Introduction
Oxidation is an essential biological process for
energy production in numerous living organisms. However, excessive
reactive oxygen species (ROS) produced in vivo during
certain oxidative reactions are strongly associated with the
etiology and/or progression of a number of diseases, such as
atherosclerosis, cancer and other degenerative diseases, and in
aging (1–4). Recently, an increasing number of
studies confirm that numerous fruits and vegetables may afford
protection against certain chronic diseases caused by oxidative
stress since they contain a wide variety of free radical scavenging
molecules, such as polyphenols and flavonoids, Vitamin C,
carotenoids and tocopherols (5,6),
which scavenge radicals by inhibiting initiation and breaking the
chain propagation or by suppressing the formation of free radicals
by binding to metal ions, reducing hydrogen peroxide and quenching
superoxide and singlet oxygen. Thus, these phytochemicals play an
important role in the prevention of these diseases (7).
The genus Actinidia consists of over
fifty-eight species widely distributed throughout the Asian
continent. Specific Actinidia species, such as A.
arguta and A. chinensis Planch, are used as health foods
and medical agents for cancer treatment (8). It has also been reported that the
root of A. eriantha possesses antitumor and immunomodulatory
activity (9,10).
Actinidia kolomikta, which grows in the wild
throughout the northern part of Indochina, is a locally famous
traditional medicine for diabetes. However, there are few studies
concerning its bioactivity. The purpose of this study was to
investigate the antioxidant and antitumor activity of the vine root
extracts processed at high and low water extraction
temperatures.
Materials and methods
Chemicals and reagents
2,2-Diphenyl-1-picryl-hydrazyl (DPPH),
Folin-Ciocalteu reagent,
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox),
propidium iodide (PI), RPMI-1640 medium, fetal bovine serum (FBS)
and penicillin-streptomycin solution were purchased from
Sigma-Aldrich, Inc. (St. Louis, MO, USA). The SOD assay kit-WST,
Cell counting kit-8 and Hoechst 33258 solution were purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Gallic acid
was purchased from Nacalai Tesque, Inc. (Kyoto, Japan).
Cell line and culture
The DLD-1 human colon cancer cell line was obtained
from the Cell Resource Center for Biomedical Research, Aging and
Cancer, Tohoku University, Japan. It was grown in RPMI-1640 medium
containing 10% FBS and 1% penicillin/streptomycin. The culture was
maintained at 37°C in a humidified 5% CO2 atmosphere
(ESPEC CO2 Incubator). The cells were cultured for 2–3
days to reach the logarithmic phase and were then used for the
experiment.
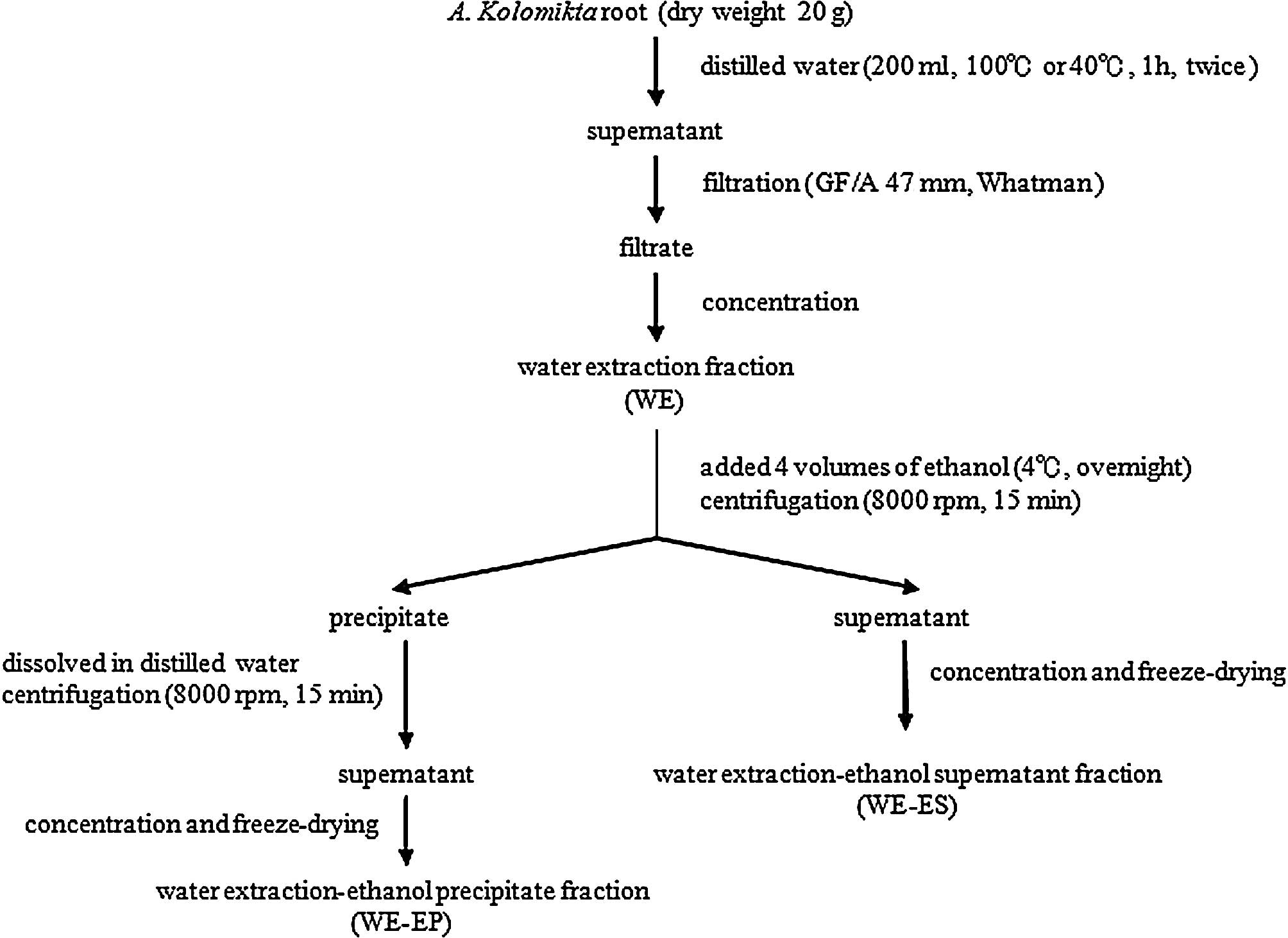
Plant material and preparation
The roots of Actinidia kolomikta were
collected from the northern part of Indochina. The scheme of the
extraction procedure is presented in Fig. 1. For the high temperature
