Introduction
Glial fibrillary acidic protein (GFAP) is expressed
in astrocytes in the central nervous system (CNS) (1) and is involved in many cellular
functioning processes, such as cell structure and movement, cell
communication and the functioning of the blood-brain barrier
(2,3). It was found that the levels of
several proteins expressed in astrocytes are altered in an
Alzheimer's disease model (4,5), and
cleavage of GFAP may contribute to astrocyte injury and damage in
the Alzheimer's disease brain (6–8).
GFAP plays an important role in mitosis, directly or indirectly, by
adjusting the filament network present in the cell. During mitosis,
the amount of phosphorylated GFAP increases and moves to the
cleavage furrow (9). In
vitro, astrocytes treated with antisense RNA against GFAP do
not form a neuron-like phenotype (10). There are multiple disorders
associated with inactive GFAP regulation, and mutations in the
coding region of the GFAP gene are associated with Alzheimer's
disease (11). Therefore, GFAP is
thought to play crucial roles in the development and
differentiation of astrocytes and is believed to be involved in the
long-term maintainance of normal CNS myelination (12). However, the mechanisms by which
GFAP is involved in the development and differentiation of
astrocytes are not yet completely understood. To better understand
the effect of GFAP expression on neurons, we generated stable PC12
cell lines overexpressing this gene.
The PC12 cell line, which was derived from a
pheochromocytoma of the rat adrenal medulla (13), is the most common cell line used as
neuron-like cells with neuron-like elongation abilities.
Maintenance of the neuronal phenotype and survival of
differentiated PC12 cells under serum-free conditions require
constant nerve growth factor (NGF) exposure. PC12 cells stop
dividing and terminally differentiate when treated with NGF. In the
present study, PC12 cells with neuron-like elongation underwent
apoptosis within 70–80 h. However, the PC12 cells overexpressing
pGFAP from the plasmid underwent cell death 120 h after NGF
exposure. The overexpression of GFAP delayed cell death in the PC12
cells.
Materials and methods
Cell culture
The PC12 cell line was purchased from the Japan
Health Science Foundation (HSRRB, Osaka, Japan) and maintained in
DMEM supplemented with heat-inactivated fetal bovine serum and
horse serum. After culture for 48 h, the cells were treated with
1.0 μM NGF (Kyowa, Tokyo, Japan) for 0, 24, 48, 72, 96 or 120 h.
All cells were grown in 5% CO2 at 37°C. The temperature
was adjusted to 42°C, and cells were observed. Apoptosis was
detected by Annexin V and propidium iodide (PI) staining using flow
cytometry (Quanta SC, Beckman Coulter, Fullerton, CA, USA).
RT real-time PCR analysis
Total RNA was extracted from the PC12 cells using
the QuickGene RNA Cultured Cell HC Kit S (Fujifilm, Kanagawa,
Japan) following the manufacturer's instructions. The final RNA
preparations were resuspended in diethylpyrocarbonate-treated water
and quantified by absorbance analysis at 260 nm. cDNA was prepared
by incubating DNase-treated total RNA (0.1 μg) with M-MLV reverse
transcriptase (Invitrogen, Carlsbad, CA, USA) in the presence of
random primers (Invitrogen). The real-time PCR reaction mixture was
prepared using a FastStart SYBR Green Master (Roche Diagnostics,
Mannheim, Germany). The primer set for amplification of a
GFAP mRNA was designed according to GenBank NM_017009, using
a forward primer at the exon 2 region, 5′-CAA GAT GAA ACC AAC CTG
AGG CT-3′, and a reverse primer at the exon 4 region, 5′-GGC TTG
GCC ACA TCC ATC T-3′ (product length 221 bp). The primer set for
amplification of a myelin basic protein (MBP) mRNA
was designed according to GenBank M25889, using a forward primer at
the exon 4–6 region, 5′-GGC AAG GAC TCA CAC ACR AGA ACT-3′, and a
reverse primer at the exon 7–8 region, 5′-GGG ACA GGC CTC TCC CCT
T-3′ (product length 154 bp). The primer set for amplification of a
class III β-tubulin mRNA was designed according to GenBank
AF459021, using a forward primer at the exon 2 region, 5′-ATA GAC
CCC AGC GGC AAC TAT GTG-3′, and a reverse primer at the exon 3
region, 5′-AGG CCT GAA TAG GTG TCC AAA GGC-3′ (product length 174
bp). The real-time PCR reaction was carried out for 45 cycles (95°C
for 20 sec, 60°C for 30 sec and 72°C for 20 sec) using an iCycler
iQ™ Real-Time Detection System (Bio-Rad, Hercules, CA, USA).
GAPDH cDNA was amplified for normalization (Applied
Biosystems, Foster City, CA, USA); mRNA levels are presented as the
mRNA copy number per μg total RNA.
