Introduction
Liver cancer is one of the most common and prevalent
human malignancies in the world. However, prevention and treatment
of liver cancer remain inadequate. In the theory and practice of
traditional Chinese Medicine, bear bile has been widely used for
fighting fever, toxins, inflammation, swelling, pain, liver
diseases and cancer (1). However,
due to increasing concerns that obtaining bile from bears is cruel
and inhuman, bear farming and bile collection has been restricted
in China and worldwide by government policies. The use of bear bile
is now illegal, as bears are listed in the Convention on
International Trade in Endangered Species of Wild Fauna and Flora
(CITES). A search for alternatives to bear bile is therefore
urgently required. Bile from other animal sources is being
considered as an alternative to bear bile. Various pharmacological
actions between animal bile and bear bile have been compared
(2); however, a comprehensive
investigation on chemical composition and cytotoxic activity is
lacking.
In the present study, a high-performance liquid
chromatography (HPLC)-evaporative light scattering detector (ELSD)
system was introduced to quantify the conjugated and free bile
acids in seven different animal bile samples. Standard chemicals
were used to identify and measure the chemical composition of the
animal bile samples, and a cell viability assay was used to
determine the cytotoxic potential of the animal bile samples as
well as the bile acids. The various chemical compositions as well
as the in vitro cytotoxic activity of the different animal
bile samples were determined. The cattle bile contained the active
components DCA, CDCA and TCDCA, and was determined to be a
potential cytotoxic agent against cancer cell growth.
Materials and methods
Chemicals and sample collection
Sodium tauroursodeoxycholate (TUDCA, T0266),
ursodeoxycholic acid (UDCA, U5127), sodium deoxycholate (DCA,
D6750), sodium taurochenodeoxycholate (TCDCA, T6260), sodium
taurodeoxycholate (TDCA), taurocholic acid (TCA, T4009), sodium
chenodeoxycholate (CDCA, C8261), sodium glycodeoxycholate (GDCA,
G9910), sodium glycochenodeoxycholate (GCDCA, G0759), sodium
glycocholate (GCA, G7132), cholic acid (CA, C1129) and taurine (TR,
T0625) were purchased from Sigma-Aldrich (USA). Bile from the
American black bear (UB), Ursus Americanus, was purchased
from Pak Shing Tong Ginseng Co. Ltd. (Hong Kong; licence no. APO/PL
1907/06). Bile from the Asiatic black bear (AB) was purchased from
Hang Hing Co. (Hong Kong; licence no. APO/PL 2384/07). Snake bile
powder (SB), pig bile powder (PB), cattle bile powder (CaB) and
chicken bile powder (ChB) were purchased from Yee Po International
(China). Bile juice from rabbit (RB) was kindly provided by the
Laboratory Animal Unit (The University of Hong Kong).
Sample preparation
Bile samples (2 g) were extracted with a 40-ml
methanol-water solution (1:1; v/v) in 50-ml centrifuge tubes for 2
h using an ultrasonic cleaner (Branson, USA) at room temperature
and were then centrifuged at 4,000 rpm for 20 min. The supernatant
(2 ml) was collected and filtered through a 0.45-μm membrane
(Millipore, USA). Pure compounds (Sigma) were dissolved in
methanol-water solution (1:1; v/v) for a final concentration of 2
mg/ml and then filtered. For analysis of bioactivity, 30 ml of
supernatant was collected, and the solvent was evaporated by a
rotary evaporator. The residue was dissolved in water containing
0.1% dimethyl sulphoxide (DMSO) (Sigma) at various concentrations.
Pure compounds were dissolved in water containing 0.1% DMSO.
