Introduction
Patients with end stage renal failure (ESRF) develop
severe anemia, including those undergoing maintenance hemodialysis
(HD). To manage anemia in HD patients, administration of
erythropoiesis-stimulating agent (ESA) was widespread in the 1990s
(1,2). The use of recombinant human
erythropoietin (rHuEpo) to treat the anemia of patients on
maintenance dialysis has been a major advance in the care of these
patients. However, this treatment is frequently blunted for various
reasons, including iron deficiency and inflammation (3).
The serum ferritin level and transferrin saturation
(TSAT) percentage are the standard laboratory tests used to
evaluate iron stores. Both the 1997 and 2001 National Kidney
Foundation's anemia guidelines proposed maintaining serum ferritin
above 100 ng/ml and TSAT above 20% via intermittent or maintenance
iron administration (1,2) in patients with chronic renal failure
(CRF). Although serum ferritin is a marker of iron status, it is
also an acute phase reactant and is consequently influenced by
inflammation and infection, which are often present in HD
patients.
Previous studies have reported that oral iron
therapy cannot meet the demands of ESA in hemodialysis patients due
to poor absorption and its interaction with other concurrently used
drugs (4,5). Intravenous (IV) iron therapy has been
widely used to maintain iron stores and to permit adequate
erythropoiesis in HD patients as it is well tolerated by many
patients (6–8).
Few reviews have compared oral vs. IV iron in HD
patients. A recent systemic review and meta-analysis suggested that
patients treated with IV iron have better hemoglobin (Hb) levels
than those treated with oral iron (9). However, some studies have found that
oral iron is well tolerated and effective in long-term HD patients
(10) and as effective as IV iron
in pre-dialysis patients (11,12).
The appropriate and standard method of iron supplementation in
managing anemia in HD patients is still under debate.
Chronic inflammation is now believed to decrease
iron absorption from the gut through increased hepcidin synthesis
(13). Hepcidin, a serum protein,
is produced by the liver and is a key regulator of iron
mobilization. In HD patients, bacterial contamination of the
dialysate leads to increased inflammatory cytokine production and
chronic inflammation (14,15). Therefore, we hypothesized that
sterile dialysate may improve the effect of oral iron
supplementation on erythropoiesis. In this study, we prospectively
evaluated the effectiveness of oral and IV iron for the management
of anemia using ultrapure dialysate in randomly selected HD
patients.
Materials and methods
Patients and treatment
This study was conducted in compliance with the
declaration of Helsinki. The institutional review board approved
the study protocol, and written informed consent was obtained from
all of the patients. A total of 24 consecutive HD patients treated
at our hospital were selected prospectively from March to September
2007. One patient was transferred to another hospital due to
personal reasons and was therefore excluded from the study.
Twenty-three patients (11 males and 12 females; median age 60
years, range 35–81) were finally included. Patients were then
randomly assigned to two treatment groups. The first group of 11
patients received oral ferrous fumarate 305 mg (equivalent to 100
mg Fe) (Nichi-iko Pharmaceutical Co. Ltd., Tokyo, Japan) once a
day, while the second group of 12 patients received IV infusions of
cideferron (50 mg Fe in 2 ml) (Nippon Zoki Pharmaceutical Co. Ltd.,
Tokyo, Japan). Cideferron (2 ml) was diluted in 100 ml of a 0.9%
sodium chloride solution and was infused during HD once a week. The
patients were administered rHuEpo (Epoetin-β; Chugai Pharmaceutical
Co. Ltd, Tokyo, Japan) intravenously at the end of HD. The rHuEpo
dose was individually adjusted to maintain a target hematocrit
(Hct) of 33–38%.
The inclusion criteria included i) patients with
anemia, ii) patients on regular hemodialysis for >6 months, and
iii) patients testing negative for occult blood in stool. Excluding
criteria included i) patients with active infection, ii) patients
with uncontrolled hypertension, iii) patients with a history of
coronary artery disease, iv) patients who changed treatment options
from oral to IV or vice-versa or who were awaiting renal
transplantation, v) pregnant or lactating women, and vi) patients
who received a blood transfusion within 1 month prior to the study.
Patients were permitted to withdraw from the study at any time for
any reason and were not replaced.
Efficacy endpoints and clinical
assessments
The primary purpose of the study was to evaluate the
efficacy of oral iron and IV iron for the management of anemia.