extraction, the roots were extracted with distilled water at 100°C
for 1 h at a ratio of 1:10 (w/v). The operation was repeated twice.
The aqueous extract was centrifuged at 6,000 rpm for 15 min and
filtered through a filter paper (GF/A, 47 mm; Whatman, UK). The
filtrate was concentrated and lyophilized to obtain the water
extraction (WE) fraction. The WE fraction was redissolved in
distilled water and was added to four volumes of 99.5% ethanol and
then stored at 4°C overnight for precipitation. The precipitate and
supernatant were separated and collected by centrifugation at 8,000
rpm for 15 min. The supernatant was concentrated and lyophilized to
obtain the water extraction-ethanol supernatant (WE-ES) fraction.
The precipitate was washed with 80% ethanol and dissolved in
distilled water. After the centrifugation, the supernatant was
concentrated and lyophilized to obtain the water extraction-ethanol
precipitate (WE-EP) fraction.
The low temperature extraction operation was the
same as the high temperature treatment, but distilled water at 40°C
was used.
Determination of total carbohydrates and
polyphenols
The content of total carbohydrates in the extracts
was determined by the phenol-sulfuric acid method (11,12),
with glucose as a standard. First, 1 ml of the extraction fraction
was collected in glass tubes and 1 ml of 5% phenol solution and 5
ml of concentrated sulphuric acid were added. After mixing for 2
min at room temperature, the tubes were boiled at 100°C for 15 min
in a hot water bath. Subsequently, the absorbance was read at 486
nm with a spectrophotometer (HACH, DR/4000 U).
The total polyphenol content was determined using
the Folin-Ciocalteu method (3). A
volume of 7.9 ml distilled water, 0.1 ml extract fraction and 0.5
ml Folin-Ciocalteu reagent (1:1 with distilled water) were added
and mixed in a tube for 1 min. Then, 1.5 ml of sodium carbonate (20
g/100 ml) was added, mixed and allowed to stand for 2 h at room
temperature in the dark. The absorbance was read at 765 nm. The
total polyphenol content was determined using gallic acid as a
standard.
SOD-like activity assay
The levels of SOD-like activity in the extracts were
measured using the SOD assay kit-WST according to the technical
manual provided by Dojindo Molecular Technologies. Briefly, in a
96-well plate, sample solution (20 μl) was added to each
sample and blank 2-well, and 20 μl double-distilled water
was added to each blank 1-and blank 3-well. Then, 200 μl of
WST working solution was added to each well. After mixing, 20
μl of dilution buffer was added to each blank 2- and blank
3-well, and 20 μl of enzyme working solution was added to
each sample- and blank 1-well. The plate was incubated at 37°C for
20 min, and the optical density (OD) was determined at 450 nm,
using a microplate reader (Model 550; BioRad, USA). The SOD-like
activity was calculated using the following equation: SOD activity
(inhibition rate %) =
{[(Ablank1-Ablank3)-(Asample-Ablank2)]/(Ablank1-Ablank3)}
× 100.