HPLC-ELSD analysis
A Dionex® HPLC system (comprising a
quaternary pump 680, an autosampler ASI-100, an injector with a 200
μl loop, a column oven STH 585 and a data system
Chromeleon® 6.40) was used in this experiment with an
ELSD (2000ES; Alltech, USA). ELSD conditions were as follows: flow
rate of purified compressed air as a nebulizing gas, 1.6 l/min;
temperature of heated drift tube, 85°C. A Nova-Pack® C18
column (300 mm × 3.9 mm I.D., particle size, 4 μm; Waters,
USA) with a Nova-Pack® C18 Guard column was used as a
solid phase. Methanol as organic solvent A and 0.5% acetic acid in
Mill-Q water (pH 3.0) as aqueous solvent B were used as mobile
phases. A three-step gradient elution was as shown in Table I. The column temperature was 40°C,
and the flow rate was kept constant at 0.9 ml/min.
 | Table I.HPLC separation conditions. |
Table I.
HPLC separation conditions.
| Time (min) | Methanol (A%) | 0.5% acetic acid in
water (pH 3.0, B%) |
|---|
| 0 | 51 | 49 |
| 10 | 51 | 49 |
| 15 | 75 | 25 |
| 35 | 75 | 25 |
Cell culture and cell viability
assay
The in vitro cytotoxic activity of the agents
(TCA, GCA, DCA, GCDCA, GDCA, TDCA, taurine, TCDCA, CDCA, UDCA,
TUDCA) and the animal bile (PB, SB, CaB, ChB, RB, AB, UB) in the
hepatocellular carcinoma cell line MHCC97-L was assessed using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
assay. Briefly, cells at 80% confluence in a 75 cm2
flask were trypsinized, and a single-cell suspension was obtained.
Cells (10,000) in 200 μl of medium per well were seeded in
96-well plates and incubated for 24 h. Cells were then incubated
along with a series of bile samples or pure compounds at various
concentrations (2, 4, 8, 16, 32, 64, 128, 256 and 512 μM)
for 24, 48 and 72 h. Wells treated with vehicle (0.1% DMSO) served
as the controls. After treatment for various time periods, 15
μl of 5 mg/ml MTT (Sigma) was added to each well and
incubated for 4 h at 37°C. The medium was then discarded, and 200
μl of DMSO (Sigma) was added and pipetted up and down to
dissolve the crystals within the wells. The absorbance was measured
at 570 nm by a Multiskan MS microplate reader (Labsystems,
Finland). Each experiment was repeated three times, and the
standard deviations were indicated as error bars. Cell viability
was calculated as the ratio of the absorbance of cells treated with
agents to that of the untreated control multiplied by 100%. A curve
was plotted as the percentage of viable cells against the
concentration of the agents.
Statistical analysis
The data were analyzed using the Student's t-test
and are expressed as the mean ± SD.
Results
Optimization of HPLC conditions
Various separation conditions were tested to obtain
the optimal resolution for the free and conjugated bile acids. A
mixed standard solution (10 μl) was eluted with different
starting ratios of mobile phases. The separation was monitored to
determine the optimal elution conditions. The optimal condition
with a starting ratio of A to B of 51 to 49% was selected for
sample analysis based on good baseline resolution and stable
duration. The optimization of HPLC conditions is presented in
Fig. 1.