Overexpression of pGFAP
The gene encoding GFAP was subcloned into the
mammalian expression vector pTriEX™-3 (Merck, San Diego, CA, USA),
and the entire sequence was verified by DNA sequencing. For
overexpression in PC12 cells, GFAP was subcloned from
pTriEX™-3 vectors into NovaBlue Singles™ competent cells (Merck).
The resultant plasmids were designated as pGFAP. Cells were seeded
at a density of 106 cells per 10-cm dish and transfected
with 8 μg of plasmid DNA using 120 μl of GeneJuice®
Transfection reagent (Merck) for 2 h at 37°C, after which time the
DMEM medium plus 10% serum was changed. After 24 h, cells were
cultured under serum-free conditions and exposed to NGF
stimulation. PC12 cells that were stably transfected with cDNAs
encoding GFAP or the empty vector (mock) were grown to confluence
in DMEM.
Results
RT real-time PCR
The levels of GFAP mRNA in the culture at
37°C ranged from 104.6 to 105.5 copies/μg
total RNA and were evaluated as the mean ± SE
(105.1±100.4) copies/μg total RNA. The levels
of GFAP mRNA in the culture at 42°C ranged from 0.0 to
102.1 copies/μg total RNA and were evaluated as the mean
± SE (101.2±100.9) copies/μg total RNA; this
difference was significant (p<0.01; Student's t-test). The
levels of MBP mRNA in the culture at 37 and 42°C were
103.9–104.6 and
103.8–104.6 copies/μg total RNA,
respectively. The levels of class III β-tubulin mRNA in the
culture at 37 and 42°C were 104.7–105.6 and
104.9–105.3 copies/μg total RNA, respectively
(Fig. 1).
Cell culture and overexpression of
pGFAP
In the mock-transfected PC12 cells exposed to NGF,
no apoptosis was induced at 0, 24 or 48 h at 37°C. After 72 h,
apoptosis was induced when the cell that expands the neurite was
cultured at 37°C (data not shown). Exposure of PC12 cells to a
temperature of 42°C for 24 h significantly decreased NGF-induced
neuron-like elongation compared to cells cultured at 37°C, while it
was possible to maintain the neuron-like elongation at 42°C in the
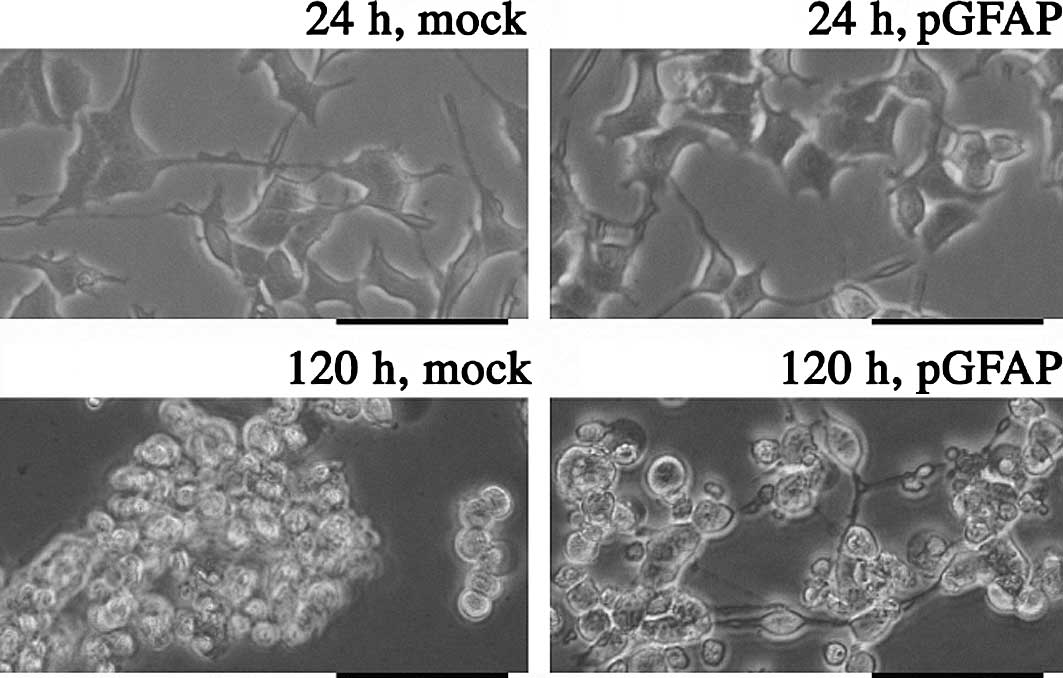
cells with pGFAP overexpression (Fig.
2). Mock-transfected PC12 cells showed similar features to
control PC12 cells when incubated at 42°C. In cells overexpressing
pGFAP, neuron-like elongation was maintained in the culture for 120
h (Fig. 3).
