Introduction
It is generally accepted that diesel exhaust
particles (DEP), derived from diesel engine-powered automobiles and
major constituents in atmospheric particulate matter, are
associated with several allergic disorders, which perhaps can be
referred to as adjuvanticity (1).
We and others have experimentally demonstrated that pulmonary
exposure to DEP aggravates allergic airway inflammation and
amplifies the lung expression of helper T (Th)2 cytokines in
vivo (2–5). However, detailed mechanisms
underlying the exacerbating effects of DEP on the allergic
pathology remain unclear, particularly when analyzed only in in
vivo samples.
DEP reportedly affect/disrupt several cell
populations such as epithelial cells (6,7),
endothelial cells (8), macrophages
(9), eosinophils (10) and mast cells/basophils (11,12),
which play roles in the pathogenesis of allergic inflammation,
mainly in vitro. Furthermore, we (13) and others (14) have shown that DEP activate
dendritic cells, one of the pivotal cell types involved in adaptive
immunity, leading to maladaptive immune responses in vitro.
However, the cellular contribution of DEP-mediated exacerbation of
allergy has not been fully clarified.
Peripheral lymphoid organs and their resident
mononuclear cells, including lymphocytes, are key players in the
pathogenesis of allergic pathology; thus, the effects of DEP on
these compartments might generate much information regarding their
adjuvant effects on allergy. Previous studies have shown that DEP
and their components directly/indirectly activate B cells to
produce IgE in vitro (15,16),
implicating B/plasma cells in the mechanism of the adjuvant effects
of DEP. However, less has been studied regarding the impact of DEP
on T cells, in particular, in the context of Th lymphocyte
differentiation/activation.
Another important issue is the fact that DEP have
carbonaceous nuclei, which absorb organic chemicals, including
polycyclic aromatic hydrocarbons (17). Previous studies have demonstrated
that organic chemicals extracted from DEP have adjuvant potential
for the production of allergen-specific immunogloblins in
vivo (18) and in vitro
(15). We previously reported that
extracted organic chemicals from DEP with benzene-ethanol (referred
to as ‘DEP-OC’), rather than residual carbonaceous nuclei of DEP
after extraction (referred to as ‘washed DEP’), predominantly
intensified allergen-related airway inflammation in mice, although
‘whole’ DEP (before extraction) exhibited much greater adjuvant
effects than DEP-OC or washed DEP (19). Nonetheless, the distinct
effect/role of DEP components in naïve immune cells in vitro
has never been reported.
Therefore, the aim of the present study was i) to
examine the effects of DEP on naïve splenic mononuclear cells and T
cells isolated from splenocytes and ii) to compare the effects of
organic chemical components (DEP-OC) and residual particles of DEP
(washed DEP).
Materials and methods
Animals
Male ICR mice at 6 weeks of age and weighing 29–33 g
(Japan Clea Co., Tokyo, Japan) were used in all experiments. They
were fed a commercial diet (Japan Clea Co.) and given water ad
libitum. Mice were housed in an animal facility maintained at
24–26°C with 55–75% humidity and a 12-h light/dark cycle. The
studies adhered to the National Institutes of Health guidelines for
the experimental use of animals. All animal studies were approved
by the Institutional Review Board of the National Institute for
Environmental Studies.
Preparation of DEP
A 4JB1-type, light-duty, four-cylinder, 2.74 L Isuzu
diesel engine (Isuzu Automobile Co., Tokyo, Japan) under computer
control was connected to a dynamometer (Meiden-sha, Tokyo, Japan).
DEP were collected in the dilution tunnel (stainless steel tubing)
of a diesel inhalation facility by scraping the inside surface of
the tubing as previously described (20). The collected DEP were referred to
as ‘whole’ DEP and stored at −80°C until use. Whole DEP were
suspended in phosphate-buffered saline (PBS; Sigma, St. Louis, MO,
USA) at pH 7.4 containing 0.05% Tween-80 (Nakarai Tesque, Kyoto,
Japan) and 0.25% dimethyl sulfoxide (DMSO) (Nakalai Tesque). The
whole DEP suspension was sonicated for 3 min with an ultrasonic
disrupter (UD-201; Tomy Seiko, Tokyo, Japan), as previously
described (21,22).
Preparation of DEP-OC and washed DEP
DEP were extracted with benzene-ethanol as
previously described (19).