Identification and quantification of free
and conjugated bile acids in animal bile samples, including bile
crystals from Asian and American bears
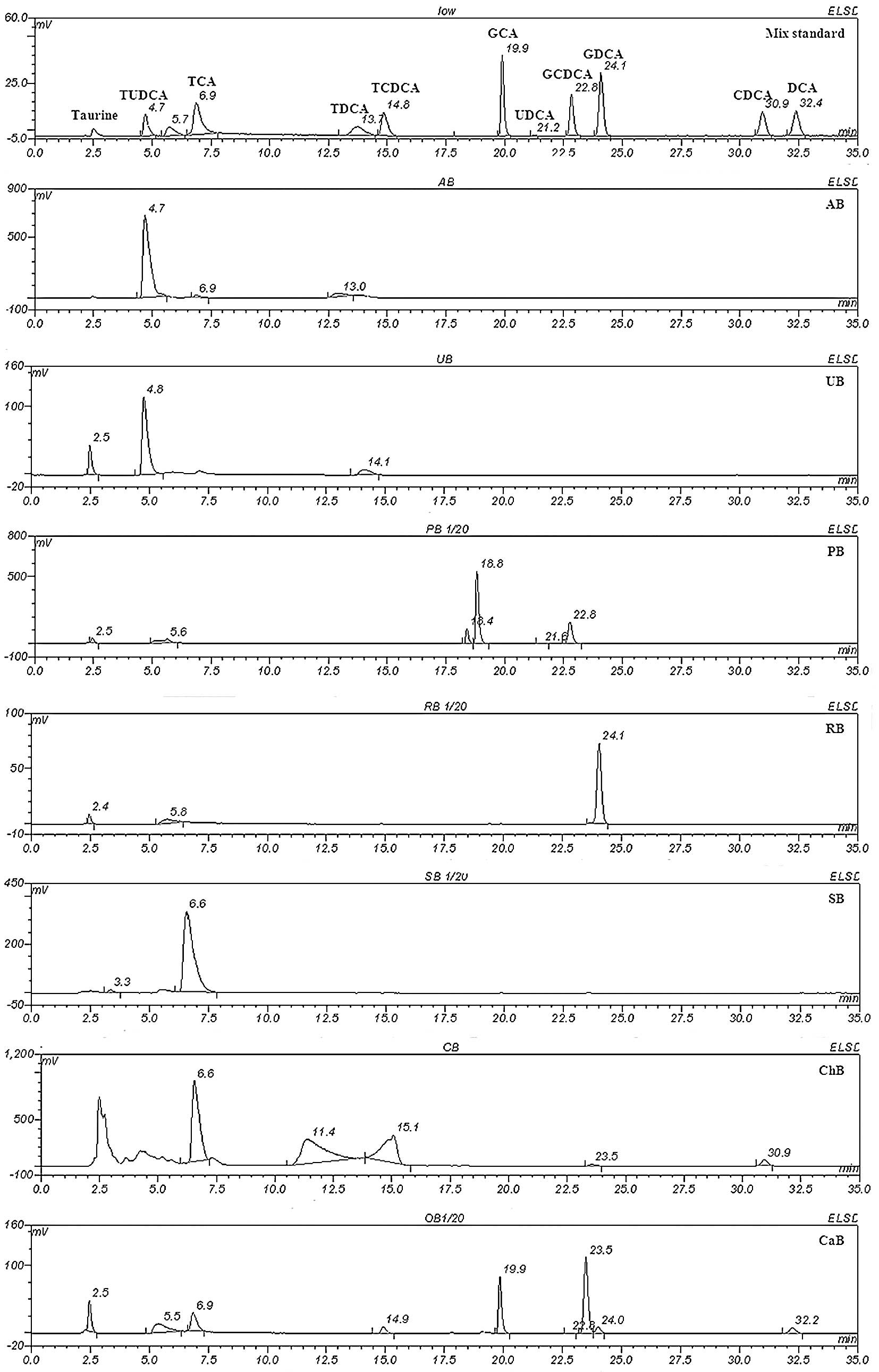
Isolation of the mixed standardized chemicals and
the seven animal bile samples was performed under the optimized
conditions (Fig. 2). An individual
standard sample was analyzed under the same conditions to identify
the peaks in the bile samples. Notably, the chemical composition of
the seven animal bile samples varied, showing extensive
differences. Both AB and UB samples contained TUDCA, a particular
component only found in bear bile juice, but not in any of the
other animal bile samples. UB also contained a small amount of
TCDCA, while AB did not. PB contained a large amount of UDCA, whose
taurine conjugated form is TUDCA, whereas CaB was composed of a
series of DCA-based chemicals, including DCA, CDCA, TDCA and TCDCA.
SB was mainly comprised of TCA, while RB contained large amounts of
GDCA. The standard curve is presented in Fig. 3, and the chemical composition of
the seven animal bile samples is shown in Table II.
 | Table II.Relative content (%) of the conjugated
and free bile acids in the seven animal bile samples. |
Table II.
