Introduction
Rifampicin (RFP) is a semisynthetic antibiotic
derived from the rifamycins, the common structure of which is a
naphthohydroquinone or naphthoquinone chromophore spanned by an
aliphatic chain. Previously, we reported the inhibitory effects of
oral RFP on human cancer progression and its anti-angiogenic
properties, which were comparable to those of the angiogenesis
inhibitor endostatin (1).
Clinically, low-dose and long-term oral administration of RFP to 6
hepatitis C virus (HCV)-related liver cirrhosis patients who were
at high risk of presenting with hepatocellular carcinoma (HCC),
resulted in only a single occurrence of HCC during the extensive
follow-up period of 97.3±29.1 (mean ± SD) months, suggesting that
RFP markedly suppressed the progression of HCC of the high-risk
patients by inhibiting angiogenesis and also its direct anticancer
activity. During that study, we noted that RFP also had a
hepatocyte-protective effect by lowering the release of hepatic
enzymes, alanine transaminase (ALT) and aspartate transaminase
(AST), which was not explicitly described in the previous report.
Here, we report the hepatocyte-protective effect of RFP by
revealing clinical data obtained from a group of advanced cirrhosis
patients, who had a high risk of presenting with HCC. We also
revealed that oral RFP had a hepatocyte-protective effect in acute
hepatocyte disorder models of rats and mice. Based on these results
and a finding that RFP has a strong free-radical oxygen scavenging
activity, we speculated that RFP has a hepatocyte-protective effect
in hepatitis patients by lowering oxidative stress, which is
greatly increased in HCV-infected liver cells (2).
Materials and methods
Clinical follow-up
The 6 patients (1)
were followed-up monthly at Tokyo Medical and Dental University
Hospital, after liver cirrhosis was diagnosed by liver biopsy or a
combination of clinical features or both. During monthly visits,
these patients underwent physical examination of serum ALT and AST
related to hepatic function. The protocol was approved by the
Ethics Committee of the Tokyo Medical and Dental University, and
informed consent was received from the patients.
Animal experiments
Animal procedures were approved by the Committee for
the Institutional Care and Use of Animals at GeneCare Research
Institute in accordance with the guidelines for animal
experimentation prepared by the Japanese Association for Laboratory
Animal Science.
Mouse concanavalin A (ConA) model
The efficacy of RFP was evaluated in a mouse
hepatocyte-disorder model induced by ConA injection. Doses of 50,
100 and 200 mg/kg RFP were orally administered to 8 mice
(C57BL/6NCrlCrlj) once a day for 4 days; a single dose of 200 mg/kg
RFP was administered to one group of 8 mice. One hour after the
last drug administration, 0.2 mg ConA in phosphate-buffered saline
was injected into the tail vein. Under ether anesthesia, blood was
collected by vena cava puncture 24 h after the ConA injection. ALT,
AST and lactose dehydrogenase (LDH) levels in the plasma were
measured.
Rat D-galactosamine (D-Gal) model
The efficacy of RFP was evaluated in a rat
hepatocyte-disorder model induced by D-Gal injection. Doses of 50,
100 and 200 mg/kg RFP were orally administered to 8 rats
[Crlj:CD(SD)] once a day for 4 days; 200 mg/kg of RFP was
administered to one group of 8 rats. One hour after the last drug
administration, 350 mg/kg of D-Gal in saline was injected into the
abdominal cavity. Under ether anesthesia, blood was collected by
vena cava puncture 24 h after the D-Gal injection. ALT, AST and LDH
levels in the plasma were measured.
Assay of anti-oxidant activity
Proton-donative anti-oxidant activity of RFP was
assessed by using stable free radical α,α-diphenyl-β-picrylhydrazyl
(DPPH) as a substrate according to the method by Nomura et
al (3). Briefly, DPPH and test
samples were mixed, and the amount of DPPH was determined
spectrophotometrically at the absorbance (A) at OD550.