Briefly, 10 g of DEP was suspended in 800 ml of benzene-ethanol
(3:1, v/v) and was ultrasonicated for 30 min. The suspension was
centrifuged at 600 × g for 20 min. The supernatants were
transferred to another tube and then were filtered with a membrane
filter (pore size 0.45 μm). This procedure was repeated twice. The
residual (solid) particles of DEP were prepared as washed DEP. The
benzene-ethanol solution was evaporated to dryness in a rotary
evaporator and then a vacuum pump, and the residue was dissolved in
100% DMSO and prepared as DEP-OC. They were also stored at −80°C
until use.
Splenocyte preparation and DEP
exposure
Spleens were removed from the ICR mice and placed in
a Petri dish with PBS. The spleens were pushed through a 200-mesh
stainless steel sheet, and the resulting cells were suspended in
PBS. Cells were collected by centrifugation at 400 × g for 10 min
at 20°C. Thereafter, red blood cells were removed by incubating
with hypotonic lysis buffer for 3 min. Cells were washed twice with
PBS and resuspended in R10 [Gibco RPMI-1640 medium (Invitrogen,
Grand Island, NY, USA), supplemented with 10% heat-inactivated
fetal bovine serum (FBS; MP Biomedicals Inc., Eschwege, Germany),
100 U/ml penicillin, 100 μg/ml streptomycin (Sigma) and 50 μM
2-mercaptoethanol (Invitrogen)]. The number of viable cells was
determined using the trypan blue dye exclusion method. Splenocytes
(1×106) were cultured in 1 ml of R10 containing DEP-OC
(0.5, 5 or 50 μg/ml), washed DEP (0.5, 5 or 50 μg/ml), whole DEP
(1, 10 or 100 μg/ml) or the control (0.1% DMSO, 0.05% Tween-80) in
12-well plates at 37°C in a 5% CO2/95% air atmosphere.
After a 24-h incubation, cells were analyzed for FACS, and the
culture supernatants were collected and frozen for subsequent
interleukin (IL)-4 and total IgE analyses. Since the organic
fraction of DEP constitutes ∼50% of total particle mass (16,23),
we set the concentration of each component.
T-cell isolation and DEP exposure
T lymphocytes were isolated by passing splenocytes
(1×108) over a nylon fiber column (Wako Pure Chemical
Industries Ltd., Osaka, Japan) based on a T-cell purification
method. This method utilizes the principle of nylon fiber's
affinity to B cells; thus, T-cell preparation of sufficient purity
and without severe damages was achieved through the preferential
adsorption of B cells to the fiber in the column (24). The column was incubated for 45 min
at 37°C in a 5% CO2/95% air atmosphere; thereafter, it
was washed with R10 and the fraction of non-adherent cells (T
cells) was collected. The T cells were then resuspended in R10. The
DEP exposure protocol and examination points and parameters (except
for IgE) in the experiments were the same as for the
splenocytes.
ELISA for interleukin (IL)-4
ELISA for IL-4 (Amersham, Buckinghamshire, UK) was
conducted according to the manufacturer's instructions. The
secondary antibodies were conjugated to horseradish peroxidase.
Values generated by subtracting readings obtained at 450 nm from
those at 550 nm were converted to pg/ml using values obtained from
standard curves generated with the limits of detection at 5
pg/ml.
Total IgE determination
Total IgE antibody level was measured by IgE-capture
ELISA (2,25). In brief, microplate wells were
coated with a rat anti-mouse IgE monoclonal antibody (BD
Biosciences Pharmingen, San Diego, CA, USA) and incubated overnight
at 4°C. After washing with PBS containing 0.05% Tween-20 (PBST;
Nakalai Tesque), microplate wells were incubated with 1% BSA-PBS
and 0.01% thimerosal at room temperature for 1 h. After washing
with PBS containing PBST, diluted samples were added to the
microplate and incubated overnight at 4°C. After washing with PBST,
biotinylated rat anti-mouse IgE was added to each well and
incubated for 1 h at room temperature with
β-D-galactosidase-conjugated streptavidin (Zymed Laboratories, San
Francisco, CA, USA). After the final washing, the wells were
incubated with 4-methylumbelliferyl-β-galactoside (Sigma) as the
enzyme substrate at 37°C for 2 h. The enzyme reaction was stopped
with 0.1 M glycine-NaOH (pH 10.3). The fluorescene intensity was
read using a microplate fluorescene reader (Fluoroskan Flow
Laboratories, Costa Mesa, CA, USA). A450 readings of the samples
were converted to nanograms per milliliter, using a standard curve
generated with double dilutions of the mouse IgE κ isotype standard
(BD Biosciences Pharmingen).