Efficacy was evaluated by an improvement in iron deficiency, such
as an increase in TSAT, serum ferritin, Hct or Hb, and a reduction
in the mean weekly erythropoietin dose. Changes in dry weight (DW)
and cardiothoracic ratio (CTR) were also evaluated. Hb, Hct and
creatinine were measured twice a month. DW and CTR were measured on
a monthly basis. TSAT, serum ferritin, serum iron, β2-microglobulin
(β2-MG) and total iron binding capacity (TIBC) were measured once
every 6 months. Patients were closely monitored for
gastrointestinal symptoms, including indigestion, abdominal pain
and constipation. Stool specimens were checked for fecal occult
blood before treatment, every 3 months, and whenever there was a
clinical suspicion of gastrointestinal hemorrhage.
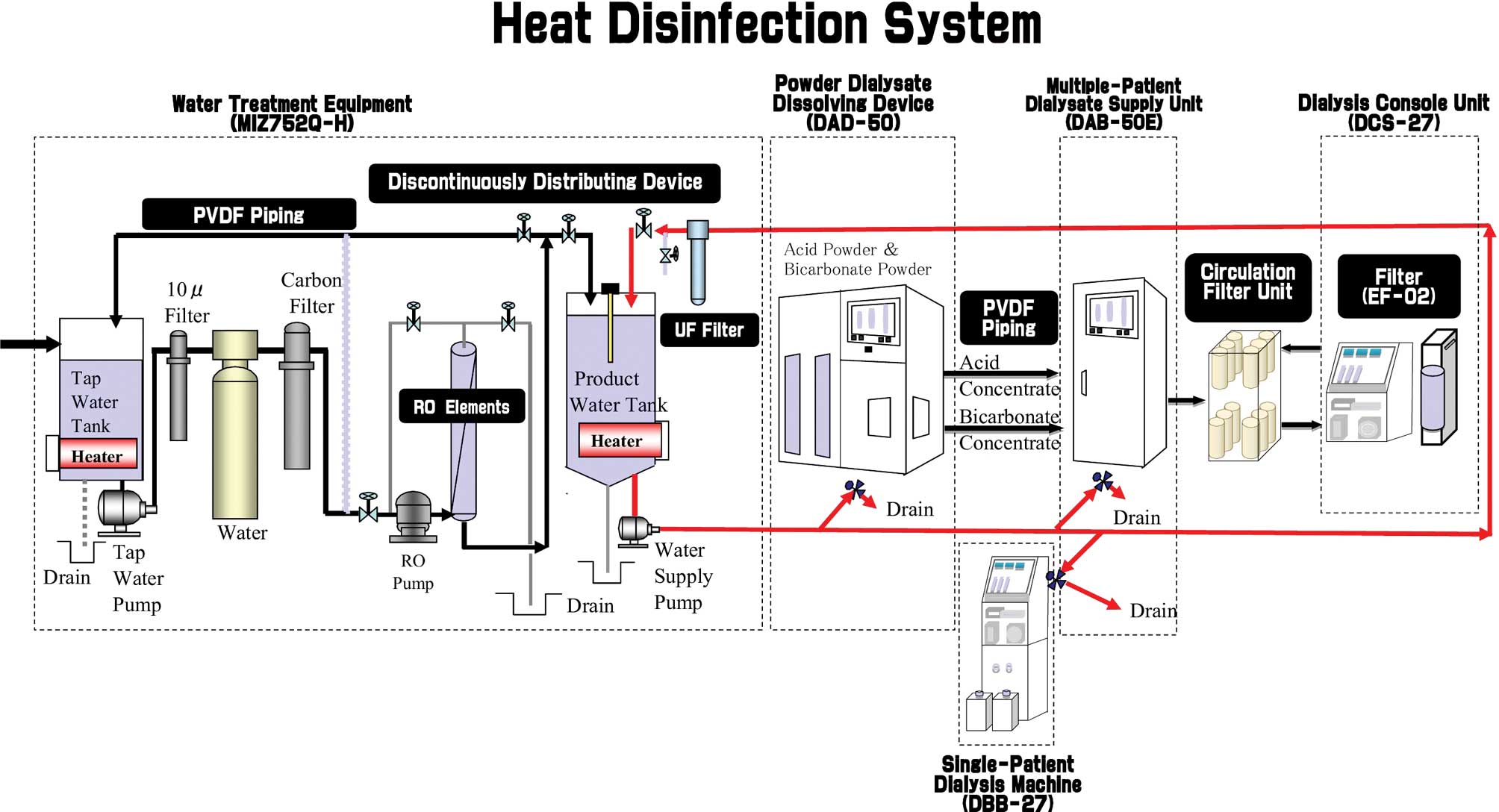
Dialysate cleaning system
At our hospital, the dialysate was cleaned using
water treatment equipment with the reverse osmosis (RO) module
MIZ752Q-H (Japan Water System Co, Tokyo, Japan), the newest model
in the series (Nikkiso Co. Ltd., Tokyo, Japan), as shown in
Fig. 1. The system is equipped
with the most recent reverse osmosis module, polyvinylidene
difluoride piping, RO water lines, automatic flushing within the
equipment and daily automatic disinfection system. Disinfection is
performed using peracetic acid and calcium hydrochloride in
combination with the heat treatment of water by boiling it at 83°C
and allowing it to be circulated for 60 min throughout the entire
piping system that comes into contact with the dialysis solutions.
