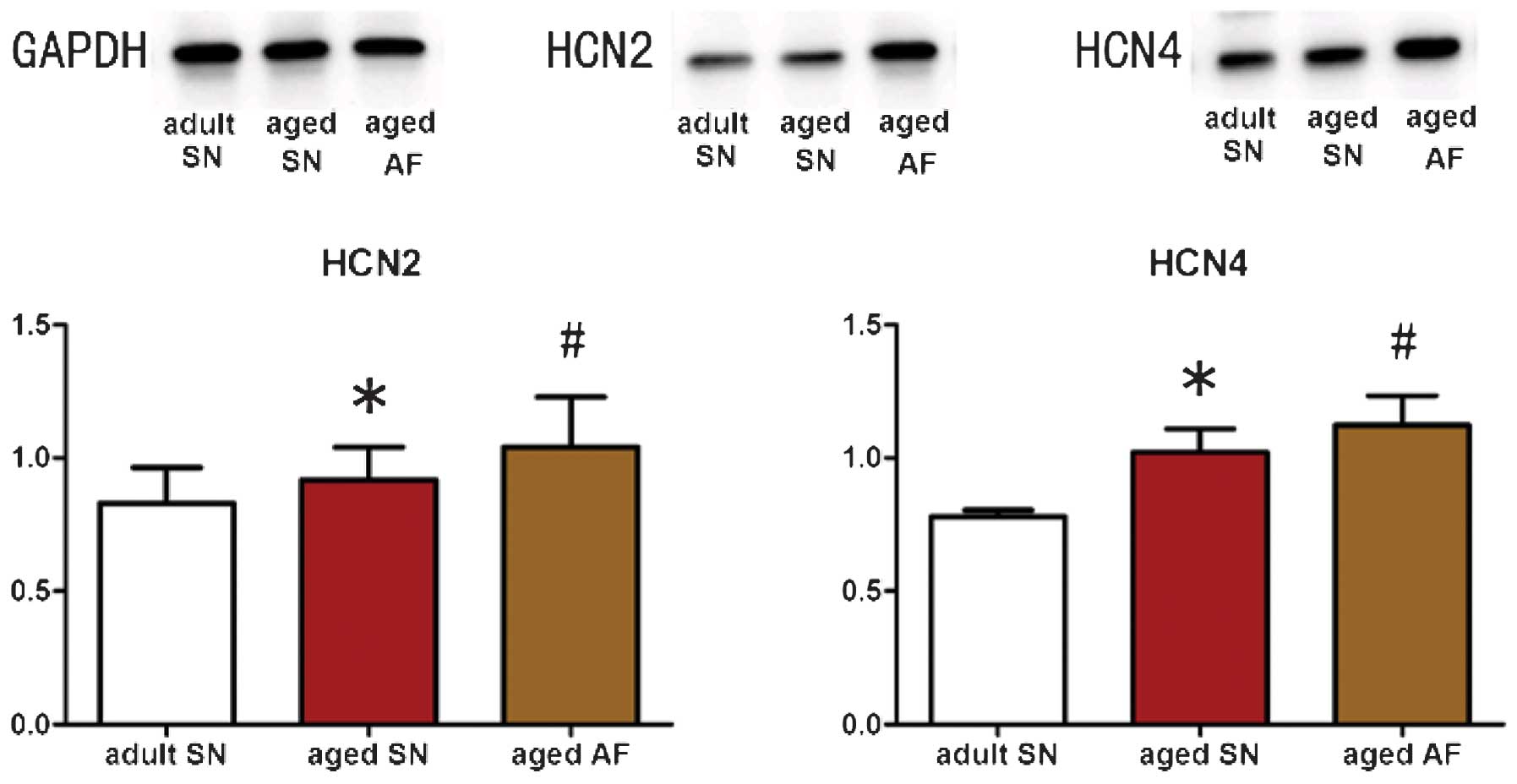
Expression levels of HCN2 and HCN4
channel proteins in the right atrial appendage vary between
groups
In accordance with the results of RT-qPCR analysis,
the aged sinus rhythm group was demonstrated to have significantly
elevated expression levels of HCN2 and HCN4 proteins compared with
those of the adult sinus rhythm group (P<0.05; HCN2, 0.92±0.12
vs. 0.83±0.13; HCN4, 1.02±0.08 vs. 0.78±0.02; Fig. 2). Similarly, the aged atrial
fibrillation group exhibited significantly enhanced HCN2 and HCN4
protein expression levels compared to those of the aged sinus
rhythm group (P<0.05; HCN2, 1.04±0.19 vs. 0.92±0.12; HCN4,
1.12±0.11 vs. 1.02±0.08; Fig.