The anti-oxidant activity was calculated by using: anti-oxidant
activity = [A550 (sample) – A550
(blank)]/[A550 (trolox) – A550 (blank)] x
trolox concentration/sample concentration. Trolox is a standard
anti-oxidant reagent.
Statistical analyses
First, homoscedasticity was tested and statistical
analyses between control and test groups were carried out using
Dunnett type multiple comparison for homoscedasticity and by
Dunnett type multiple comparison (joint type) for
non-homoscedasticity. For histopathological studies, statistical
analyses were carried out using the Wilcoxon test. For comparison,
vitamins C and E, silibinin and trolox were used as anti-oxidant
reagents.
Results
Clinical study
The liver function of the 6 HCC high-risk patients
with HCV-related cirrhosis was studied by monitoring the serum
values of ALT and AST. Detailed clinical information of the 6
patients was described in our previous study (1). All 6 patients had hepatitis virus
type C of 1b genotype, suggesting resistance to interferon-α
therapy; either they were resistant to interferon-α or their
interferon-α therapy was discontinued because of adverse reactions.
Most patients received administration of ursodeoxycholic acid
(UDCA) before or during administration of RFP (Fig. 1Aa–Fa, dotted lines). They received
low-dose RFP therapy of 150 or 300 mg daily during most of the
follow-up period from 121 to 209 months. Notably, serum levels of
α-fetoprotein, a marker of the growth of hepatocytes and HCC cells,
decreased after RFP administration in 3 patients. Notably, 5
patients showed no sign of presenting with HCC during the long REP
treatment, despite a high statistical frequency of the occurence of
HCC in patients with advanced cirrhosis. Only 1 patient had HCC
during the long follow-up period of 97.3±29.1 months. These
clinical results strongly suggest that RFP is effective in
suppressing HCC, as previously reported (1).
In this context, we investigated whether low-dose
and long-term RFP therapy also improves liver function of these
high-risk patients with HCV-related cirrhosis.
Patient 1 (Fig. 1A)
During ∼90 months of RFP therapy, the values of ALT
and AST tended to improve from 0 to ∼40 months of RFP
administration: AST from 100 to ∼50 IU/l; ALT from 80 to ∼50 IU/l.
However, the strict evaluation of the efficacy of long-term
administration of RFP became difficult after the first 40 months
when the patient developed bile duct stenosis and acute
pancreatitis and received treatment for these two conditions.
Patient 2 (Fig. 1B)
ALT and AST values improved markedly during 0–80
months of RFP administration: AST values decreased from 200 to
<100 IU/l; ALT from 150 to <50 IU/l. This patient received
treatment of UDCA before and sometimes during RFP therapy, but the
treatment of UDCA alone apparently failed to lower the ALT and AST
values. RFP lowered the ALT and AST values without UDCA
Patient 3 (Fig. 1C)
ALT and AST values markedly improved throughout ∼80
months of RFP therapy: AST from 100 to approximately 50 IU/l; ALT
from 175 to ∼50 IU/l. This patient received treatment of UDCA
before and during RFP treatment, but the treatment of UDCA alone
failed to lower the ALT and AST values.
Patient 4 (Fig. 1D)
High values of ALT (>150 IU/l) and AST (>100
IU/l), which were often observed before RFP administration, were
not observed throughout ∼60 months of RFP therapy (150 mg/day), and
most values remained <100 IU/l. This patient received treatment
of UDCA before and during the period of RFP treatment, but the
treatment of UDCA alone did not seem to lower the ALT and AST
values.
Patient 5 (Fig. 1E)
ALT and AST values markedly improved throughout ∼145
months of RFP therapy: AST values decreased from >250 to <100
IU/l; ALT values decreased from >150 to ∼50 IU/l by 150 mg/day
administration of RFP. This patient received treatment of UDCA
before and during RFP treatment, but the results showed that UDCA
alone was ineffective in lowering ALT and AST values.