The system was operated automatically every day, enabling the
hygienic mixing of solutions within an entirely enclosed piping
system. The heat disinfection was performed 2.5 h before dialysis,
which allowed the optimal temperature of the dialysate to be
administered into the patients. The crevice-free adhesion method
allows pipes to be joined together without creating a difference in
level between the two pipes, thus reducing the problem of
micro-organisms adhering to and multiplying in the joints between
pipes. Therefore, in our clinic, we assure safety by providing an
outstandingly clean dialysate. This model provides a superior level
of cleanliness to the processing water.
Bacteriological analysis
Bacterial analysis was performed by trained staff
three times a week by collecting the dialyzed samples from
different sites of the circulation system. The bacterial count was
evaluated by a pour-plate method using Trypticase Soy Agar at 20
and 37°C for at least 5 days. Testing for fecal indicator bacteria,
such as fecal coliforms, enterococci and pseudomonas aeruginosa,
was conducted. Endotoxin concentrations were measured using a
chromogenic limulus amebocyte lysate assay (Mini-LAL kit; Wako
Junyaku, Osaka, Japan) by the kinetic turbidimetric method
(LAL-5000; Associates of Cape Cod, MA, USA). This test system is
calibrated in endotoxin units (EU) per ml. The detection limit was
0.03 EU/ml.
Statistical analysis
Data were expressed as the mean ± standard
deviation. The paired Student's t-test was used to evaluate the
changes from baseline of each group, and the unpaired t-test was
used to compare the changes between the oral and IV iron groups.
Probability values of <0.05 were considered statistically
significant.
Results
Efficacy
The baseline characteristics of the different
parameters are summarized in Table
I. At the end of the 6-month treatment, patients receiving oral
iron had a significant increase from the baseline value of TSAT
(20.09±8.92 to 29.73±7.24; p=0.011) and ferritin (32.64±15.45 to
115.36±28.18; p=0.0001). On the other hand, patients receiving IV
iron had a significant increase from the baseline value of TSAT
(17.42±6.1 to 33.75±8.64; p=0.0001), ferritin (57.75±26.68 to
183.5±47.53; p=0.0002) and serum iron (55.5±15.8 to 67.3±16;
p=0.021). In both treatment groups, Hb, Hct and DW were increased,
but did not reach statistical significance, as shown in Table II. Patients receiving oral iron and
IV iron had a significantly decreased erythropoietin dose from
baseline (5,590.91±1,513.58 to 3,727.27±1,618.08; p=0.011 and
6,775.83±2,292.23 to 4,375.0±2,473.73; p=0.027, respectively).
TIBC, β2-MG, creatinine and CTR tended to decrease in both
treatment groups, but did not reach statistical significance. We
also analyzed changes after therapy of the different parameters
between the two treatment groups, and found no statistically
significant differences in any of the parameters (data not
shown).
 | Table I.Baseline characteristics of the 23
patients. |
Table I.