FACS analysis
For FACS analysis, the following monoclonal
antibodies were used: IL-4R (CD124: mIL4R-M1, PE-conjugated), CD69
(H1. 2F3), CD40 Ligand (L) (CD154: gp39: MR1, PE-conjugated) and
CD19 (FITC-conjugated) (all from BD Biosciences Pharmingen). Cells
(1–3×105) were resuspended in 100 μl PBS with 0.3% BSA
and 0.05% sodium azide (Wako Pure Chemical Industries Ltd.) and
stained with antibodies at 1 μg for 30 min on ice. After
incubation, the cells were washed, and fluorescence was measured
using a FACSCalibur (Becton Dickinson and Company, NJ, USA). For
each sample, fluorescence data from 10,000 cells were collected,
and positive cells were expressed as the percentage of total
events.
Statistical analysis
Data are expressed as the mean ± SEM of four animals
from one experiment, representative of three experiments.
Differences among groups were analyzed by ANOVA (Stat View version
4.0; Abacus Concepts, Inc., Berkeley, CA, USA). When significant
differences were detected, post hoc comparisons within each
group were evaluated with the Fisher's LSD test. Significance was
assigned to P-values <0.05.
Results
Effects of DEP on IL-4 production from
naïve splenocytes and their T cells
To determine the effects of DEP exposure on naïve
splenocytes and their T cells in the context of the Th2 milieu, we
compared the protein levels of IL-4 in the supernatants 24 h after
co-culture (Fig. 1). Exposure to
whole DEP and their components elevated IL-4 levels in the
supernatants from splenocytes and isolated T cells in a
concentration-dependent manner as compared to IL-4 levels in the
cells exposed to the control medium [P<0.01 vs. whole DEP (100
μg/ml), DEP-OC (50 μg/ml) or washed DEP (50 μg/ml), except for
T-cells exposed to DEP-OC (50 μg/ml) (P<0.05)].
 | Figure 1.Effects of whole diesel exhaust
particles (whole DEP) and their components [organic chemicals in
DEP extracted with dicloromethane (DEP-OC)] or residual
carbonaceous nuclei of DEP (washed DEP) on interleukin (IL)-4
production in splenocytes and T cells isolated from the
splenocytes. The collection of splenocytes and T-cell isolation was
conducted as described in Materials and methods. Splenocytes
(1×106) or isolated T cells were cultured in 1 ml of R10
containing DEP-OC (0.5, 5 or 50 μg/ml), washed DEP (0.5, 5 or 50
μg/ml), whole DEP (1, 10 or 100 μg/ml) or control (0.1% DMSO, 0.05%
Tween-80) in 12-well plates at 37°C in a 5% CO2/95% air
atmosphere. After a 24-h incubation, the culture supernatants were
collected, and IL-4 levels were measured by ELISA. Data are the
mean ± SEM of four individual cultures from four animals,
representative of three independent experiments.
*P<0.05, **P<0.01 vs. medium. |
Effects of DEP on total IgE production
from naïve splenocytes
To further determine whether DEP exposure biases
these mononuclear cells towards a Th2 response, we compared protein
levels of total IgE in the supernatants from the splenocytes 24 h
after co-culture (Fig. 2). Whole
DEP and their components increased the total IgE level in a
concentration-dependent manner (data not shown). As compared to
exposure to medium alone, exposure to whole DEP and their
components at each maximal concentration elevated the value in the
supernatants (P<0.01). However, in this context, whole DEP did
not reveal synergistic effects of DEP-OC plus washed DEP, as
observed in the previous in vivo study (19).
Effects of DEP on CD19 expression on
naïve splenocytes and IL-4R, CD69 and CD40L expression on naïve
splenocytes and their T cells
To determine the effects of exposure to DEP on the
surface expression of molecules related to lymphocyte activation 24
h after the culture of naïve splenocytes and their T cells, we
analyzed the expression patterns of CD19 (on splenocytes; Fig. 2), IL-4R, CD69 and CD40L (on
splenocytes and T cells) by FACS (Fig.
3). Exposure to whole DEP, washed DEP or DEP-OC significantly
increased CD19 expression on splenocytes as compared to the level
of CD19 expression on splenocytes exposed to the control medium
(P<0.01; Fig. 2). The
expression levels of IL-4R, CD69 or CD40L were significantly higher
on both splenocytes and isolated T cells treated with whole DEP
(100 μg/ml) or DEP-OC (50 μg/ml) than the expression levels on
cells treated with medium alone (P<0.01). Also, the level was
higher on cells treated with washed DEP (P<0.01 for IL-4R on
both cells, CD69 or CD40L on splenocytes; P<0.05 for CD40L on T
cells) compared to the level on cells treated with medium; however,
the level was lower on cells treated with DEP-OC (P<0.01 for
IL-4R on splenocytes, CD69 or CD40L; data not shown).
