Introduction
During the course of studies on the antiviral
activities of various natural products and their components
(1–6), we previously characterized the
antiviral activity of ascorbic acid against the multiplication of a
variety of DNA and RNA viruses under the defined in vitro
conditions. We found that the antiviral activity of ascorbic acid
was not due to its antioxidant activity since dehydroascorbic acid,
an oxidized form of ascorbic acid without any reducing ability,
also showed noticeable antiviral activities against those viruses
(5). Previous characterization
revealed that dehydroascorbic acid showed even stronger antiviral
activity, though a much less cytotoxic effect in vitro than
ascorbic acid. In addition, dehydroascorbic acid does not induce
the formation of highly toxic free hydroxyl radicals, even in the
presence of metal ions in culture medium. Considering a potential
therapeutic use of dehydroascorbic acid, we further characterized
the mode of antiviral activities of this compound.
Materials and methods
Cells and viruses
MDCK, HEp-2 and Vero cells were grown in Eagle's
minimum essential medium (MEM) containing 5% fetal bovine serum
(FBS). Herpes simplex virus type 1, strain F (HSV-1), influenza
virus A/Aichi (H3N2) and polio-virus type 1,
Sabin vaccine strain, were used throughout the experiments. The
viruses were propagated in Vero cells (for HSV-1 and poliovirus) in
MEM supplemented with 0.5% FBS, or in MDCK cells (for influenza
virus) in MEM supplemented with 0.1% bovine serum albumin (BSA) and
acetylated trypsin (4 μg/ml). The viruses were stored at
−80°C until use. The amount of each virus was measured by a plaque
assay as described previously (7–9).
Effect of the reagent on the virus
yields
Dehydroascorbic acid was obtained from Wako
Chemicals. The reagent solution (1.0 M or 100 mM) was prepared by
dissolving the reagents in hot water, and its acidity was
neutralized with 1 N sodium hydroxide solution followed by
filtration through a Millipore Dimex membrane (pore size, 0.22
μm).
Monolayered cells in 35-mm dishes were infected with
the viruses at an indicated multiplicity of infection (MOI). The
infected cells were further incubated at 37°C (for HSV-1 and
influenza virus) or 35.5°C (for poliovirus) for the indicated times
in the serum-free MEM containing 0.1% BSA and the indicated
concentrations of the reagent. For the experiments with influenza
virus, acetylated trypsin (4 μg/ml) was also added to the
medium for the proteolytic activation of virus infectivity. At the
indicated times, the infected cells with the culture medium (for
HSV-1 and poliovirus) or the culture medium only (for influenza A
virus) were harvested, and the amount of total progeny virus in the
culture was determined as described previously (7–9).
Determination of cytopathic effects and
cell death
Confluent monolayers of HEp-2 cells were incubated
at 37°C for 24 h in the serum-free MEM containing 0.1% BSA and the
indicated concentrations of reagent. Cytopathic effects were
determined by microscopic observation of the cells, in which
approximate numbers of rounded cells on monolayers were estimated
under a phase-contrast microscope.
To determine the extent of cell death, the
monolayered cells were trypsinized to obtain a single-cell
suspension. After the addition of MEM containing 5% FBS to the
suspension to neutralize the trypsin and to stabilize the cells,
the number of living or dead cells was determined by a
dye-exclusion method with trypan blue.
Results and Discussion
Effect of dehydroascorbic acid on the
multiplication of DNA and RNA viruses
In a previous study (5), we found that dehydroascorbic acid had
antiviral activity against HSV-1 in vitro, which was
stronger than that of ascorbic acid. To further characterize the
antiviral activities of the reagent, we tested three viruses of
completely different types: HSV-1 (Family of Herpesviridae),
influenza virus type A (Family of Orthomyxoviridae) and
poliovirus (Family of Picornaviridae). HSV-1 is a
double-stranded DNA virus (10),
while influenza virus is a negative-stranded RNA virus (11); both are large enveloped viruses and
require the cell nucleus for virus multiplication. By contrast,
poliovirus is a small non-enveloped virus carrying a
positive-stranded RNA as a genome, and replicates in the cytoplasm
of infected cells (12).