Patient 6 (Fig. 1F)
High levels of ALT (150–200 IU/l) and AST (>150
IU/l), which were often observed before RFP administration, were
not observed throughout ∼76 months of RFP therapy, and most values
remained <100 IU/l. This patient received treatment of UDCA
before and during the RFP treatment. The treatment of UDCA alone
tended to lower ALT and AST values, while the effects were not as
consistent as those obtained with combined UDCA and RFP
therapy.
These results indicated that low-dose (150 mg/day)
and long-term oral administration of RFP markedly decreased ALT and
AST values. All patients received oral administration of UDCA
before and throughout REP therapy, but UDCA alone was ineffective
in lowering the values of ALT and AST of most patients (Fig. 1B–F). By contrast, the results of
patients 1 and 2 revealed that administration of RFP alone lowered
the values of ALT and AST. All these results together indicated
that RFP therapy improved liver function of the HCV-related
cirrhosis patients with a high-risk of presenting with HCC. The
decrease in ALT and AST values was not considered to be due to a
decrease in hepatic enzymes associated with hepatic cirrhosis, as
no marked decrease in platelets was observed during RFP therapy
(data not shown). The stabilization of platelet values during
long-term RFP administration supports the idea that progression of
hepatic cirrhosis is prevented by RFP therapy.
Effect of RFP studied in the
experimental animal hepatocyte-disorder models
We studied the effect of oral administration of RFP
in animal models of acute hepatic disorder. A mouse
hepatocyte-disorder model induced by ConA and a rat
hepatocyte-disorder model induced by D-Gal were used in the
following studies.
Effect of RFP on Con A-induced hepatic
disorder in mice
Biochemical study
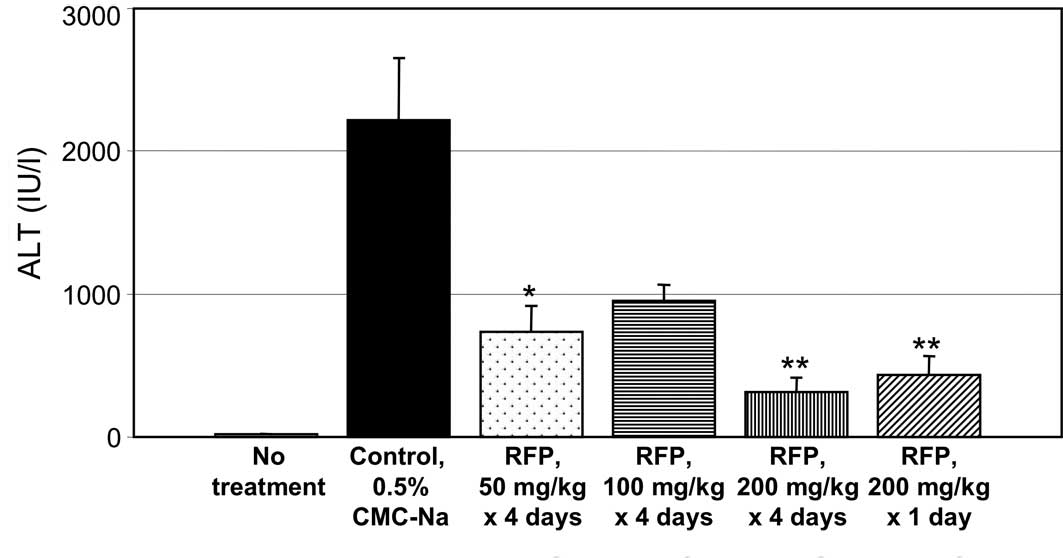
Fig. 2 shows the
effects of RFP on the plasma levels of biochemical markers of liver
function in ConA-induced hepatic disorder mice. The mouse hepatic
disorder is induced by ConA that activates T cells and therefore
has a common background with human hepatitis C, as both disorders
are induced by immune reactions (4). Pre-treatment with oral RFP at 50
mg/mouse x 4 days and 100 mg/mouse x 4 days prevented the increase
in the plasma levels of ALT (Fig.