Discussion
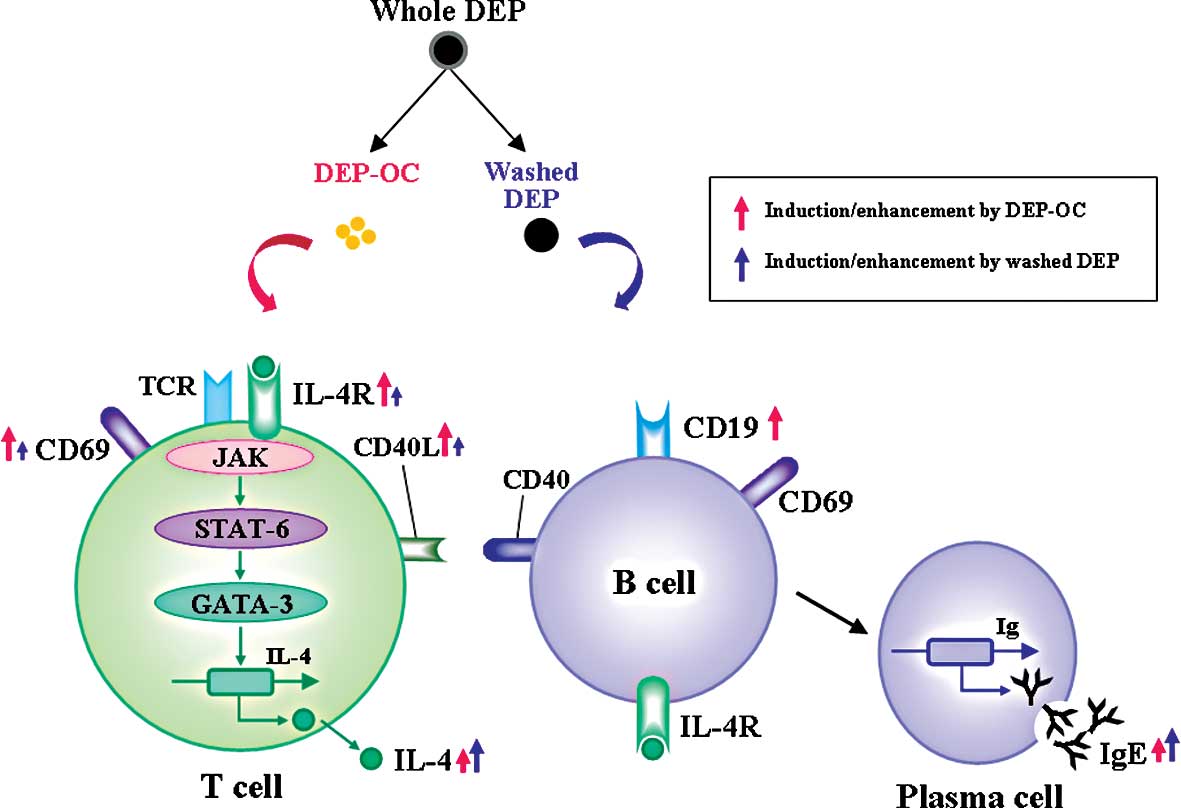
The present study demonstrated that DEP exposure
induces IL-4 and IgE production and several surface markers related
to lymphocyte activation by mouse naïve mononuclear cells and/or
isolated T cells in vitro. Studies on their constituents
show that both DEP components induce IL-4 production by splenocytes
and isolated T cells and consequent IgE synthesis in splenocytes;
however, the effect is generally greater for washed DEP than for
DEP-OC. In contrast, DEP-OC only increased the surface expression
of CD19 on splenocytes. Furthermore, although both components
increased the surface expression of IL-4R, CD69 and CD40L on both
splenocytes and isolated T cells, the impact was significantly
greater for DEP-OC than for washed DEP with an overall trend.