Fig. 1 shows the
effects of dehydroascorbic acid on the relative virus yields of
these three viruses when the cells infected with each of the
viruses were incubated in the medium containing the indicated
concentrations of the reagent. The multiplication of all three
viruses was suppressed to various degrees depending on the virus,
although these viruses showed a similar degree of sensitivity to
ascorbic acid as in the previous study (5). The virus yield decreased with
increasing concentrations of dehydroascorbic acid: with 10 mM of
the reagent, the yield of influenza virus, HSV-1 and poliovirus was
approximately one thousandth, one tenth and half that in the
absence of the reagent, respectively, indicating that influenza
virus is the most sensitive and poliovirus is the least sensitive
of these viruses. These results clearly show that dehydroascorbic
acid inhibits the multiplication of viruses of widely different
structures (regardless of whether they are enveloped or
non-enveloped, double-stranded DNA- or single-stranded RNA-genome)
and also that it inhibits virus multiplication whether the
replication and transcription of the viral genome occur in the
nucleus or in the cytoplasm of the infected cells. It is also worth
noting that the antiviral activity of the reagent was apparently
independent of the cell type, as the multiplication of the
influenza virus was examined in MDCK cells (derived from canine
kidney cells), while that of HSV-1 and poliovirus was examined in
HEp-2 cells (derived from human cervical carcinoma).
Virucidal effect of the reagent
To examine the direct effect of dehydroascorbic acid
on the infectivity of viruses, HSV-1 or poliovirus was incubated in
the buffered solutions containing the reagent at various
concentrations. As shown in Fig.
2, neither the infectivity of HSV-1 nor of poliovirus was
inactivated by incubation with the reagent even at 10 mM,
indicating that dehydroascorbic acid does not directly inactivate
these viruses. Similar results were observed when the viruses were
incubated in buffer at a neutral pH (data not shown).
Cytopathic effects of the reagent
Ascorbic acid is known to generate hydroxyl
radicals, even in the presence of a trace amount of ferric ion in
medium. We previously reported that ascorbic acid causes a severe
cytotoxic effect in cells, probably due to these hydroxyl radicals
(5). Since dehydroascorbic acid
also induced a significant degree of cytopathic effects (cell
rounding and detachment from the dish surface) on the
virus-infected cells, we examined the effects of the reagent on the
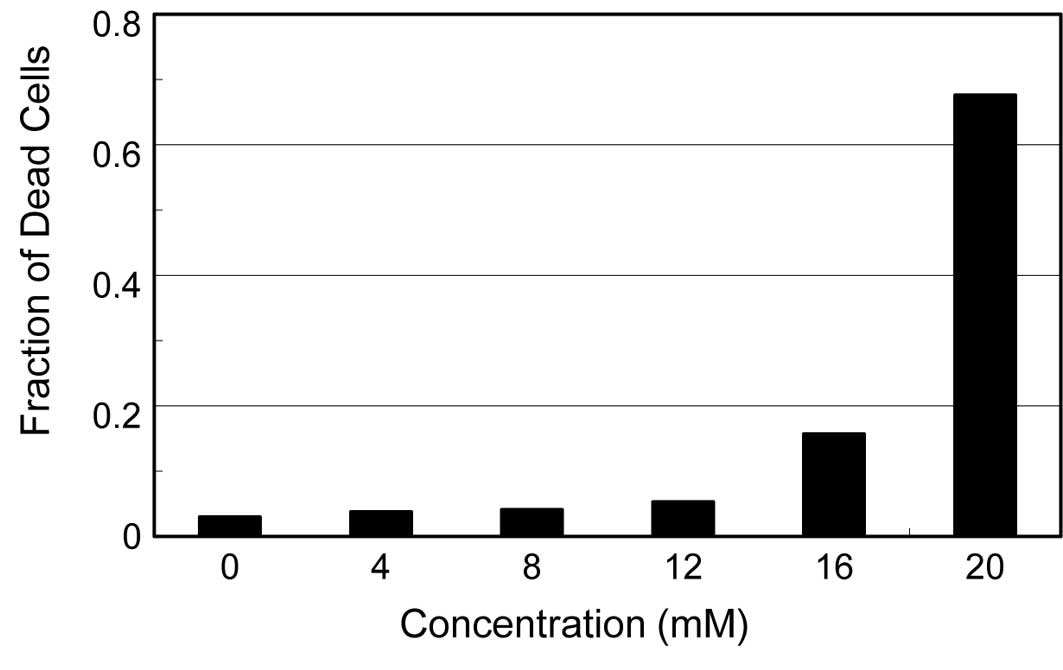
viability of the uninfected cells. As shown in Fig. 3, HEp-2 cells incubated in medium
containing various concentrations of dehydroascorbic acid for 24 h
showed no increase in the fraction of dead cells even at 12 mM of
reagent, although a significant amount of dead cells appeared at 16
mM and increased drastically to approximately 70% at 20 mM. These
results indicate that, although the reagent induces significant
cytopathic effects on the virus-infected HEp-2 and MDCK cells, the
cytotoxic effect of this reagent is likely insufficient to directly
affect the multiplication of the viruses in the infected cells,
considering the concentration of dehydroascorbic acid required for
the significant antiviral activities, i.e., approximately 10
mM.