Relative content (%) of the conjugated
and free bile acids in the seven animal bile samples.
| UDCA | DCA | TCA | GCA | TDCA | CDCA | GDCA | GCDCA | TUDCA | TCDCA |
|---|
| PB | 16.112 | | | | | | | 34.691 | | |
| SB | | | 94.857 | | | | | | | |
| RB | | | | | | | 18.356 | | | |
| CaB | | 8.615 | 15.058 | 18.020 | | | | 1.777 | | 9.881 |
| ChB | | 6.940 | | | 11.446 | 1.242 | | | | 7.182 |
| AB | | | | | | | | 6.522 | | |
| UB | | | | | | | | 2.344 | | 0.685 |
Cytotoxic effect of animal bile samples
on hepatocellular carcinoma MHCC97-L cells
Since bear bile is regarded as a therapeutic agent
for liver diseases according to classic Chinese Medical theory, and
has been used for the treatment of liver cancer by ancient and
modern Chinese Medical practitioners (3), the cytotoxic effect of bile from two
bear species and other animals on the hepatocellular carcinoma cell
line MHCC97-L was examined using the MTT assay. Our results
revealed moderate cytotoxic effects for both types of bear bile.
Consistent with the difference in their chemical composition, UB
(IC50= ∼200 μM) exhibited more significant
cytotoxic activity than AB (IC50= ∼400 μM). Bile
from pig or cattle is usually used as an alternative to bear bile
due to concerns for protecting endangered species. Our results
revealed that both CaB and PB exhibited potent cytotoxic activity
in MHCC97-L cells after a 72-h treatment (Table III). In contrast, both SB and RB
revealed pro-proliferative activity in MHCC97-L cells; SB had a
more extensive promotional effect on cell proliferation than RB
(Fig. 4).
 | Table III.IC50 of animal bile in
hepatocellular carcinoma MHCC97-L cells. |
Table III.
IC50 of animal bile in
hepatocellular carcinoma MHCC97-L cells.
| IC50 | AB (μM) | UB (μM) | PB (μM) | CaB (μM) |
|---|
| 24 h | 487.97 | 512.02 | NA | 106.41 |
| 48 h | 374.14 | 202.58 | 330.86 | 72.74 |
| 72 h | 378.92 | 263.52 | 58.29 | 45.47 |
Cytotoxic effect of free and conjugated
acids on the growth of hepatocellular carcinoma MHCC97-L cells
The cytotoxic activity of free and conjugated bile
acids on human carcinoma has been well documented in previous in
vitro and in vivo studies (3,4). In
order to provide a systematic report on the cytotoxic activity of
bile acids from animal bile in the liver cancer cell line MHCC97-L,
we conducted experiments to examine the in vitro
cytotoxicity of ten free and conjugated bile acids, which were
originally isolated from animal bile. The results are presented in
Fig. 5. DCA, CDCA and TCDCA
demonstrated a significant cytotoxic activity in MHCC97-L cells,
while TDCA, GDCA and GCDCA exhibited lower cytotoxic activity, even
though they share similar chemical structure. UDCA and its taurine
conjugated form, TUDCA, revealed no cytotoxic activity in MHCC97-L
cells, whereas TCA and GCA even exhibited a weak stimulative
activity on MHCC97-L cell proliferation. The IC50 values
of DCA, CDCA and TCDCA are presented in Table IV.
 | Table IV.IC50 of bile acids in
hepatocellular carcinoma MHCC97-L cells. |
Table IV.
IC50 of bile acids in
hepatocellular carcinoma MHCC97-L cells.
| IC50 | DCA (μM) | CDCA (μM) | TCDCA
(μM) |
|---|
| 24 h | 258.68 | 100.01 | 122.43 |
| 48 h | 244.29 | 101.57 | 119.22 |
| 72 h | 212.20 | 95.17 | 109.61 |
Discussion
Bear bile and bile extractions belong to the
category of animal drugs in Traditional Chinese Medicine. The use
of bear bile in Chinese Medical practice has a long history in
attenuating fever, toxification, inflammation, swelling, pain,
liver diseases and cancer (1). The
use of bear bile is now illegal since bears are classified as
endangered animal species in CITES. The identification of
alternatives to bear bile is therefore necessary. Bile from other
animals is considered an alternative due to its similar origin
(2,5,6).