With respect to the adjuvant activity of DEP on
atopic pathophysiology, there are two major aspects, i.e., impacts
on allergens/antigens and on host immunity. DEP reportedly absorb
several allergens, such as Lol p1, Bet v 1, Der p 1, Fel d 1 and
Can f 1 (26,27). Furthermore, D'Amato et al
demonstrated that, by adhering to the surface of airborne
allergens, such as pollen grains and/or plant-derived paucimicronic
components, air pollutants including DEP, modify their antigenic
properties (28), although the
underlying mechanisms remain to be determined. On the other hand,
DEP and/or DE were reported to directly/indirectly influence
several host cell populations, particularly immune cells, leading
to the progression/development/exacerbation of allergic disorders
in vitro. For example, DEP and their components directly
activated B cells to enhance IgE production (29,16),
chemical constituents of DEP induced IL-4 production/release by
human-derived basophils (12) and
DE exposure decreased IL-12 (Th1 cytokine) production from alveolar
macrophages or a macrophage cell line (RAW264.7 cell) in
vitro (30) as well as that in
lung homogenates in vivo (2). Furthermore, DEP was found to suppress
T-bet expression and interferon-γ production by isolated T cells
from healthy humans, implicating DEP in promoting Th2-skewed
responses in lymphocytes (31,32).
In the present study, whole DEP and their components (washed DEP
and DEP-OC) induced IL-4 production and IgE synthesis from
splenocytes. Our present study was, accordingly, consistent with
these previous studies (31,32)
with an overall trend; however, in the present study, we first
demonstrated that DEP components differentially contributed to the
adjuvant effects on allergic reaction, i.e., washed DEP and DEP-OC
induced a Th2 response (IL-4 production with IgE de novo
synthesis) in splenocytes, including T cells, whereas DEP-OC had a
marked potential to up-regulate IL-4R (with CD69, CD40L and CD19)
on these cells, which conceivably culminates in the promotion of
Th2-skewed immune reactions and consequent atopic immunopathology
(Fig. 4). In addition, we and
another group found that DEP activated dendritic cells,
professional antigen-presenting cells, to favor allergen-specific
Th2 immunogenicity in vitro (33,13).
Thus, the present study also expanded the previous ones in that DEP
and their components favor a Th2 milieu in naïve splenic
mononuclear cells and T cells isolated from splenocytes, even in
the absence of antigen-presenting cells (plus allergen), indicating
that these toxicants have the potential to promote an allergic
pathophysiology irrespective of allergen sensitization and/or DC/T
cell interaction.
We previously demonstrated that DEP components
differentially affect allergic lung inflammation in vivo,
i.e., DEP-OC predominantly exacerbated allergic inflammation,
whereas washed DEP did not aggravate the pathology (19). Also, in the present study, DEP-OC
induced IL-4/IgE production/synthesis, implying a role in the Th2
milieu even in the absence of allergen-priming. Furthermore, DEP-OC
markedly induced the surface expression of CD19, suggesting a role
in B cell-plasma cell activation. These in vitro effects of
DEP-OC support the adjuvant property against Th2-mediated
pathobiology in vivo (19)
and also might be one of the mechanisms explaining the phenomenon
(19). Notably, however, washed
DEP also significantly elevated IL-4 production with IgE synthesis
in the present study. Conversely, our previous in vivo study
demonstrated that pulmonary exposure to washed DEP in asthmatic
mice did not increase the lung expression of Th2-type cytokines,
such as IL-4, IL-5 and IL-13, but did exacerbate Th1-type
cytokines, such as interferon-γ (19). These inconsistent results may be
explained by a difference between in vitro and in
vivo studies. As compared to an in vivo condition
(19), washed DEP easily come into
contact with immune cells and stimulate/activate them in
vitro. In support of this, we confirmed that carbon (black)
nanoparticles, which are ultrafine particles less than 100 nm in
mass median aerodynamic diameter compared to washed DEP (200 nm),
exacerbate allergic airway inflammation even in vivo
(25). Furthermore, the
enhancement was greater with smaller (14 nm) than with larger (56
nm) nanoparticles (25),
suggesting that more mobile particles have a higher adjuvant
potential. On the contrary, a soluble component, DEP-OC, may
influence systemic allergic response (broadly) likely reaching the
spleen, thereby yielding profound adverse effects on allergic
pathophysiology in vivo. Alternatively, DEP-derived
carbonaceous compounds (washed DEP) may have antiallergic
properties against alveolar macrophages and/or lung resident cells,
including endothelial cells, epithelial cells and fibroblasts
rather than mononuclear cells, which can hinder the enhancing
effects in vivo. Further studies are required to address
this issue.