Baseline characteristics of the 23
patients.
| Characteristics | All patients
(n=23) | Oral iron group
(n=11) | IV iron group
(n=12) |
|---|
| Age (years) | 60.3±11.9 | 59.5±10.7 | 61±13.3 |
| Gender | | | |
| Male | 11 | 5 | 6 |
| Female | 12 | 6 | 6 |
| Duration of dialysis
(months) | 98.4±80.4 | 114±93.6 | 85.7±68.4 |
| Primary renal
disorders | | | |
| Diabetic
nephropathy | 12 | 6 | 6 |
| Chronic
glomerulonephrites | 9 | 4 | 5 |
| Renal
sclerosis | 1 | 0 | 1 |
 | Table II.Outcome of different parameters before
and after the therapy of the 23 patients. |
Table II.
Outcome of different parameters before
and after the therapy of the 23 patients.
| Variables | Oral iron (n=11)
| IV iron (n=12)
|
|---|
| Before therapy | After therapy | p-value | Before therapy | After therapy | p-value |
|---|
| TSAT (%) | 20.09±8.92 | 29.73±7.24 | 0.0110 | 17.42±6.10 | 33.75±8.64 | 0.0001 |
| Serum ferritin
(ng/ml) | 32.64±15.45 | 115.36±28.18 | 0.0001 | 57.75±26.68 | 183.5±47.53 | 0.0002 |
| Serum iron
(μg/dl) | 57.8±26.4 | 59.2±23.5 | 0.9000 | 55.5±15.8 | 67.3±16 | 0.0210 |
| Serum TIBC
(μg/dl) | 245.9±41.6 | 236±36.3 | 0.6100 | 259±40 | 218.4±47.6 | 0.0600 |
| β2-MG (mg/l) | 31.1±7.7 | 28.7±4.7 | 0.3900 | 26.1±2.3 | 24.1±6.8 | 0.6900 |
| Creatinine
(mg/dl) | 11.2±2.3 | 11.1±2.1 | 0.8600 | 9.4±2.8 | 9.3±2.6 | 0.9500 |
| Hemoglobin
(g/dl) | 10.16±1.28 | 11.1±0.6 | 0.0620 | 9.57±1.64 | 10.74±1.09 | 0.0510 |
| Hematocrit (%) | 33.47±2.67 | 35.4±2.53 | 0.0970 | 30.98±5.17 | 34.38±3.47 | 0.0700 |
| Dry weight (kg) | 55.15±8.11 | 55.98±7.13 | 0.8100 | 57.86±17.09 | 57.98±16.69 | 0.9800 |
| CTR (%) | 49.73±5.24 | 48.44±4.76 | 0.5500 | 48.03±2.54 | 48.78±3.23 | 0.5200 |
| Erythropoeitin
(IU/week) |
5,590.91±1,513.58 |
3,727.27±1,618.08 | 0.0110 |
6,775.83±2,292.23 |
4,375.00±2,473.73 | 0.0240 |
Safety
Three patients in the oral iron group and 1 patient
in the IV iron group were reported to have a mild or moderate
gastrointestinal disorder; however, it was tolerated and was
corrected with diet. There were no deaths or severe adverse effects
during the study, nor did any patients discontinue the study due to
drug-related side effects.
Quality of dialysate
The results of the microbacterial analysis from the
dialyzed sample consistently showed a bacterial count of <0.1
colony forming units (cfu) per ml and EU of <0.03 (data not
shown). According to the Japanese Society for Dialysis Therapy, the
bacterial count should be <100 cfu/ml in standard dialysate and
<0.1 cfu/ml in ultrapure dialysate (16). Thus, our clinic provided ultrapure
dialysate for HD patients on a routine basis.
Discussion
The results of the present study suggest that oral
iron is as effective as IV iron in managing anemia in CRF patients
undergoing HD using ultrapure dialysate. The baseline value of TSAT
and ferritin was significanlty increased in each group. In
addition, the mean weekly erythropoietin dose was significantly
decreased from the baseline in each group, indicating that oral
iron may indeed be as effective as IV iron.
Previous studies have reported that patients
undergoing HD showed a better response to IV iron; however,
patients with CRF showed no difference between oral and IV iron
(9). Another study demonstrated
that IV and oral iron caused a similar correction in anemia over 6
weeks (17). Several studies have
reported that IV iron has greater efficacy with rHuEpo than oral
iron, perhaps as a result of reduced iron absorption from the gut
and poor patient compliance with oral medication (4–8,18).