2A), AST (B) and LDH (C) in the mouse model of ConA-induced
experimental liver disorder. RFP at 200 mg/kg x 4 days or single
administration of 200 mg/kg x 1 day also prevented ConA-induced
increase in serum ALT, AST and LDH levels.
Histopathological study
Nest coagulation necrosis of mouse hepatocytes was
induced by ConA treatment. The effect of RFP to protect this
hepatocyte disorder was evaluated by histopathological studies. The
results were: i) ConA alone resulted in 5 severe cases and 3
moderate cases; ii) ConA and then oral administrations of RFP at 50
mg/mouse x 4 days RFP resulted in 1 severe case, 6 moderate and 1
weak case; iii) ConA and then RFP at 100 mg/mouse x 4 days resulted
in 2 moderate cases, 4 weak, 1 slight and 1 case without nest
coagulation; iv) ConA and then RFP at 200 mg/mouse x 4 days
resulted in 6 weak cases and 2 slight cases; v) ConA and then RFP
at 200 mg/mouse x 1 day resulted in 2 moderate cases, 2 weak, 2
slight and 2 cases without nest coagulation.
Statistical analysis using the Steel test of nest
coagulation necrosis showed a significant difference (p<0.01)
between the group treated with ConA alone and the groups treated
with ConA together with RFP at 100 mg/mouse x 4 days, 200 mg/mouse
x 4 days and 200 mg/mouse x 1 day. These histopathological results
indicate that RFP markedly improves hepatic disorder induced by
ConA in mice.
Effect of RFP on D-Gal-induced hepatic
disorder in rats
Biochemical study
Fig. 3A–C shows the
effects of RFP on liver function in D-Gal-induced hepatic disorder
model rats. The plasma levels of ALT, AST and LDH were measured to
monitor the liver-protective effect of oral RFP. D-gal induces
various cytokines, including tumor necrosis factor responsible for
hepatic injury (5).
Administrations of RFP at 50 mg/rat x 4 days and 100 mg/rat x 4
days prevented an increase in the plasma levels of ALT (Fig. 3A), AST (B) and LDH (C) in
D-Gal-induced experimental hepatic disorder in rats. RFP
pre-treatment with 200 mg/kg x 4 days or a single pre-treatment
with 200 mg/kg strongly prevented an increase in serum ALT, AST and
LDH levels in D-Gal-induced experimental hepatic disorder in
rats.
Histopathological study
D-Gal-treated rats had hepatocytes that showed pale
staining of cytoplasm and nuclei. The rats also had hepatocytes
that showed diffuse necrosis and inflammatory intralobular cellular
infiltration. Administration of RFP resulted in the following
therapeutic effects: i) the group of rats treated with D-Gal and
then with RFP at 200 mg/kg x 4 day showed significantly (p<0.05)
reduced pale staining of cytoplasm and nuclei of hepatocytes, and
inflammatory intralobular cellular infiltration, compared to the
group treated with D-Gal alone; ii) the group of rats treated with
D-Gal and then with RFP of 200 mg/kg x 1 day showed markedly
(p<0.01) reduced pale staining of cytoplasm and nuclei of
hepatocytes, inflammatory intralobular cellular infiltration and
significantly reduced (p<0.05) diffuse necrosis of hepatocytes
compared with the group treated with D-Gal alone.
These results indicate that oral administration of
RFP protects rat liver from D-Gal-induced hepatic injury.