CD69 is an early activation marker of hematopoietic
cells. The molecule is reportedly linked to the activation of Th1
lineage in vitro (34) and
Th1-dominant immunopathology in vivo (35). CD40-CD40L (CD154) interaction is
crucial in the regulation of crosstalk between both dendritic
(DC)-T and DC-B cells (36). In
the present study, DEP-OC exposure dramatically up-regulated CD69
and CD40L on both splenocytes and T cells. It is attractive to
speculate that the amplifying effect on CD69 expression partially
contributes to relatively weaker IL-4 synthesis by DEP-OC causally
via altering Th1/Th2 homeostasis than that by washed DEP.
Alternatively, DEP-OC may fully show their adjuvant effects on
allergic reactions in cases of (allergen)/antigen-presenting
cells/lymphocytes rather than naïve (physiological)
conditions/cells. Future investigations are necessary employing the
same protocol involving Th2 cells with or without
allergen-priming.
There are some limitations regarding this study. We
used splenocytes and isolated T cells from these cells, not
completely matching the real situation in terms of the exposure
pattern, as humans are exposed to particulate matter (PM),
including DEP mainly through the airway route. However, our recent
ex vivo studies showed that pulmonary exposure to DEP
potentiates CD4 polarization (37)
with an allergenspecific Th2 response in splenocytes of asthmatic
mice (13), suggesting that these
extrathoracic lymphoid cells are a target. In the future,
nevertheless, the same protocol as in the present study should be
employed involving draining lymph node cells around respiratory
systems, such as cervical and mediastinal nodes. In any case, the
present study may provide a clue for future novel screening tests
to detect the pro-allergic activity of other conceivable
pollutants, such as airborne PM, nanomaterials and environmental
chemicals to elicit allergic reactions. Nonetheless, investigation
of intracellular signaling regarding the differential effects of
DEP components in depth to identify molecular targets is warranted.
We are currently examining this using a T-cell line (Jurkat cells).
In the cell line, whole DEP and washed DEP significantly and DEP-OC
modestly activated ERK1/2 of MAP kinase (unpublished data)
consistent with a previous report (32).
In summary, whole DEP and their components induced
IL-4 production with IgE synthesis from naïve splenocytes and/or
splenocyte-derived T cells in vitro. The effects were
stronger with residual carbonaceous nuclei in DEP than with organic
chemical components, whereas, organic chemical components markedly
increased the surface expression of molecules related to B-cell
activation, such as CD19, and T-cell activation, such as IL-4R,
CD69 and CD40L, on these cells. These data indicate the
pro-allergic effects of DEP on lymphoid-lineage cells, even in the
absence of allergen-priming in vitro, and raise the
possibility that each component in DEP differentially affects
immune cells, which culminates in an increase in the DEP-mediated
exacerbation of allergic inflammatory conditions with maladaptive
Th2 immunity.
Acknowledgements
This study was partly funded by grants
from the Grant-in-Aid (#20120014 to H. Takano) for Scientific
Research on Innovative Areas. The authors thank Rie Yanagisawa,
Naoko Ueki, Satomi Abe and Rieko Shibahara for the assistance
throughout the study.
References
|
1.
|
Ichinose T, Furuyama A and Sagai M:
Biological effects of diesel exhaust particles (DEP). II. Acute
toxicity of DEP introduced into lung by intratracheal instillation.
Toxicology. 99:153–167. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Takano H, Yoshikawa T, Ichinose T,
Miyabara Y, Imaoka K and Sagai M: Diesel exhaust particles enhance
antigen-induced airway inflammation and local cytokine expression
in mice. Am J Respir Crit Care Med. 156:36–42. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ichinose T, Takano H, Miyabara Y and Sagai
M: Long-term exposure to diesel exhaust enhances antigen-induced
eosinophilic inflammation and epithelial damage in the murine
airway. Toxicol Sci. 44:70–79. 1998. View Article : Google Scholar
|
|
4.
|
Miyabara Y, Takano H, Ichinose T, Lim HB
and Sagai M: Diesel exhaust enhances allergic airway inflammation
and hyperresponsiveness in mice. Am J Respir Crit Care Med.
157:1138–1144. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Takano H, Ichinose T, Miyabara Y,
Yoshikawa T and Sagai M: Diesel exhaust particles enhance airway
responsiveness following allergen exposure in mice. Immunopharmacol
Immunotoxicol. 20:329–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Terada N, Maesako K, Hiruma K, Hamano N,
Houki G, Konno A, Ikeda T and Sai M: Diesel exhaust particulates
enhance eosinophil adhesion to nasal epithelial cells and cause
degranulation. Int Arch Allergy Immunol. 114:167–174. 1997.