Ablank1, Ablank2,
Ablank3 and Asample are the absorbances of
blank 1-, blank 2-, blank 3- and sample-wells. The SOD activity (1
unit) was defined as the amount of enzyme having a 50% inhibitory
effect on WST-1.
Measurement of the DPPH scavenging
activity
The DPPH scavenging activity was measured according
to Nakajima et al (13) and
Yang et al (14). Volumes
of 80 μl of 0.5 mM DPPH in MeOH solutions and 80 μl
of 0.1 M MES buffer in 50% MeOH (pH 6.0) were added to a 96-well
plate, and an aliquot of sample solution was added for a total
volume of 200 μl. After 10 min of reaction, the OD was
measured at 570 nm with a microplate reader. The DPPH
radical-scavenging activity was calculated using the following
equation: DPPH-scavenging activity (%) =
(1-Asample/Acontrol) × 100.
The scavenging activity of the sample is expressed
as a 50% effective concentration (EC50), which
represents the sample concentration (μg/ml) inhibiting 50%
of the DPPH radical activity.
Cell proliferation assay
The DLD-1 cells were grown in RPMI-1640 medium at
37°C in a 5% CO2 atmosphere to logarithmic phase. The
cells were harvested, and an aliquot (100 μl) of the DLD-1
cell suspension (5×104 cells/ml) was dispensed into a
96-well plate and pre-incubated at 37°C in a 5% CO2
atmosphere for 24 h. The cells were then exposed to various
concentrations (25, 50, 100, 200 and 400 μg/ml) of the
extracts for 12, 24 and 48 h. After drug exposure, 10 μl of
Cell Counting reagent solution was added, and incubation was
carried out at 37°C for 4 h. The cell numbers were quantitated by
reading the OD at 450 nm.
Flow cytometric assay
The flow cytometric assay was performed according to
Zhang et al (15) with some
modifications. DLD-1 cells (1×105 cells/ml) were
incubated in a 6-well plate with the extracts at concentrations of
25 and 100 μg/ ml for 48 h. The cells were harvested and
washed with cold PBS (-) and then fixed in 70% ethanol at 4°C for 4
h. Cells were strained with PI solution (20 μg/ml) at 4°C
for 30 min. DNA histograms were generated by flow cytometry (BD
LSR; BD Biosciences). Data from 10,000 cells per sample were
collected, and the percentage of apoptotic cells was obtained with
the CellQuest software (Becton Dickinson).
Nuclear morphological observation
DLD-1 cells (1×105/ml) were incubated in
6-well plate with the extracts at concentrations of 25 and 100
μg/ml for 48 h. The cells were subsequently harvested and
stained with Hoechst 33258 (10 μg/ml) for 10 min. Nuclear
morphological changes were observed using a Leica fluorescence
microscope.
Statistical analysis
Experiments were conducted in triplicate, and the
results were expressed as the mean ± SD. Statistical significance
was calculated by a two-tailed Student’s t-test (16). p<0.05 was considered
significant.
Results
Extraction yield and total polysaccharide
and polyphenol contents
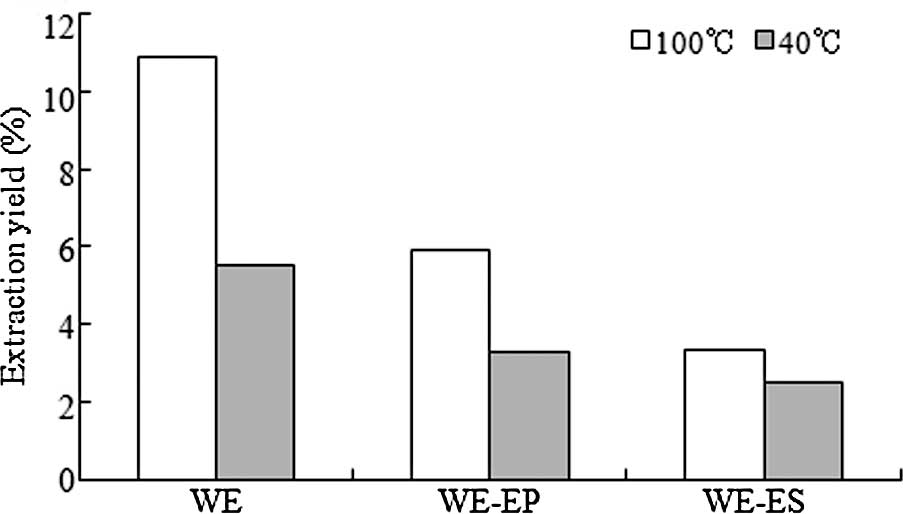
The extraction yields are shown in Fig. 2. Yields of WE, WE-EP and WE-ES
produced using the 100°C procedure were 10.9, 5.89 and 3.35%, while
the yields of the fractions produced using the 40°C procedure
reached only 5.53, 3.28 and 2.5%. Thus, the extraction yields
produced using the 100°C procedure were higher than those produced
using the 40°C extraction procedure.