Discussion
In the present study, GFAP was found to play an
important role, directly or indirectly, in the protection of in
vitro PC12 cultured cells from damage. Unfortunately, apoptosis
progresses rapidly and the relation between the level of GFAP and
the degree of inhibition of apoptosis is not yet understood.
However, it appears that the existence of GFAP inhibited apoptosis
in the PC12 cells with neuron-like elongation. Heat stress at a
temperature of 42°C caused the PC12 cells to die. Neuron-like
elongation was lost in the cultures at 42°C, and these cells
underwent apoptosis. Severe stress was found to play a role in the
pathogenesis of neurodegenerative diseases, such as Alzheimer's
disease. If neuronal cells can be made sufficiently resistant to
stress, then the pathogenesis of neuro-degenerative diseases should
be slowed or decreased. Our results corroborate those of other
researchers who have shown that GFAP is necessary for the long-term
maintenance of the normal CNS (14–16).
The mechanism by which GFAP overexpression inhibits
the apoptosis of PC12 cells is uncertain. However, we propose that
this novel function of GFAP plays an important role in the ability
of astrocytes to protect neuronal cells against apoptosis. These
data support the concept that GFAP is responsible for many of the
progressive astroglial changes that appear after CNS injury and
disease. Further evaluation of this and other possible roles of
GFAP, as well as the regulation of GFAP expression, are crucially
important for the development of new strategies for maintaining
homeostasis in the nervous system.
References
|
1.
|
Rodríguez JJ, Olabarria M, Chvatal A and
Verkhratsky A: Astroglia in dementia and Alzheimer's disease. Cell
Death Differ. 16:378–385. 2009.
|
|
2.
|
Bignami A, Eng LF, Dahl D and Uyeda CT:
Localization of the glial fibrillary acidic protein in astrocytes
by immunofluorescence. Brain Res. 43:429–435. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Antanitus DS, Choi BH and Lapham LW:
Immunofluorescence staining of astrocytes in vitro using antiserum
to glial fibrillary acidic protein. Brain Res. 89:363–367. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Counts SE and Mufson EJ: The role of nerve
growth factor receptors in cholinergic basal forebrain degeneration
in prodromal Alzheimer disease. J Neuropathol Exp Neurol.
64:263–272. 2005.PubMed/NCBI
|
|
5.
|
Angelopoulos P, Agouridaki H, Vaiopoulos
H, et al: Cytokines in Alzheimer's disease and vascular dementia.
Int J Neurosci. 118:1659–1672. 2008.
|
|
6.
|
Eng LF and Ghirnikar RS: GFAP and
astrogliosis. Brain Pathol. 4:229–237. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Mouser PE, Head E, Ha KH and Rohn TT:
Caspase-mediated cleavage of glial fibrillary acidic protein within
degenerating astrocytes of the Alzheimer's disease brain. Am J
Pathol. 168:936–946. 2006.PubMed/NCBI
|
|
8.
|
Quinlan RA, Brenner M, Goldman JE and
Messing A: GFAP and its role in Alexander disease. Exp Cell Res.
313:2077–2087. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Tardy M, Fages C, Le Prince G, Rolland B
and Nunez J: Regulation of the glial fibrillary acidic protein
(GFAP) and of its encoding mRNA in the developing brain and in
cultured astrocytes. Adv Exp Med Biol. 265:41–52. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Lefrançois T, Fages C, Peschanski M and
Tardy M: Neuritic outgrowth associated with astroglial phenotypic
changes induced by antisense glial fibrillary acidic protein (GFAP)
mRNA in injured neuron-astrocyte cocultures. J Neurosci.
17:4121–4128. 1997.
|
|
11.
|
Brenner M, Johnson AB, Boespflug-Tanguy O,
et al: Mutations in GFAP encoding glial fibrillary acidic protein
are associated with Alexander disease. Nat Genet. 27:117–120. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Goss JR, Finch CE and Morgan DG:
Age-related changes in glial fibrillary acidic protein mRNA in the
mouse brain. Neurobiol Aging. 12:165–170. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar
|
|
14.
|
Pekny M, Levéen P, Pekna M, et al: Mice
lacking glial fibrillary acidic protein display astrocytes devoid
of intermediate filaments but develop and reproduce normally. EMBO
J. 14:1590–1598. 1995.PubMed/NCBI
|
|
15.
|
Shibuki K, Gomi H, Chen L, et al:
Deficient cerebellar long-term depression impaired eyeblink
conditioning and normal motor coordination in GFAP mutant mice.
Neuron. 16:587–599. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Liedtke W, Edelmann W, Bieri PL, et al:
GFAP is necessary for the integrity of CNS white matter
architecture and long-term maintenance of myelination. Neuron.
17:607–615. 1996. View Article : Google Scholar : PubMed/NCBI
|