View Article : Google Scholar
|
|
7.
|
Takizawa H, Abe S, Okazaki H, Kohyama T,
Sugawara I, Saito Y, Ohtoshi T, Kawasaki S, Desaki M, Nakahara K,
Yamamoto K, Matsushima K, Tanaka M, Sagai M and Kudoh S: Diesel
exhaust particles upregulate eotaxin gene expression in human
bronchial epithelial cells via nuclear factor-kappa B-dependent
pathway. Am J Physiol Lung Cell Mol Physiol. 284:L1055–L1062. 2003.
View Article : Google Scholar
|
|
8.
|
Terada N, Hamano N, Maesako KI, Hiruma K,
Hohki G, Suzuki K, Ishikawa K and Konno A: Diesel exhaust
particulates upregulate histamine receptor mRNA and increase
histamine-induced IL-8 and GM-CSF production in nasal epithelial
cells and endothelial cells. Clin Exp Allergy. 29:52–59. 1999.
View Article : Google Scholar
|
|
9.
|
Beck-Speier I, Dayal N, Karg E, Maier KL,
Schumann G, Schulz H, Semmler M, Takenaka S, Stettmaier K, Bors W,
Ghio A, Samet JM and Heyder J: Oxidative stress and lipid mediators
induced in alveolar macrophages by ultrafine particles. Free Radic
Biol Med. 38:1080–1092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hirota R, Akimaru K and Nakamura H: In
vitro toxicity evaluation of diesel exhaust particles on human
eosinophilic cell. Toxicol In Vitro. 22:988–994. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Saneyoshi K, Nohara O, Imai T, Shiraishi
F, Moriyama H and Fujimaki H: IL-4 and IL-6 production of bone
marrow-derived mast cells is enhanced by treatment with
environmental pollutants. Int Arch Allergy Immunol. 114:237–245.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Devouassoux G, Saxon A, Metcalfe DD,
Prussin C, Colomb MG, Brambilla C and Diaz-Sanchez D: Chemical
constituents of diesel exhaust particles induce IL-4 production and
histamine release by human basophils. J Allergy Clin Immunol.
109:847–853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Inoue K, Koike E, Takano H, Yanagisawa R,
Ichinose T and Yoshikawa T: Effects of diesel exhaust particles on
antigen-presenting cells and antigen-specific Th immunity in mice.
Exp Biol Med. 234:200–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Porter M, Karp M, Killedar S, Bauer SM,
Guo J, Williams D, Breysse P, Georas SN and Williams MA:
Diesel-enriched particulate matter functionally activates human
dendritic cells. Am J Respir Cell Mol Biol. 37:706–719. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Takenaka H, Zhang K, Diaz-Sanchez D, Tsien
A and Saxon A: Enhanced human IgE production results from exposure
to the aromatic hydrocarbons from diesel exhaust: direct effects on
B-cell IgE production. J Allergy Clin Immunol. 95:103–115. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Tsien A, Diaz-Sanchez D, Ma J and Saxon A:
The organic component of diesel exhaust particles and phenanthrene,
a major polyaromatic hydrocarbon constituent, enhances IgE
production by IgE-secreting EBV-transformed human B cells in vitro.
Toxicol Appl Pharmacol. 142:256–263. 1997. View Article : Google Scholar
|
|
17.
|
IARC: Monographs on the Evaluation of
Carcinogenic Risks to Humans: Diesel and Gasoline Exhaust and Some
Nitoarenes. Lyon, France: pp. 1989
|
|
18.
|
Heo Y, Saxon A and Hankinson O: Effect of
diesel exhaust particles and their components on the
allergen-specific IgE and IgG1 response in mice. Toxicology.
159:143–158. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Yanagisawa R, Takano H, Inoue K, Ichinose
T, Sadakane K, Yoshino S, Yamaki K, Yoshikawa T and Hayakawa K:
Components of diesel exhaust particles differentially affect
Th1/Th2 response in a murine model of allergic airway inflammation.
Clin Exp Allergy. 36:386–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sagai M, Furuyama A and Ichinose T:
Biological effects of diesel exhaust particles (DEP). III.
Pathogenesis of asthma like symptoms in mice. Free Radic Biol Med.
21:199–209. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Takano H, Yanagisawa R, Ichinose T,
Sadakane K, Yoshino S, Yoshikawa T and Morita M: Diesel exhaust
particles enhance lung injury related to bacterial endotoxin
through expression of proinflammatory cytokines, chemokines, and
intercellular adhesion molecule-1. Am J Respir Crit Care Med.