However, a comprehensive study on the chemical composition and
bioactivity of animal bile is necessary.
Studies have revealed that PB solution has similar
pharmacological action as bear bile in regards to its
anti-inflmmatory, anticonvulsive and analgesic activities (1). It has been reported that PB is used
as an alternative to bear bile in specific Chinese Medicinal
formulas (2). In the present
study, we found that PB contains a large amount of UDCA, the
unconjugated form of TUDCA, which is only produced in bears. Both
UDCA and TUDCA have been previously found to have
anti-inflammatory, anti-apoptotic, cell protective and
anticholestatic properties (7–11).
In the present study, no significant cytotoxic activity of PB, as
well as UDCA and TUDCA, was observed.
The chemical composition of CaB, another type of
bile that is usually used as an alternative to bear bile, was found
to differ from that of the bear bile in our study. CaB, which
mainly contains DCA, CDCA and TCDCA, had excellent cytotoxicity
against hepatocellular carcinoma MHCC97-L cells. DCA, CDCA and
TCDCA have been reported to inhibit growth, induce apoptosis and
suppress metastasis in breast, esophageal, and colon cancers
(12–14). Chinese Medicine literature has
reported that bile may attenuate liver diseases and cancer
(1). Our study on the cytotoxic
activity of CaB, as well as its active components, DCA, CDCA and
TCDCA, reveals for the first time that these three bile acid
derivates and CaB are potential agents for liver tumor treatment.
Similar results were also found for ChB.
RB was previously found to be another alternative
source to bear bile (6). However,
in the present study, we found that RB had a totally distinct
chemical composition to bear bile. RB exhibited no cytotoxic
activity and even weakly promoted MHCC97-L cell proliferation,
which is consistent with the activity of GDCA (main active compound
in RB). Notably, we observed a strong and constant stimulation of
MHCC97-L cell proliferation by SB, in which TCA is the major and
only component identified by the HPLC analysis. TCA was found to
promote the occurrence of cholangiocarcinoma induced by
diisopropanolnitrosamine in hamsters (15), although the exact mechanism needs
further investigation.
In conclusion, the use of bile from other animal
sources as an alternative to bear bile has been considered based on
their similar chemical or pharmacological profiles. The chemical
composition and in vitro cytotoxic activity of seven animal
bile samples, PB, SB, RB, CaB, ChB, AB and UB, were evaluated in
this study. Both free and conjugated bile acids in the animal bile
samples were evaluated. HPLC-ELSD analysis revealed the distinct
chemical composition of the different animal bile samples. A cell
viability assay revealed that bile from cattle exhibits more marked
inhibitory activity on hepatocellular carcinoma cell growth and
proliferation than bear bile. DCA, CDCA and TCDCA are the major
active compounds in cattle bile. Our results support the potential
of cattle bile as an alternative to bear bile in liver cancer
prevention and therapy.
Acknowledgements
This study was supported by grants
from the Research Council of the University of Hong Kong (project
codes 200811159197 and 200907176140), the Research Grant Council
(RGC) of Hong Kong SAR, China (project code 764708M), Pong Ding
Yueng Endowment Fund for Education and Research in Chinese-Western
Medicine (project code: 20005274) and Hong Kong Government-Matching
Grant Scheme (4th phase, project code: 20740314). The cell line
MHCC97-L was a kind gift from the Liver Cancer Institute of Fudan
University, Shanghai, China. The authors are grateful for the
support of Professors Sai-Wah Tsao, Kwan Man, Yung-Chi Cheng,
Chi-Ming Che and Allan S.Y. Lau. The authors would like to express
thanks to Dr Ka-Yu Siu, Ms. Cindy Lee, Mr. Keith Wong and Mr.
Freddy Tsang for their technical support.