Although new formulations of IV iron have drastically reduced the
risk of immediate adverse effects, a variety of long-term
consequences of IV iron have been reported in the literature
(15,19). Furthermore, several studies have
demonstrated that the administration of IV iron releases free iron,
which may react with hydrogen peroxide to produce hydroxyl radical
oxidants and may lead to increased oxidative stress that can
potentially damage cellular lipids, nucleic acids, proteins and
carbohydrates (20,21). IV iron bypasses the physiologic
controls of iron absorption and storage, thereby exposing the
patient to higher levels of circulating free iron and higher tissue
levels of iron. A number of authors recently reviewed the link
between free or stored iron and a variety of disease states,
including cardiovascular disease (18,22),
carcinogenesis (19,23) and infection (15,24).
The chemical and microbiological quality of water
used in dialysate plays a crucial role in the mortality and
morbidity of patients on HD. During dialysis, a patient receives
90–150 l of water a day; insufficient chemical and microbiological
quality of water leads to infections. Beside cardiovascular
disease, infection is the most common cause of death in dialysis
patients. Endotoxin derived from gram-negative bacteria may
penetrate the dialyser membrane and may be responsible for a
pyrogenic reaction (25). Thus, we
speculate that the highly ultrapure dialysate used for HD in our
clinic contributed to the proper utilization of oral iron
absorption from the gut by controlling intestinal inflammation, and
ensured the control of anemia through oral iron
supplementation.
The present study showed that, by using ultrapure
dialysate, oral iron improves iron deficiencies and significantly
reduces the erythropoietin dose from the baseline, similarly to IV
iron. As oral iron is less expensive, safer and easier to
administer, and equally or more competent as IV iron, the use of
oral iron as a first-line therapy is preferred. However, IV iron
should be considered as an alternative for patients who do not
tolerate oral iron or for whom iron deficiency persists despite an
adequate dose of oral iron. There are a few limitations to our
study. The total population of the patients was small, and the
period of observation for efficacy was short. The erythropoietin
dose administered was not the same in the two treatment groups;
rather it was modified depending on the Hct level.
In summary, with the help of ultrapure dialysate,
oral iron is indeed as effective as intravenous iron in managing
anemia in HD patients, as it maintains the iron indices and is well
tolerated. Our hospital currently focuses on the use of oral iron
supplementation as a first-line therapy to control anemia in ESRF
patients undergoing HD.
Acknowledgements
We thank Mr. Takahiro Nakama (Nikkiso
Comp. Ltd., Tokyo, Japan) and Mr. Takenori Suzuki (JWS Comp.,
Tokyo, Japan) for help with making the layout figure of the water
treatment equipment. We also thank Mr. Kenichi Nishimura, Ms. Junko
Imaizumi and Ms. Taeko Kobayashi of the Sanshi Group Hikari Clinic
for their expertise. Part of the data was presented at the 53rd
Congress of the Japanese Society of Dialysis and Treatment, Kobe,
Japan.
References
|
1.
|
Van Wyck D, Eckardt KU, Uhlig K, Rocco M
and Levin A: Appraisal of evidence and control of bias in the
kidney disease outcomes quality initiative guideline development
process. Clin J Am Soc Nephrol. 2:8–10. 2007.PubMed/NCBI
|
|
2.
|
Fishbane S, Kalantar-Zadeh K and Nissenson
AR: Serum ferritin in chronic kidney disease: reconsidering the
upper limit for iron treatment. Semin Dial. 17:336–341. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Kalantar-Zadeh K, McAllister CJ, Lehn RS,
Lee GH, Nissenson AR and Kopple JD: Effect of
malnutrition-inflammation complex syndrome on EPO
hyporesponsiveness in maintenance hemodialysis patients. Am J
Kidney Dis. 42:761–773. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Fishbane S and Maesaka JK: Iron management
in end-stage renal disease. Am J Kidney Dis. 29:319–333. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wingard RL, Parker RA, Ismail N and Hakim
RM: Efficacy of oral iron therapy in patients receiving recombinant
human erythropoietin. Am J Kidney Dis. 25:433–439. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kapoian T, O'Mara NB, Singh AK, et al:
Ferric gluconate reduces epoetin requirements in hemodialysis
patients with elevated ferritin. J Am Soc Nephrol. 19:372–379.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Macdougall IC: Intravenous administration
of iron in epoetin-treated haemodialysis patients – which drugs,
which regimen? Nephrol Dial Transplant. 15:1743–1745. 2000.