2).
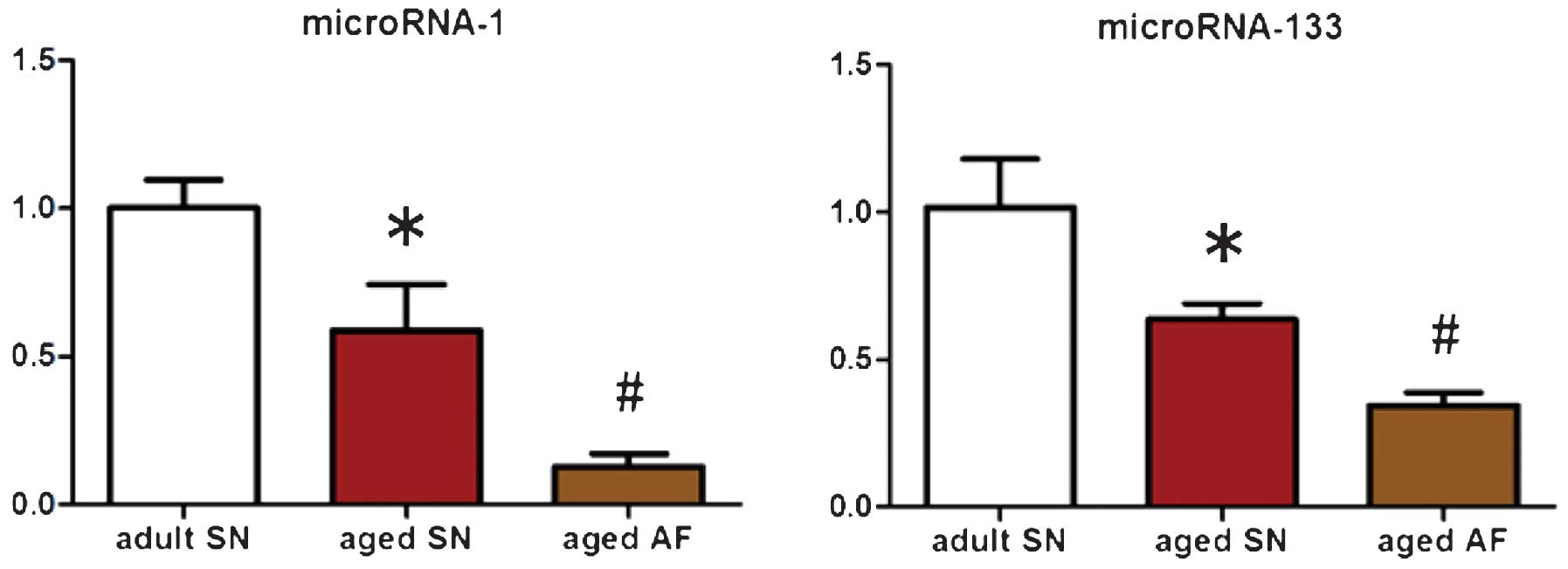
Expression levels of miR-1 and miR-133 in
the right atrial appendage vary between groups
Patients in the aged sinus rhythm group exhibited
significantly lower expression levels of miR-1 and miR-133 compared
to those of the adult sinus rhythm group (P<0.05; miRNA-1,
0.59±0.16 vs. 1.00±0.09; miRNA-133, 0.64±0.05 vs. 1.01±0.17;
Fig. 3). Compared with those of
the aged sinus rhythm group, the aged atrial fibrillation group
also exhibited significantly lower expression levels of miRNA-1 and
-133 (P<0.05; miRNA-1, 0.13±0.04 vs. 0.59±0.16; miRNA-133,
0.34±0.04 vs. 0.64±0.05; Fig.
3).
Discussion
The results of the present study suggested that the
mRNA and protein expression levels of HCN2 and HCN4 channels
increased in the right atrial appendage with age, whereas the
expression levels of post-transcriptional regulators miR-1 and
miR-133 declined with age. These age-associated alterations in
expression were even more pronounced in aged atrial fibrillation
patients compared with those of aged sinus rhythm patients,
implicating elevated HCN activity and reduced miR-1/133-mediated
regulation of HCN expression in the pathogenesis of atrial
fibrillation.