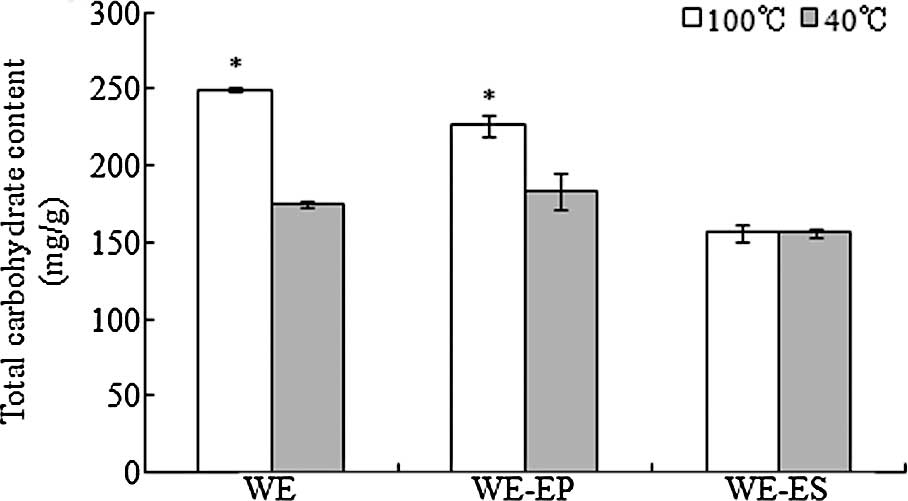
The total carbohydrate contents are shown in
Fig. 3. The total carbohydrate
contents of the WE and WE-EP fractions produced using the 100°C
extraction procedure were 249.6 and 226.6 mg/g, respectively, which
were significantly higher than the total carbohydrate contents of
WE (175.1 mg/g) and WE-EP (183.0 mg/g) produced using the 40°C
extraction procedure. The results also revealed that the total
carbohydrate content in the WE-EP fraction was higher than that in
the WE-ES fraction.
The total polyphenol contents are shown in Fig. 4. The polyphenol contents of the WE,
WE-EP and WE-ES fractions produced using the 100°C extraction
procedure were higher than the polyphenol contents of the fractions
produced using 40°C. Moreover, the polyphenol contents of WE and
WE-ES produced using the 100°C extraction procedure were
significantly higher than those of the extracts produced using the
40°C extraction procedure (p<0.05). The polyphenol content of
the WE-ES fraction was higher than that of WE-EP. Data revealed
that the polyphenol content of WE-ES produced using the 100°C
treatment consisted of 201.9 mg gallic acid/g, which was the
highest content compared to all of the other fractions.
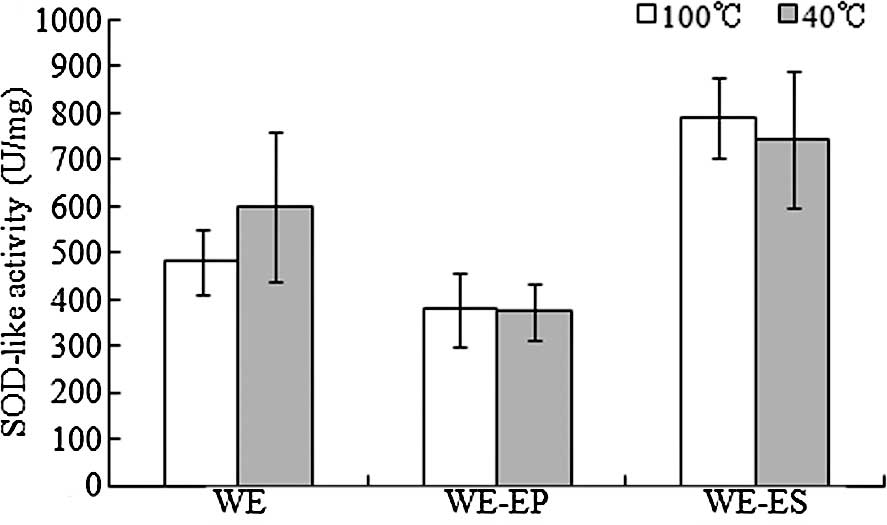
SOD-like activity
As shown in Fig. 5,
compared to the WE and WE-EP fractions, the WE-ES fraction
exhibited a higher antioxidant activity; the SOD-like activities of
the WE-ES fraction produced using the 100°C and 40°C extraction
procedures were 790.3 and 742.6 U/mg, respectively, while the WE-EP
fraction exhibited the lowest SOD-like activities (377.9 and 374.1
U/mg, respectively, for the WE-EP fractions produced using the
100°C and 40°C extraction procedures).