Anti-oxidant activity of RFP
To understand the chemical nature of RFP that may
explain the mechanism behind several therapeutic effects of RFP
observed in clinical studies with liver cirrhosis patients and in
experiments with murine models of acute hepatic injury, we measured
the anti-oxidant activity of RFP and compared the values to those
of several compounds that have anti-oxidative properties. The
anti-oxidant activities of RFP, vitamins E and C and silibinin as
expressed by the specific activity of mol/mol trolox (a
water-soluble vitamin E derivative) were 0.95±0.04, 1.11±0.10,
1.11±0.04 and 0.33±0.08 (n=3), respectively (Table I). These results indicated that RFP
has anti-oxidant activity comparable to vitamins C and E, and is
approximately three times stronger than the activity of silibinin,
which was found to exhibit anti-inflammatory and anti-fibrogenic
effects on human hepatic stellate cells (6).
 | Table I.Anti-oxidant activity of
rifampicin. |
Table I.
Anti-oxidant activity of
rifampicin.
| Specific activity
(mol/mol)a |
|---|
| Rifampicin | 0.95±0.04 |
| Vitamin E | 1.11±0.10 |
| Vitamin C | 1.11±0.14 |
| Silibinin | 0.33±0.08 |
Discussion
This study showed that low-dose and long-term oral
administration of RFP markedly improved plasma hepatitis markers in
6 HCV-related liver cirrhosis patients with a high risk of
presenting with HCC. Also, RFP markedly improved acute hepatocyte
disorder in mouse and rat models induced by ConA and D-Gal,
respectively. Huang et al (7) reported that RFP cures liver injury in
mice caused by carbon tetrachloride. These results suggest that RFP
has an anti-inflammatory effect on hepatocyte disorder by
preventing the release of hepatic enzymes, including ALT and
AST.
RFP has a naphthohydroquinone chromophore spanned by
an aliphatic chain and is implicated to have anti-oxidant and
free-radical scavenging activities (8). This study showed that RFP indeed has
a strong anti-oxidant activity, comparable to the activities of
vitamins C and E, which is approximately three times stronger than
silibinin (also known as sylimarin or silybin), an
anti-inflammatory agent for human hepatic stellate cells (6).
Peroxynitrite, formed by the reaction of superoxide
and nitric oxide, is an important tissue-damaging species generated
at sites of inflammation. Whiteman and Halliwell (9) examined in vitro the ability of
RFP to protect against peroxynitrite-dependent inactivation of
α1-antiproteinase and to inhibit tyrosine nitration by
peroxynitrite. They showed that RFP was highly protective in both
assay systems, suggesting that RFP prevents tissue injury at sites
of inflammation by scavenging peroxynitrite. Kalpana et al
(10) showed that ascorbic acid
(vitamin C) and RFP have free-radical scavenging activity by using
DPPH in vitro and a deproteinated blood method. Data have
accumulated that implicate the participation of oxidants and free
radicals in the pathogenesis of hepatitis (11). Morisco et al (12) studied the effect of interferon-α
and ribavirin treatment on the oxidative state in 52 chronic
hepatitis C patients who received a combination of the two drugs
for 6 or 12 months. The results revealed that patients with a
successive long-term response had a significantly lower basal serum
hydroperoxide concentration than non-responders, and that the mean
hydroperoxide concentration decreased significantly during the
treatment. Berkson (13) reported
a conservative triple anti-oxidant approach to the treatment of
hepatitis C; 3 patients who received this therapy recovered quickly
and their laboratory values improved markedly. RFP has the
excellent property of accumulating in the liver (14), which supports the notion that the
effect of RFP is markedly increased and thus is preferentially
efficient in the liver.
These results collectively support strongly the idea
that hepatocyte-protective effects of RFP on human chronic
hepatitis C and on acute hepatocyte disorder in the murine
experimental models of this study are due to its anti-oxidant and
free-radical scavenging activities. Although the pathological
reasons behind these human and animal hepatic disorders differ in
many details, the observed hepatocyte-protective effect of RFP
seems to derive from the fundamental reaction acting on a common
and basic cellular event. Destruction of cells occurs in various
inflammatory events caused by free radicals produced endogenously
by lymphokines and cytokines. This is exemplified by the
destruction of cells in inflammation, which includes attack on
cells by other exogenous chemical substances, such as carbon
tetrachloride.