Dehydroascorbic acid-sensitive step in
the HSV-1 multiplication
Previously, we quantitatively characterized the
kinetics of viral DNA replication, the encapsidation of viral DNA,
the envelopment of nucleocapsids and the formation of infectious
progeny virus in HSV-1-infected cells (13), and revealed that viral DNA
replication occurs exclusively between 3 and 6 h post infection
(p.i.) and that a large amount of DNA accumulates in the infected
cells when the replication of the virus DNA is completed. The
formation of nucleocapsids as well as the envelopment of these
nucleocapsids begins at 5 h p.i., simultaneously with the formation
of infectious progeny virus, and the amount of the progeny virus
increases with time until approximately 14 h p.i.
To examine the target of the antiviral activity of
dehydroascorbic acid in the multiplication process of HSV-1, a
‘time of addition’ experiment was carried out. As shown in Fig. 4, the reagents were added to the
infected culture at various times after the infection, and the
virus yield at the end of virus multiplication was compared to the
virus yield at the time of the addition of reagent. The amounts of
progeny virus were suppressed even when the infected cells received
the reagent in the late stages of the infection, such as at 10 or
12 h p.i. A small but significant increase in the amount of progeny
virus was observed after the addition of the reagent at any time
point during infection, except at 0 h p.i; for example, the amount
of the infectious virus at 10 h p.i. was 5.0. When dehydroascorbic
acid was added at this time point and cell culture was continued
for 23.5 h, the final virus yield was 10.7, indicating that progeny
virus formation continued. However, this value of 10.7 was much
less than the virus yield without the addition of dehydroascorbic
acid (70.4), though it was almost similar to the amount of the
infectious virus formed at 12 h p.i. (13.6). Thus it is evident
that, although the addition of dehydroascorbic acid did not
completely stop progeny formation, it greatly suppressed the
formation even when added at the late stages of infection. These
results clearly show that i) the reagent interferes with virus
multiplication even after the completion of viral DNA replication
(i.e., at 6 h p.i.) and ii) the formation of progeny virus does not
cease immediately after the addition of the reagent.
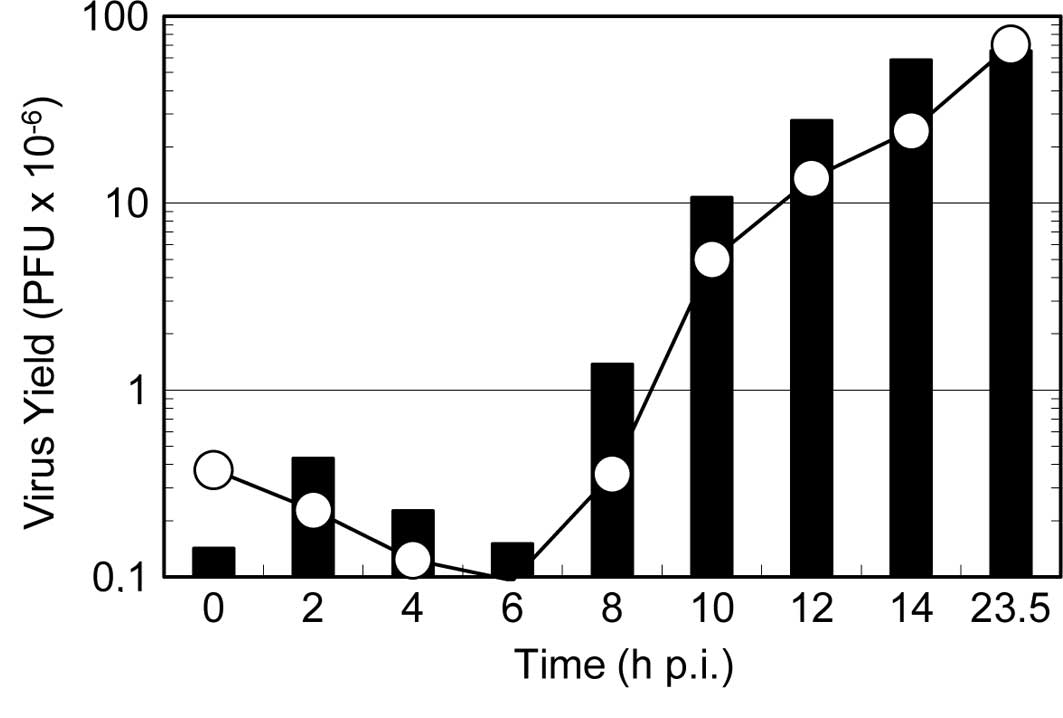
To further clarify the mode of action of the reagent
on viral multiplication, a one-step growth curve was examined in
the presence of the reagent. As shown in Fig. 5, the addition of the reagent at the
beginning of virus multiplication resulted in a significant delay
in the onset of the progeny virus formation (i.e., an extension of
the latent period) and decreased the final yield of progeny virus.
By contrast, when the reagent was added at 8 h p.i., the formation
of progeny virus continued steadily for an additional 2 h and then
ceased completely. These results are consistent with the results in
Fig. 4, indicating that the