DPPH radical-scavenging activity
The DPPH radical-scavenging activity is shown in
Fig. 6. The positive control
Trolox revealed the strongest DPPH radical-scavenging activity
(EC50, 31.7 μg/ml), and the WE-ES fraction
produced using the 100°C extraction procedure had the second
strongest radical-scavenging activity (EC50, 87.4
μg/ml). Moreover, all of the fractions (WE, WE-EP and WE-ES)
produced using the 100°C extraction procedure revealed significant
higher DPPD radical-scavenging activities compared to the fractions
produced at 40°C (p<0.05).
Anti-proliferative effects on DLD-1
cells
The WE-EP and WE-ES fractions were used for the
experiment of anti-proliferation on the DLD-1 cells (Fig. 7). In this experiment, the extracts
at various concentrations (25, 50, 100, 200 and 400 μg/ml)
were used. The results revealed that, the higher the concentration
of the extract, the lower the cell survival rate. Treatment at the
concentration of 400 μg/ml revealed the strongest inhibitory
effect on the DLD-1 cells. This suggests that the WE-EP and WE-ES
fractions (produced using the 100°C and 40°C extraction procedures)
exhibited anti-proliferative effects on DLD-1 cells in a
dose-dependent manner. Moreover, the WE-ES fraction inhibited DLD-1
cell proliferation in a time-dependent manner, compared to WE-EP,
for which a slight recovery of DLD-1 cell proliferation at 48 h was
noted. When DLD-1 cells were treated with the WE-EP and WE-ES
fractions for 12 h, a decrease in DLD-1 cell proliferation was
noted. However, after the 48-h treatment, the cell survival rates
increased in the DLD-1 cells exposed to WE-EP. For example, when
the DLD-1 cells were treated with the extracts at the concentration
of 400 μg/ml for 24 h, the cell survival rates induced by
WE-EP and WE-ES (produced using the 100°C extraction procedure)
were 30.7 and 27.0%, respectively. At 48 h, the cell survival rates
were 36.5 and 17.35%, respectively. The same trend was observed
when cells were treated with fractions produced using the 40°C
extraction procedure. Based on these results, the
anti-proliferative effect of the WE-ES fraction was stronger than
that of WE-EP.
Apoptosis assay
Results of the apoptosis assay are shown in Fig. 8. When the DLD-1 cells were treated
with the WE-EP fraction (produced using the 100°C extraction
procedure) at concentrations of 25 and 100 μg/ml for 48 h,
the percentage of apoptotic cells decreased from 1.24 to 0.8% while
the percentage increased from 0.94 to 1.72% upon treatment of WE-EP
produced using the 40°C extraction procedure). These data did not
reveal a significant increase. In comparison with the WE-EP
treatment, when DLD-1 cells were exposed to WE-ES (produced using
the 100°C extraction procedure) at concentrations of 25 and 100
μg/ml for 48 h, the percentage of apoptotic cells increased