Aberrant changes in pacemaker currents contribute to
the generation of rapid arrhythmias. The HCN channels conduct a
mixed K+/Na+ depolarizing current (If), which
is activated by hyperpolarization and cyclic adenosine
monophosphate (5). The activation
of HCN channels contributes towards membrane depolarization during
the myocardial diastolic period (6). Despite the vital role of If in
cardiac pacemaker activity, the molecular structure of the HCN
channels underlying this activity was only relatively recently
elucidated (7). Early studies
suggested that the expression levels of HCN channel proteins were
low in normal myocardial cells outside of the sinoatrial node
(8); however, this hypothesis was
challenged by subsequent studies. Porciatti et al (9) successfully recorded If currents in
the human right atrium using whole cell patch-clamping, and
Zorn-Paulya et al (10)
confirmed the existence of HCNs in the left atrium. The kinetics of
If differed between the left auricula and left atrial wall,
suggesting regional heterogeneity of HCN subtype distribution.
Zorn-Paulya et al (10)
detected HCN2 and HCN4 expression in the human left atrial
appendage muscle, which produced an If conferring strong pacemaker
cell characteristics to left atrial myocytes. The If, and the
associated HCN channels, is also closely associated with arrhythmia
(11). A variety of pathological
processes lead to abnormally enhanced myocardial HCN channel
expression and If augmentation in the atrium or ventricle (12,13).
These elevated expression levels may enhance the autorhythmia of
myocytes and strengthen ectopic premature beats. Kuwahara et
al (14) revealed that the
transcriptional inhibitor neuron-restrictive silencer factor (NRSF)
acted as a regulator of fetal cardiac gene expression. A
dnNRSF-expressing transgenic mouse model with gradually progressive
myocardial disease was demonstrated to overexpress HCN2 and HCN4 in
the ventricle (15). These mice
died of sudden arrhythmia aged eight weeks, indicating that HCN2
and HCN4 overexpression in the ventricles may contribute to
ventricular arrhythmias (15).
Zicha et al (12) analyzed
atrial myocytes in a canine model of rapid ventricular pacemaker
activity and demonstrated that If enhancement, and the associated
HCN channel overexpression, contributed to heart failure-induced
ventricular arrhythmia.
The pacemaker currents likely participate in the
pathogenesis of age-associated atrial fibrillation. The
HCN-mediated If current is expressed at high levels in working
myocytes exhibiting ectopic premature beats (16). In the present study, whether these
channels also contribute to age-associated atrial fibrillation was
evaluated. Stillitano et al (4) compared right atrial HCN expression
between patients that had undergone bypass surgery with persistent
atrial fibrillation or sinus rhythm and found that HCN expression
was significantly higher in patients with persistent atrial
fibrillation, while levels of miR-1 were lower. The prevalence of
atrial fibrillation was positively correlated with age (1). Similarly, the prevalence of
sinoatrial node disorder (SND) increases with age (17), and SND is frequently accompanied by
atrial fibrillation and rapid arrhythmias. Age-associated
degeneration of the sinoatrial node also contributes to atrial
electrical remodeling (18).
Therefore, atrial electrophysiological characteristics and ion
channel expression patterns are altered with age (2,3,19,20).
Consequently, it was hypothesized that age-associated atrial
fibrillation may be correlated with age-associated sinoatrial node
degeneration; in addition, it was speculated that with age, atrial
and pulmonary HCN channel expression levels may be elevated and the
If current may be enhanced, which would lead to an increase in
ectopic autorhythmia and may trigger atrial fibrillation. Li et
al (21) established canine
models with age-associated atrial fibrillation or age-associated
sinus rhythm, and demonstrated that the If current and HCN4 mRNA
expression levels were significantly higher in the age-associated
sinus rhythm model, indicating that HCN and the HCN-mediated If,
particularly the HCN4 channel current component, may be involved in
the pathogenesis of age-associated atrial fibrillation.
In the present study, it was demonstrated that the
expression levels of HCN2 and HCN4 mRNA and protein were enhanced
in the right atrial appendage of aged sinus rhythm patients
compared to those of adult sinus rhythm patients, confirming the
presence of the hypothesized age-associated elevation in HCN
expression. Furthermore, aged atrial fibrillation patients were
found to exhibit higher HCN mRNA and protein expression levels than
those of aged sinus rhythm patients. Due to ethical constraints,
samples were only collected from the right atrial appendage;
however, it was speculated that HCN channel and If current
densities may be elevated at other sites of the atrium and in the
pulmonary vein. The enhanced expression of atrial HCN and the
strengthened If current may result in ventricular premature beats
or atrial tachycardia; therefore inducing atrial fibrillation.