RFP protects against acute liver injury induced in
mice by carbon tetrachloride (7,15).
In the models of this study, RFP reduced plasma levels of ALT and
AST, and hepatocyte necrosis. Takeda et al (15) speculated that RFP suppresses the
expression of cytochrome P2E1 and protects CCl4-mediated
DNA damage in hepatocytes by inhibiting cytochrome P2E1-mediated
formation of free radicals from CCl4.
Cytokines resulting from ConA or D-Gal treatment
participate in ConA-induced hepatocyte disorder of mice and
D-Gal-induced hepatocyte disorder of rats (4,5).
Activated T lymphocytes appear to be responsible for liver damage
in chronic hepatitis, and the mouse and rat systems are sometimes
considered to be hepatitis models in which activated T lymphocytes
participate (16). For instance,
ConA is a T-cell mitogen and induces release of systemic tumor
necrosis factor, interferon-γ, interleukin 12 and various other
cytokines. Passive immunization against interleukin 12 (17) or pre-treatment with
immunosuppressive drugs to suppress tumor necrosis factor protects
mice from liver injury (18),
supporting the idea that cytokines participate in liver injury of
mice induced by ConA. Suppressive effects of RFP on the immune
system have been reported. For instance, secretion of tumor
necrosis factor-α is suppressed markedly by RFP in
lipopolysaccharide-stimulated monocytes (19,20).
These results suggest that RFP indirectly suppresses liver injury
by inhibiting secretion of cytokines. Therefore, in the present
study RFP possibly suppressed hepatocyte disorder in the murine
models also by inhibiting cytokine release, in addition to its
anti-inflammatory effects by its anti-oxidant and free-radical
scavenging activities.
In our previous clinical study (1), low-dose and long-term oral
administration of RFP to 6 HCC high-risk patients with HCV-related
liver cirrhosis, who were also the targets of this study, resulted
in reduced occurrence of HCC, in which the angiogenesis-inhibitory
effect of RFP was implicated (1,21).
Since angiogenesis is well known to be stimulated in inflammatory
lesions, the angiogenesis-inhibitory effect of RFP is considered to
be partly due to its anti-inflammatory effect. Conversely,
long-term suppression of inflammation in chronic hepatitis patients
might also have contributed to the suppression of HCC in these
patients. Recently, Pal et al (2) analyzed the evidence of oxidative
stress in association with HCV-induced chronic inflammation. They
reported that human hepatoma cells infected with HCV showed 30- to
60-fold increases in the level of reactive oxygen species (ROS) and
6-fold increases in oxidatively modified guanosine levels compared
to uninfected cells. RFP is apparently multifunctional, but the
mechanisms of RFP to cure chronic hepatitis C and to prevent HCC
generation may be partly related to RFP scavenging ROS in
HCV-infected hepatic cells.
Acknowledgements
We thank Dr Tsuyoshi Hara (previously
of the staff of GeneCare Research Institute Co., Ltd.) for his
contribution to the assay of anti-oxidant activity. This study was
partially supported by a Fund from the Japan Science and Technology
Agency (JST).
References
|
1.
|
Shichiri M, Fukai N, Kono Y and Tanaka Y:
Rifampicin as an oral angiogenesis inhibitor targeting hepatic
cancers. Cancer Res. 69:4760–4768. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pal S, Polyak SJ, Bano N, et al: Hepatitis
C virus induces oxidative stress, DNA damage and modulates the DNA
repair enzyme NEIL1. J Gastroenterol Hepatol. 25:627–634. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Nomura T, Kikuchi M, Kubodera A and
Kawakami Y: Proton-donative antioxidant activity of fucoxanthin
with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem Mol Biol Int.
42:361–370. 1997.PubMed/NCBI
|
|
4.
|
Tiegs G and Gantner F: Immunotoxicology of
T cell-dependent experimental liver injury. Exp Toxicol Pathol.