165:1329–1335. 2002. View Article : Google Scholar
|
|
22.
|
Yanagisawa R, Takano H, Inoue K, Ichinose
T, Sadakane K, Yoshino S, Yamaki K, Kumagai Y, Uchiyama K,
Yoshikawa T and Morita M: Enhancement of acute lung injury related
to bacterial endotoxin by components of diesel exhaust particles.
Thorax. 58:605–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kawasaki S, Takizawa H, Takami K, Desaki
M, Okazaki H, Kasama T, Kobayashi K, Yamamoto K, Nakahara K, Tanaka
M, Sagai M and Ohtoshi T: Benzene-extracted components are
important for the major activity of diesel exhaust particles:
effect on interleukin-8 gene expression in human bronchial
epithelial cells. Am J Respir Cell Mol Biol. 24:419–426. 2001.
View Article : Google Scholar
|
|
24.
|
Julius MH, Simpson E and Herzenberg LA: A
rapid method for the isolation of functional thymus-derived murine
lymphocytes. Eur J Immunol. 3:645–649. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Inoue K, Takano H, Yanagisawa R, Sakurai
M, Ichinose T, Sadakane K and Yoshikawa T: Effects of nano
particles on antigen-related airway inflammation in mice. Respir
Res. 6:1062005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Knox RB, Suphioglu C, Taylor P, Desai R,
Watson HC, Peng JL and Bursill LA: Major grass pollen allergen Lol
p 1 binds to diesel exhaust particles: implications for asthma and
air pollution. Clin Exp Allergy. 27:246–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ormstad H, Johansen BV and Gaarder PI:
Airborne house dust particles and diesel exhaust particles as
allergen carriers. Clin Exp Allergy. 28:702–708. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
D'Amato G, Liccardi G, D'Amato M and
Cazzola M: Outdoor air pollution, climatic changes and allergic
bronchial asthma. Eur Respir J. 20:763–776. 2002.PubMed/NCBI
|
|
29.
|
Zhang K, Clark EA and Saxon A: CD40
stimulation provides an IFN-gamma-independent and IL-4-dependent
differentiation signal directly to human B cells for IgE
production. J Immunol. 146:1836–1842. 1991.
|
|
30.
|
Saito Y, Azuma A, Kudo S, Takizawa H and
Sugawara I: Effects of diesel exhaust on murine alveolar
macrophages and a macrophage cell line. Exp Lung Res. 28:201–217.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Ohtani T, Nakagawa S, Kurosawa M, Mizuashi
M, Ozawa M and Aiba S: Cellular basis of the role of diesel exhaust
particles in inducing Th2-dominant response. J Immunol.
174:2412–2419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Sasaki Y, Ohtani T, Ito Y, Mizuashi M,
Nakagawa S, Furukawa T, Horii A and Aiba S: Molecular events in
human T cells treated with diesel exhaust particles or formaldehyde
that underlie their diminished interferon-gamma and interleukin-10
production. Int Arch Allergy Immunol. 148:239–250. 2009. View Article : Google Scholar
|
|
33.
|
Chan RC, Wang M, Li N, Yanagawa Y, Onoe K,
Lee JJ and Nel AE: Pro-oxidative diesel exhaust particle chemicals
inhibit LPS-induced dendritic cell responses involved in T-helper
differentiation. J Allergy Clin Immunol. 118:455–465. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Harimaya A, Himi T, Fujii N, Tarkkanen J,
Carlson P, Ylikoski J and Mattila P: Induction of CD69 expression
and Th1 cytokines release from human peripheral blood lymphocytes
after in vitro stimulation with Alloiococcus otitidis and
three middle ear pathogens. FEMS Immunol Med Microbiol. 43:385–392.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hernandez-Garcia C, Fernandez-Gutierrez B,
Morado IC, Banares AA and Jover JA: The CD69 activation pathway in
rheumatoid arthritis synovial fluid T cells. Arthritis Rheum.
39:1277–1286. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Ma DY and Clark EA: The role of CD40 and
CD154/CD40L in dendritic cells. Semin Immunol. 21:265–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Inoue K, Koike E, Yanagisawa R and Takano
H: Effects of pulmonary exposure to diesel exhaust particles on
extrathoracic CD4 polarization in asthmatic mice. Immunopharmacol
Immunotoxicol. 31:71–74. 2009. View Article : Google Scholar : PubMed/NCBI
|