reagent inhibits virus multiplication even at the late stages of
the virus multiplication, and that the formation of progeny
infectious virus continues for 2 h after the addition of the
reagent to the infected culture.
Previously, we observed similar kinetics when the
multiplication of HSV-1 was inhibited by the addition of ammonium
chloride (7) or Brefeldin A
(14) at the stage of the
envelopment of viral nucleocapsids after the completion of viral
DNA replication. These two reagents are known to inhibit the
function of the Golgi apparatus of cells where the envelopment of
HSV nucleocapsids takes place (15). The similarity of the kinetics
suggests that dehydroascorbic acid inhibits the formation of
progeny infectious virus at the stage of the envelopment of
nucleocapsids at the Golgi apparatus of the infected cells,
although additional contribution of some other step(s) in the
multiplication process cannot be excluded.
In this study, we showed that dehydroascorbic acid
inhibits the multiplication of several viruses of widely different
structures and replication strategies. Previous characterization of
the antiviral effects of ascorbic acid (5) revealed that the antiviral effect of
ascorbic acid is, at least in part, a secondary result of the
cytotoxic effect of the reagent. In contrast to ascorbic acid, the
antiviral effect of dehydroascorbic acid is not a secondary effect
of cytotoxicity, but is more likely specific interference in a
certain virus-cell interaction, probably due to its binding to the
virus or to molecules involved in viral replication. The results
shown in Figs. 4 and 5 reveal that dehydroascorbic acid
interferes with the multiplication of HSV-1 after the completion of
viral DNA replication, probably at the stage of the envelopment of
nucleocapsids (i.e., the assembly of progeny virus particles).
Dehydroascorbic acid has been reported to have the ability to bind
to proteins (16,17) and to inhibit certain kinases and
enzymes (18–20), suggesting that it may inhibit
certain protein(s) necessary for virus-host interactions, and may
thereby interfere with virus multiplication.
Acknowledgements
The authors thank Dr Tsutomu Arakawa
for stimulating discussions and for assistance with manuscript
editing.
References
|
1.
|
Uozaki M, Yamasaki H, Katsuyama Y, Higuchi
M, Higuchi T and Koyama AH: Antiviral effect of octyl gallate
against DNA and RNA viruses. Antiviral Res. 73:85–91. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yamasaki H, Uozaki M, Katsuyama Y,
Utsunomiya H, Arakawa T, Higuchi M, Higuti T and Koyama AH:
Antiviral effect of octyl gallate against influenza and other RNA
viruses. Int J Mol Med. 19:685–688. 2007.PubMed/NCBI
|
|
3.
|
Utsunomiya H, Ichinose M, Uozaki M,
Tsujimoto K, Yamasaki H and Koyama AH: Antiviral activities of
coffee extracts in vitro. Food Chem Toxicol. 46:1919–1924. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Murayama M, Tujimoto K, Uozaki M,
Katsuyama Y, Yamasaki H, Utsunomiya H and Koyama AH: Effect of
caffeine on the multiplication of DNA and RNA viruses. Mol Med Rep.