from 3.26 to 25.68% while the percentage of apoptotic cells
increased from 6.09 to 24.25% when treated with WE-ES produced at
40°C. This suggests that WE-ES induces DLD-1 cell apoptosis in a
dose-dependent manner.
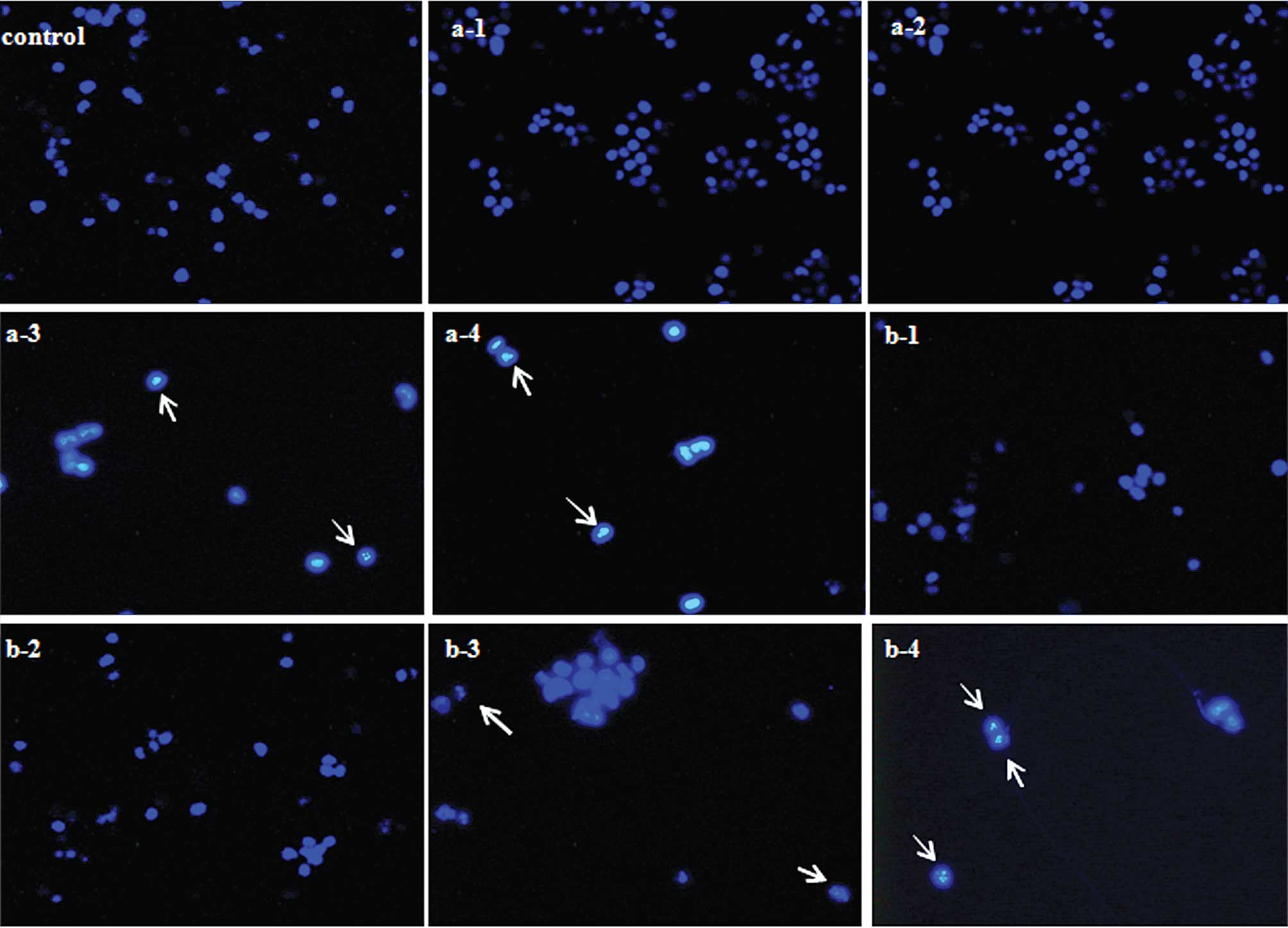
Changes in apoptotic cell morphology
The criteria used to identify pro-apoptosis includes
nuclear shrinkage and chromatin condensation (17). As shown in Fig. 9, nuclear fragmentation was clearly
noted when the DLD-1 cells were exposed to WE-ES (25 and 100
μg/ml) for 48 h, while upon treatment with WE-EP at 25 and
100 μg/ml for 48 h, apoptotic cells were hardly
observed.
Discussion
The extraction yields and the polysaccharide and
polyphenol contents of the extracts produced using the 100°C
procedure were higher than those of the extracts produced using the
40°C treatment. The total carbohydrate content of the WE-EP
fraction was significantly higher than that of the WE-ES fraction.
Since polysaccharides do not dissolve in 80% ethanol, WE-EP can be
considered as a crude polysaccharide fraction. However, based on
the results (Fig. 4), the WE-EP
fraction still contained a certain amount of polyphenols. On the
other hand, the polyphenol content of the WE-ES fraction was higher
than that of WE-EP, but there were also some polysaccharides in the
WE-ES fraction. Thus, crude polysaccharides were mostly contained
in the WE-EP fraction, while in the WE-ES fraction, the polyphenol
content was marked. Plant polysaccharides and polyphenols are
antioxidants (18), and they
scavenge radicals by inhibiting initiation and breaking chain
propagation by suppressing formation of free radicals by binding to
metal ions, reducing hydrogen peroxide and quenching superoxide and
singlet oxygen. In this study, the WE-EP and WE-ES fractions
exhibited SOD-like activity and DPPH radical-scavenging activity,
while the WE-ES fraction revealed higher antioxidant activity than
WE-EP. It has been reported that polyphenols are strong radical
scavengers and metal chelators in model chemical systems (19). Furthermore, it has been reported
that there is a positive significant linear relationship between
antioxidant activity and total polyphenol content; polyphenols are
the dominant antioxidant components in medicinal herbs (20). As our data revealed, WE-ES produced
using the 100°C extraction procedure exhibited the highest
polyphenol content (Fig. 4) and
the highest SOD-like activity and DPPH radical-scavenging activity
(Figs. 5 and 6). Thus, the high polyphenol content in
the WE-ES fraction resulted in the high antioxidant activity. In
the present study, compared to the 40°C extraction procedure, the
100°C extraction process was more effective at extracting
polysaccharides and polyphenols to obtain a higher antioxidant
content. This finding is similar to that in the study of Sousa
et al (21).