|
|
8.
|
Coyne DW, Kapoian T, Suki W, et al: Ferric
gluconate is highly efficacious in anemic hemodialysis patients
with high serum ferritin and low transferrin saturation: results of
the Dialysis Patients' Response to IV Iron with Elevated Ferritin
(DRIVE) Study. J Am Soc Nephrol. 18:975–984. 2007.PubMed/NCBI
|
|
9.
|
Rozen-Zvi B, Gafter-Gvili A, Paul M,
Leibovici L, Shpilberg O and Gafter U: Intravenous versus oral iron
supplementation for the treatment of anemia in CRF: systematic
review and meta-analysis. Am J Kidney Dis. 52:897–906. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lenga I, Lok C, Marticorena R, Hunter J,
Dacouris N and Goldstein M: Role of oral iron in the management of
long-term hemodialysis patients. Clin J Am Soc Nephrol. 2:688–693.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stoves J, Inglis H and Newstead CG: A
randomized study of oral vs intravenous iron supplementation in
patients with progressive renal insufficiency treated with
erythropoietin. Nephrol Dial Transplant. 16:967–974. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Charytan C, Qunibi W and Bailie GR:
Comparison of intravenous iron sucrose to oral iron in the
treatment of anemic patients with chronic kidney disease not on
dialysis. Nephron Clin Pract. 100:55–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Muñoz M, Villar I and García-Erce JA: An
update on iron physiology. World J Gastroenterol. 15:4617–4626.
2009.
|
|
14.
|
Pérez-García R, Martín-Malo A, Fort J, et
al: Baseline characteristics of an incident haemodialysis
population in Spain: results from ANSWER – a multicentre,
prospective, observational cohort study. Nephrol Dial Transplant.
24:578–588. 2009.PubMed/NCBI
|
|
15.
|
Malyszko J and Mysliwiec M: Hepcidin in
anemia and inflammation in chronic kidney disease. Kidney Blood
Press Res. 30:15–30. 2007. View Article : Google Scholar
|
|
16.
|
Nakai S, Masakane I, Shigematsu T, et al:
An overview of regular dialysis treatment in Japan (as of 31
December 2007). Ther Apher Dial. 13:457–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Agarwal R, Rizkala AR, Bastani B, Kaskas
MO, Leehey DJ and Besarab A: A randomized controlled trial of oral
versus intravenous iron in chronic kidney disease. Am J Nephrol.
26:445–454. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Boddy K, Lawson DH, Linton AL and Will G:
Iron metabolism in patients with chronic renal failure. Clin Sci.
39:115–121. 1970.PubMed/NCBI
|
|
19.
|
Hezode C, Cazeneuve C, Coue O, et al:
Liver iron accumulation in patients with chronic active hepatitis
C: prevalence and role of hemochromatosis gene mutations and
relationship with hepatic histological lesions. J Hepatol.
31:979–984. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lim PS, Wei YH, Yu YL and Kho B: Enhanced
oxidative stress in haemodialysis patients receiving intravenous
iron therapy. Nephrol Dial Transplant. 14:2680–2687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Nascimento MM, Suliman ME, Bruchfeld A, et
al: The influence of hepatitis C and iron replacement therapy on
plasma pentosidine levels in haemodialysis patients. Nephrol Dial
Transplant. 19:3112–3116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Feldman HI, Santanna J, Guo W, et al: Iron
administration and clinical outcomes in hemodialysis patients. J Am
Soc Nephrol. 13:734–744. 2002.PubMed/NCBI
|
|
23.
|
Williams P and Griffiths E: Bacterial
transferrin receptors – structure, function and contribution to
virulence. Med Microbiol Immunol. 181:301–322. 1992.
|
|
24.
|
Patruta SI, Edlinger R, Sunder-Plassmann G
and Hörl WH: Neutrophil impairment associated with iron therapy in
hemodialysis patients with functional iron deficiency. J Am Soc
Nephrol. 9:655–663. 1998.PubMed/NCBI
|
|
25.
|
Lonnemann G, Krautzig S and Koch KM:
Quality of water and dialysate in haemodialysis. Nephrol Dial
Transplant. 11:946–949. 1996. View Article : Google Scholar : PubMed/NCBI
|