1:251–255. 2008.PubMed/NCBI
|
|
5.
|
Furuya A, Uozaki M, Yamasaki H, Arakawa T,
Arita M and Koyama AH: Antiviral effects of ascorbic acid and
dehydroascorbic acids in vitro. Int J Mol Med. 22:541–545.
2008.PubMed/NCBI
|
|
6.
|
Tsujimoto K, Sakuma C, Uozaki M, Yamasaki
H, Utsunomiya H, Oka K and Koyama AH: Antiviral effect of
pyridinium formate, a novel component of coffee extracts. Int J Mol
Med. 25:459–463. 2010.PubMed/NCBI
|
|
7.
|
Koyama AH and Uchida T: The effect of
ammonium chloride on the multiplication of herpes simplex virus
type 1 in Vero cells. Virus Res. 13:271–282. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kurokawa M, Koyama AH, Yasuoka S and
Adachi A: Influenza virus overcomes apoptosis by rapid
multiplication. Int J Mol Med. 3:527–530. 1999.PubMed/NCBI
|
|
9.
|
Koyama AH, Irie H, Ueno F, Ogawa M, Nomoto
A and Adachi A: Suppression of apoptotic and necrotic cell death by
poliovirus. J Gen Virol. 82:2965–2972. 2001.PubMed/NCBI
|
|
10.
|
Roizman B and Knipe DM: Herpes simplex
virus and their replication. Fields Virology. 4th edition. Fields
BN, Knipe DM and Howley PM: Lippincott-Raven; New York: pp.
2399–2460. 2001
|
|
11.
|
Lamb RA and Kruchikokug RM:
Orthomyxoviridae: the viruses and their replication. Fields
Virology. 4th edition. Fields BN, Knipe DM and Howley PM:
Lippincott-Raven; New York: pp. 1487–1530. 2001
|
|
12.
|
Racaniello VR: Picornaviridae: the viruses
and their replication. Fields Virology. 4th edition. Fields BN,
Knipe DM and Howley PM: Lippincott-Raven; New York: pp. 685–722.
2001
|
|
13.
|
Koyama AH and Uchida T: Quantitative
studies on the maturation process of herpes simplex virus type 1 in
Vero cells. Virus Res. 10:281–286. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Koyama AH and Uchida T: Inhibition by
Brefeldin A of the envelopment of nucleocapsids in herpes simplex
virus type 1-infected Vero cells. Arch Virol. 135:305–317. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Leuzinger H, Ziegler U, Schraner EM,
Fraefel C, Glauser DL, Heid I, Achermann M, Mueller M and Wild P:
Herpes simplex virus 1 envelopment follows two diverse pathways. J
Virol. 79:13047–13059. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Meucci E, Martorana GE, Ursitti A,
Miggiano GA, Mordente A and Castelli A: Vitamin C-bovine serum
albumin binding behavior. Ital J Biochem. 36:75–81. 1987.PubMed/NCBI
|
|
17.
|
Lozinsky E, Novoselsky A, Glaser R, Shames
AI, Likhtenshtein GI and Meyerstein D: Effect of ionic strength on
the binding of ascorbate to albumin. Biochim Biophys Acta.
1571:239–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fiorani M, De Sanctis R, Scarlatti F,
Vallorani L, De Bellis R, Serafini G, Bianchi M and Stocchi V:
Dehydroascorbic acid irreversibly inhibits hexokinase activity. Mol
Cell Biochem. 209:145–153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Neault JF, Benkiran A, Malonga H and
Tajmir-Riahl HA: The effects of anions on the solution structure of
Na, K-ATPase. J Biomol Struc Dyn. 19:95–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Carcamo JM, Pedraza A, Borquez-Ojeda O,
Zhang B, Sanchez R and Golde DW: Vitamin C is a kinase inhibitor:
dehydroascorbic acid inhibits IkappaBalpha kinase beta. Mol Cell
Biol. 24:6645–6652. 2004. View Article : Google Scholar : PubMed/NCBI
|