HCN channels are regulated by miRs. miRs inhibit the
translation of target genes by binding to the complementary
sequence of the 3′ untranslated region or by directly modulating
mRNA degradation (22). Girmatsion
et al (23) and Stillitano
et al (4) demonstrated that
the expression of miR-1 was downregulated in patients with
persistent atrial fibrillation. miR-1 and -133 are dually regulated
in muscle (24), and their
expression levels are correlated with the expression of HCN2 and
HCN4 channel proteins (25,26).
miR-1 and -133 exert inhibitory effects upon HCN2, while miR-1 is
also able to downregulate HCN4 expression. Therefore, the
overexpression of exogenous miR-1 and miR-133 is able to suppress
HCN2 and HCN4 expression (26).
Preliminary canine studies by our group (27), revealed that miR-1 and miR-133
expression altered during aging; revealing that expression levels
were significantly lower in the aged canine atrium than those in
the adult canine atrium. In the present study, the expression
levels of miR-1 and miR-133 were found to be lower in the right
atrial appendage of aged sinoatrial fibrillation patients than
those of the adult sinus rhythm patients, and lower still in adult
sinus rhythm patients. This negative correlation between
miR-1/miR-133 and HCN2/HCN4 suggested that the downregulation of
miR-1 and miR-133 contributed to HCN2 and HCN4 upregulation during
aging.
The results of the present study demonstrated a
potential role for miR-1 and miR-133 in age-associated HCN channel
upregulation in the human atrium. In patients with aged atrial
fibrillation, the expression of HCN2 and HCN4 channels was enhanced
compared with aged and adult sinoatrial fibrillation patients,
whereas expression levels of miR-1 and miR-133 were lower. It was
therefore suggested that this age-associated increase in HCN2 and
HCN4 expression enhanced the If current and therefore may increase
the incidence of ventricular premature beats and atrial
tachycardia, triggering atrial fibrillation. Furthermore, these
changes in HCN channel and miR expression may be associated with
degeneration of the sinoatrial node. Ivabradine, a specific
inhibitor of If, is able to reduce the frequency of spontaneous
action potentials mediated by the pulmonary vein If current
(28). Multiple animal experiments
have confirmed that ivabradine is also capable of decreasing
ventricular arrhythmias. However, whether ivabradine may represent
an effective treatment for age-associated atrial fibrillation
remains to be elucidated. Relevant animal experiments are required
in order to conclude whether ivabradine may represent a potential
therapeutic. These findings therefore improve current knowledge of
the association between HCN channels and age-associated atrial
fibrillation.
In the present study, only right atrial appendage
samples were collected, and therefore whether these changes in
expression continue throughout the atrium and pulmonary vein
remains unknown. Furthermore, channel expression was investigated
at the mRNA and protein levels but the effects of these changes on
If current magnitude and kinetics was not investigated. Finally,
sinoatrial node function was not evaluated in these patients;
therefore, the potential contribution of SND or age-associated
sinoatrial node degeneration to these expression changes and
clinical conditions were not able to be assessed.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (no. 81260069).
References
|
1
|
Fuster V, Rydén LE, Cannom DS, et al
American College of Cardiology; American Heart Association Task
Force; European Society of Cardiology Committee for Practice
Guidelines; European Heart Rhythm Association; Heart Rhythm
Society: ACC/AHA/ESC 2006 guidelines for the management of patients
with a trial fibrillation: full text: a report of the American
College of Cardiology/American Heart Association Task Force on
practice guidelines and the European Society of Cardiology
Committee for Practice Guidelines (Writing Committee to Revise the
2001 guidelines for the management of patients with atrial
fibrillation) developed in collaboration with the European Heart
Rhythm Association and the Heart Rhythm Society. Europace.
8:651–745. 2006.PubMed/NCBI
|
|
2
|
Su N, Duan J, Moffat MP and Narayanan N:
Age-related changes in electrophysiological responses to muscarinic
receptor stimulation in rat myocardium. Can J Physiol Pharmacol.
73:1430–1436. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dun W, Yagi T, Rosen MR, et al: Calcium
and potassium currents in cells from adult and aged canine right
atria. Cardiovasc Res. 58:526–534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stillitano F, Lonardo G, Giunti G, et al:
Chronic atrial fibrillation alters the functional properties of If
in the human atrium. J Cardiovasc Electrophysiol. 24:1391–1400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Biel M, Wahl-Schott C, Michalakis S and
Zong X: Hyperpolarization-activated cation channels: from genes to
function. Physiol Rev. 89:847–885. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
DiFrancesco D: Funny channels in the
control of cardiac rhythm and mode of action of selective blockers.
Pharmacol Res. 53:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ludwig A, Zong X, Jeglitsch M, et al: A
family of hyperpolarization-activated mammalian cation channels.