48:471–476. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Czaja MJ, Flanders KC, Biempica L, Klein
C, Zern MA and Weiner FR: Expression of tumor necrosis factor-alpha
and transforming growth factor-beta 1 in acute liver injury. Growth
Factors. 1:219–226. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Trappoliere M, Caligiuri A, Schmid M, et
al: Silybin, a component of sylimarin, exerts anti-inflammatory and
anti-fibrogenic effects on human hepatic stellate cells. J Hepatol.
50:1102–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Huang R, Okuno H, Takasu M, Shiozaki Y and
Inoue K: Protective effect of rifampicin against acute liver injury
induced by carbon tetrachloride in mice. Jpn J Pharmacol.
69:325–334. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Acocella G: Clinical pharmacokinetics of
rifampicin. Clin Pharmacokinet. 3:108–127. 1978. View Article : Google Scholar
|
|
9.
|
Whiteman M and Halliwell B: Prevention of
peroxynitrite-dependent tyrosine nitration and inactivation of
alpha1-antiproteinase by antibiotics. Free Radic Res. 26:49–56.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Kalpana T, Karunakar N, Reddy MS,
Prabhakar MC and Krishna DR: Assessment of antioxidant activity of
some antileprotic drugs. Arzneimittelforschung. 51:633–637.
2001.PubMed/NCBI
|
|
11.
|
Loguercio C and Federico A: Oxidative
stress in viral and alcoholic hepatitis. Free Radic Biol Med.
34:1–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Morisco F, Verde V, Fogliano V, et al:
Oxidative status in chronic hepatitis C: the influence of antiviral
therapy and prognostic value of serum hydroperoxide assay. Free
Radic Res. 38:573–580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Berkson BM: A conservative triple
antioxidant approach to the treatment of hepatitis C. Combination
of alpha lipoic acid (thioctic acid), silymarin, and selenium:
three case histories. Med Klin. 94(Suppl 3): 84–89. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Iseri E, Ercan MT, Kas HS and Hincal AA:
The in vivo distribution of the 99mTc-labelled albumin and gelatin
microspheres of a tuberculostatic agent, rifampicin. Boll Chim
Farm. 130:66–70. 1991.PubMed/NCBI
|
|
15.
|
Takeda K, Watanabe J, Inoue K and Kanamura
S: Rifampicin suppresses hepatic CYP2E1 expression and minimizes
DNA injury caused by carbon tetrachloride in perivenular
hepatocytes of mice. Alcohol Clin Exp Res. 24(Suppl 4): 87–92.
2000.PubMed/NCBI
|
|
16.
|
Tiegs G: Experimental hepatitis and role
of cytokines. Acta Gastroenterol Belg. 60:176–179. 1997.PubMed/NCBI
|
|
17.
|
Nicoletti F, Di Marco R, Zaccone P, et al:
Murine concanavalin A-induced hepatitis is prevented by interleukin
12 (IL-12) antibody and exacerbated by exogenous IL-12 through an
interferon-gamma-dependent mechanism. Hepatology. 32:728–733. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ishiwata Y, Yokochi S, Hashimoto H,
Ninomiya F and Suzuki T: Protection against concanavalin A-induced
murine liver injury by the organic germanium compound,
propagermanium. Scand J Immunol. 48:605–614. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Doria G and Agarossi G: Inhibition of the
immune response in vitro by rifampicin and derivatives. Scand J
Respir Dis. (Suppl 84): 23–26. 1973.PubMed/NCBI
|
|
20.
|
Ziglam HM, Daniels I and Finch RG:
Immunomodulating activity of rifampicin. J Chemother. 16:357–361.
2004. View Article : Google Scholar
|
|
21.
|
Shichiri M and Tanaka Y: Inhibition of
cancer progression by rifampicin: involvement of antiangiogenic and
anti-tumor effects. Cell Cycle. 9:64–68. 2010. View Article : Google Scholar : PubMed/NCBI
|