The four extracts, WE-EP and WE-ES produced using
100°C and 40°C extraction procedures, were used for evaluating the
anti-proliferative effect on DLD-1 cells. All of the fractions
inhibited DLD-1 cell proliferation, and their anti-proliferative
effect was in a dose-dependent manner. Furthermore, WE-ES inhibited
DLD-1 cell proliferation in a time-dependent manner, compared to
WE-EP, for which a slight recovery of DLD-1 cell proliferation at
48 h was noted. Further purification steps with DEAE Sephadex A-50
column chromatography on WE-EP (crude polysaccharide fraction)
revealed that the high concentration of purified polysaccharides
did not exhibit a significant anti-proliferative effect on DLD-1
cells (data not shown). This suggests that the polyphenols in the
fractions conferred the main inhibitory effect on DLD-1 cell
proliferation. Recently, it was reported that several plant-derived
polyphenols may possess antioxidant, antitumor and
apoptosis-inducing properties (22). For example, HL-60 cells treated
with tea polyphenols were found to undergo morphological changes
and chromatin fragmention characteristic of apoptosis (23). Apoptosis is the process of
programmed cell death that occurs in multicellular organisms.
Biochemical events lead to characteristic cell changes (morphology)
and cell death. These changes include blebbing, loss of cell
membrane asymmetry and attachment, cell shrinkage, nuclear
fragmentation, chromatin condensation and chromosomal DNA
fragmentation. Induction of apoptosis is thus considered as a
strategy for cancer control. In this study, treatment with WE-EP
revealed no significant changes in the percentage of apoptotic
cells. In contrast, treatment with WE-ES resulted in an increase in
apoptotic cells. Thus, the apoptosis-inducing effect of WE-ES on
DLD-1 cells occurs in a dose-dependent manner. Furthermore, based
on the results of nuclear morphological changes, nuclear
fragmentation was only clearly observed when DLD-1 cells were
exposed to the WE-ES fraction (25 and 100 μg/ml) for 48 h.
Thus, these results suggest that the WE-ES fraction inhibits the
proliferation of DLD-1 cells by the induction of apoptosis.
Future studies are required to purify and analyze
the active components of WE-ES. Polysaccharides are reported to
play an important role in the immune system, and they are currently
believed to confer an antitumor effect by stimulating the host
immunomodulatory activity and enhancing the host immune response to
inhibit tumor growth (24). Thus,
it is considered that polysaccharides have an indirect inhibitory
effect on cancer cells. Thus, our future study will examine the
immunomodulatory activity of WE-EP on macrophages.
In conclusion, in this study, the water extracts
from the root of A. kolomikta exhibited antioxidant
activities and inhibitory effects on DLD-1 colon cancer cell
proliferation. Moreover, WE-ES clearly inhibited the proliferation
of DLD-1 cells by inducing apoptosis. Medicinal plant extracts are
considered as non-toxic and do not cause major side effects.
Actinidia kolomikta is a species of wild plant which is
widely distributed throughout Asia. This initial study on the
bioactivity of wild Actinidia kolomikta supports its
potential use in cancer therapies or as a natural health food with
antioxidant actions.
Acknowledgements
The materials were collected from
their natural habitat by Dr Keo Intabon, Graduate School of Life
and Environmental Sciences, University of Tsukuba, Japan.
References
|
1.
|
Moskovitz J, Yim MB and Chock PB: Free
radicals and disease. Arch Biochem Biophys. 397:354–359. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sun J, Yao JY, Huang SX, Long X, Wang JB
and Garcia EG: Antioxidant activity of polyphenol and anthocyanin
extracts from fruits of Kadsura coccinea (Lem). Food Chem.
117:276–281. 2009. View Article : Google Scholar
|
|
3.
|
Mau JL, Lin HC and Song SF: Antioxidant
properties of several specialty mushrooms. Food Res Int.
35:519–526. 2002. View Article : Google Scholar
|
|
4.
|
Hu TJ, Wei XJ, Zhang X, Cheng FS, Shuai
XH, Zhang L and Kang L: Protective effect of Potentilla anserine
polysaccharide (PAP) on hydrogen peroxide induced apoptosis in
murine splenic lymphocytes. Carbohydr Polym. 79:356–361. 2010.
View Article : Google Scholar
|
|
5.
|
PJiménez J, Arranz S, Tabernero M,
Díaz-Rubio ME, Serrano J, Goñi I and Saura-Calixto F: Updated
methodology to determine antioxidant capacity in plant foods, oils
and beverages: Extraction, measurement and expression of results.