Nature. 393:587–591. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ludwig A, Zong X, Hofmann F and Biel M:
Structure and function of cardiac pacemaker channels. Cell Physiol
Biochem. 9:179–186. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porciatti F, Pelzmann B, Cerbai E, et al:
The pacemaker current I(f) in single human atrial myocytes and the
effect of beta-adrenoceptor and A1-adenosine receptor stimulation.
Br J Pharmacol. 122:963–969. 1997. View Article : Google Scholar
|
|
10
|
Zorn-Pauly K, Schaffer P, Pelzmann B, et
al: If in left human atrium: a potential contributor to atrial
ectopy. Cardiovasc Res. 64:250–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roubille F and Tardif JC: New therapeutic
targets in cardiology: heart failure and arrhythmia: HCN channels.
Circulation. 127:1986–1996. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zicha S, Fernández-Velasco M, Lonardo G,
et al: Sinus node dysfunction and hyperpolarization-activated
(HCN)channel subunit remodeling in a canine heart failure model.
Cardiovasc Res. 66:472–481. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yeh YH, Burstein B, Qi XY, et al: Funny
current downregulation and sinus node dysfunction associated with
atrial tachyarrhythmia. Circulation. 119:1576–1585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuwahara K, Saito Y, Ogawa E, et al: The
neuron-restrictive silencer element-neuron-restrictive silencer
factor system regulates basal and endothelin 1-inducible atrial
natriuretic peptide gene expression in ventricular myocytes. Mol
Cell Biol. 21:2085–2097. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuwabara Y, Kuwahara K, Takano M, et al:
Increased expression of HCN channels in the ventricular myocardium
contributes to enhanced arrhythmicity in mouse failing hearts. J Am
Heart Assoc. 2:e0001502013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stieber J, Hofmann F and Ludwig A:
Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc
Med. 14:23–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Semelka M, Gera J and Usman S: Sick sinus
syndrome: a review. Am Fam Physician. 87:691–696. 2013.PubMed/NCBI
|
|
18
|
Li G, Liu E, Liu T, et al: Atrial
electrical remodeling in a canine model of sinus node dysfunction.
Int J Cardiol. 146:32–36. 2011. View Article : Google Scholar
|
|
19
|
Teh AW, Kalman JM, Lee G, et al:
Electroanatomic remodeling of the pulmonary veins associated with
age. Europace. 14:46–51. 2012. View Article : Google Scholar
|
|
20
|
Tuan TC, Chang SL, Tsao HM, et al: The
impact of age on the electroanatomical characteristics and outcome
of catheter ablation in patients with atrial fibrillation. J
Cardiovasc Electrophysiol. 21:966–972. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li JY, Wang HJ, Xu B, et al:
Hyperpolarization activated cation current (I(f)) in cardiac
myocytes from pulmonary vein sleeves in the canine with atrial
fibrillation. J Geriatr Cardiol. 9:366–374. 2012.
|
|
22
|
Lee HJ: Exceptional stories of microRNAs.
Exceptional stories of microRNAs. Exp Biol Med (Maywood).
238:339–343. 2013. View Article : Google Scholar
|
|
23
|
Girmatsion Z, Biliczki P, Bonauer A, et
al: Changes in microRNA-1 expression and IK1 up-regulation in human
atrial fibrillation. Heart Rhythm. 6:1802–1809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Callis TE, Deng Z, Chen JF and Wang DZ:
Muscling through the microRNA world. Exp Biol Med (Maywood).
233:131–138. 2008. View Article : Google Scholar
|
|
25
|
Suffredini S, Stillitano F, Comini L, et
al: Long-term treatment with ivabradine in post-myocardial
infarcted rats counteracts f-channel overexpression. Br J
Pharmacol. 165:1457–1466. 2012. View Article : Google Scholar :
|
|
26
|
Luo X, Lin H, Pan Z, et al:
Down-regulation of miR-1/miR-133 contributes to re-expression of
pacemaker channel genes HCN2 and HCN4 in hypertrophic heart. J Biol
Chem. 283:20045–20052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu GJ, Gan TY, Tang BP, et al: Changes in
microRNAs expression are involved in age-related atrial structural
remodeling and atrial fibrillation. Chin Med J (Engl).
126:1458–1463. 2013.
|
|
28
|
Suenari K, Cheng CC, Chen YC, Lin YK,
Nakano Y, Kihara Y, Chen SA and Chen YJ: Effects of ivabradine on
the pulmonary vein electrical activity and modulation of pacemaker
currents and calcium homeostasis. J Cardiovasc Electrophysiol.
23:200–206. 2012. View Article : Google Scholar
|