References
|
1.
|
Feng Y, Siu K, Wang N, et al: Bear bile:
dilemma of traditional medicinal use and animal protection. J
Ethnobiol Ethnomed. 5:2–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Li YW, Zhu XY, But PP and Yeung HW:
Ethnopharmacology of bear gall bladder. I. J Ethnopharm. 47:27–31.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yee SB, Yeo WJ, Park BS, Kim JY, Baek SJ,
Kim YC, Seo SY, Lee SH, Kim JH, Suh H, Kim ND, Lim YJ and Yoo YH:
Synthetic chenodeoxycholic acid derivatives inhibit glioblastoma
multiform tumor growth in vitro and in vivo. Int J
Oncol. 27:653–659. 2005.PubMed/NCBI
|
|
4.
|
Fimognari C, Lenzi M, Cantelli-Forti G and
Hrelia P: Apoptosis and modulation of cell cycle control by bile
acids in human leukemia T cells. Ann NY Acad Sci. 1171:264–269.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yang CM, Wang HZ, Hu RL and Feng ZS:
Studies on the bile-acid compositions of several fowl and domestic
animal bile. Xinjiang Agricul Sci. 43:467–472. 2006.
|
|
6.
|
Gu XC, Li MD, Chang JQ and Cui GZ:
Comparison between pharmacologic action of rabbit bile and bear
bile. Chin J Chin Materia Medicia. 19:556–558. 1994.PubMed/NCBI
|
|
7.
|
Wimmer R, Hohenester S, Pusl T, et al:
Tauroursodeoxycholic acid exerts anticholestatic effects by a
cooperative cPKC alpha-/PKA-dependent mechanism in rat liver. Gut.
571448–571454. 2008.PubMed/NCBI
|
|
8.
|
Lim SC, Choi JE, Kang HS and Si H:
Ursodeoxycholic acid switches oxaliplatin-induced necrosis to
apoptosis by inhibiting reactive oxygen species production and
activating p53-caspase 8 pathway in HepG2 hepatocellular carcinoma.
Int J Cancer. 126:1582–1595. 2010.
|
|
9.
|
Pusl T, Vennegeerts T, Wimmer R, et al:
Tauroursodeoxycholic acid reduces bile acid-induced apoptosis by
modulation of AP-1. Biochem Biophys Res Commun. 367:208–212. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Rivard AL, Steer CJ, Kren BT, Rodrigues
CM, Castro RE, Bianco RW and Low WC: Administration of
tauroursodeoxycholic acid (TUDCA) reduces apoptosis following
myocardial infarction in rat. Am J Chin Med. 35:279–295. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Solá S, Castro RE, Kren BT, Steer CJ and
Rodrigues CM: Modulation of nuclear steroid receptors by
ursodeoxycholic acid inhibits TGF-beta1-induced E2F-1/p53-mediated
apoptosis of rat hepatocytes. Biochemistry. 43:8429–8438.
2006.PubMed/NCBI
|
|
12.
|
Sun RC, Fadia M, Dahlstrom JE, Parish CR,
Board PG and Blackburn AC: Reversal of the glycolytic phenotype by
dichloroacetate inhibits metastatic breast cancer cell growth in
vitro and in vivo. Breast Cancer Res Treat. 120:253–260. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang R, Gong J, Wang H and Wang L: Bile
salts inhibit growth and induce apoptosis of human esophageal
cancer cell line. World J Gastroenterol. 11:5109–5116.
2005.PubMed/NCBI
|
|
14.
|
Park SE, Choi HJ, Yee SB, Chung HY, Suh H,
Choi YH, Yoo YH and Kim ND: Synthetic bile acid derivatives inhibit
cell proliferation and induce apoptosis in HT-29 human colon cancer
cells. Int J Oncol. 25:231–236. 2004.PubMed/NCBI
|
|
15.
|
Kinami Y, Ashida Y, Gotoda H, Seto K,
Kojima Y and Takashima S: Promoting effects of bile acid load on
the occurrence of cholangiocarcinoma induced by
diisopropanolnitrosamine in hamsters. Oncology. 50:46–51. 1993.
View Article : Google Scholar : PubMed/NCBI
|