Food Res Int. 41:274–285. 2008.
|
|
6.
|
Hadi SM, Bhat SH, Azmi AS, Hanif S, Shamim
U and Ullah MF: Oxidative breakage of cellular DNA by plant
polyphenols: A putative mechanism for anticancer properties. Semin
Cancer Biol. 17:370–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Du GR, Li MJ, Ma FW and Liang D:
Antioxidant capacity and the relationship with polyphenol and
Vitamin C in Actinidia fruits. Food Chem. 113:557–562. 2009.
View Article : Google Scholar
|
|
8.
|
Graham JG, Quinn ML, Fabricant DS and
Farnsworth NR: Plants used against cancer – an extension of the
work of Jonathan Hartwell. J Ethnopharmacol. 73:347–377. 2000.
|
|
9.
|
Xu HS, Yao L, Sun HX and Wu YW: Chemical
composition and antitumor activity of different polysaccharides
from the roots of Actinidia eriantha. Carbohydr Polym.
78:316–322. 2009. View Article : Google Scholar
|
|
10.
|
Xu HS, Wu YW, Xu SF, Sun HX, Chen FY and
Yao L: Antitumor and immunomodulatory activity of polysaccharides
from the roots of Actinidia eriantha. J Ethnopharmacol.
125:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mauro M: Estimation of total carbohydrate
amount in environmental samples by the phenol-sulphuric acid method
assisted by multivariate calibration. Chemom Intell Lab Syst.
79:84–90. 2005. View Article : Google Scholar
|
|
12.
|
Rhee SJ, Cho SY, Kim KM, Cha DS and Park
HJ: A comparative study of analytical methods for alkali-soluble
β-glucan in medicinal mushroom, Chaga (Inonotus obliquus).
LWT-Food Sci Technol. 41:545–549. 2008.
|
|
13.
|
Nakajima Y, Sato Y and Konishi T:
Antioxidant small phenolic ingredients in Inonotus obliquus
(persoon) Pilat (Chaga). Chem Pharm Bull. 55:1222–1226. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Yang B, Wang JS, Zhao MM, Liu Y, Wang W
and Jiang YM: Identification of polysaccharides from pericarp
tissues of litchi (Litchi chinensis Sonn) fruit in relation
to their antioxidant activities. Carbohydr Res. 341:634–638. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhang M, Chen HX, Huang J, Li Z, Zhu CP
and Zhang SH: Effect of lycium barbarum polysaccharide on
human hepatoma QGY7703 cells: inhibition of proliferation and
induction of apoptosis. Life Sci. 76:2115–2124. 2005.
|
|
16.
|
Hu HH, Zhang ZY, Lei ZF, Yang YN and
Sugiura N: Comparative study of antioxidant activity and
antiproliferative effect of hot water and ethanol extracts from the
mushroom Inonotus obliquus. J Biosci Bioeng. 107:42–48.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bennani H, Drissi A, Giton F, Kheuang L,
Fiet J and Adlouni A: Antiproliferative effect of polyphenols and
sterols of virgin argan oil on human prostate cancer cell lines.
Cancer Detect Prev. 31:64–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang ZJ and Luo DH: Antioxidant activities
of different fractions of polysaccharide purified from
Gynostemma pentaphyllum Makino. Carbohydr Polym. 68:54–58.
2007. View Article : Google Scholar
|
|
19.
|
Su XG, Duan J, Jiang YM, Shi J and Kakuda
Y: Effects of soaking conditions on the antioxidant potentials of
oolong tea. J Food Compos Anal. 19:348–353. 2006. View Article : Google Scholar
|
|
20.
|
Cai YZ, Luo Q, Sun M and Corke H:
Antioxidant activity and phenolic compounds of 112 traditional
Chinese medicinal plants associated with anticancer. Life Sci.
74:2157–2184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sousa A, Ferreira ICFR, Barros L, Bento A
and Alberto J: Pereira effect of solvent and extraction
temperatures on the antioxidant potential of traditional stoned
table olives “alcaparras”. LWT-Food Sci Technol. 41:739–745.
2008.
|
|
22.
|
Kilani-Jaziri S, Neffati A, Limem I,
Boubaker J, Skandrani I, Sghair MB, Bouhlel I, Bhouri W, Mariotte
AM, Ghedira K, Franca MD and Chekir-Ghedira L: Relationship
correlation of antioxidant and antiproliferative capacity of
Cyperus rotundus products towards K562 erythroleukemia
cells. Chem-Biol Interact. 181:85–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Zhao Y, Cao J, Ma H and Liu JW: Apoptosis
induced by tea polyphenols in HL-60 cells. Cancer Lett.
121:163–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Han SB, Park SK, Ahn HJ, Yoon YD, Kim YH,
Lee JJ, Lee KH, Moon JS, Kim HC and Kim HM: Characterization of B
cell membrane receptors of polysaccharide isolated from the root of
Acanthopanax koreanum. Int Immunopharmacol. 3:683–691. 2003.
View Article : Google Scholar : PubMed/NCBI
|