Introduction
Genes that are frequently up-regulated in colorectal
cancer (CRC) can be identified by genome-wide analysis with cDNA
microarray profiling. This strategy has been used to identify gene
products that are essential for the proliferation and/or survival
of CRC cells (1). Two novel
tumor-associated antigens (TAAs), RNF43 (ring finger protein 43)
and TOMM34 (34-kDa translocase of the outer mitochondrial
membrane), were found to be up-regulated in more than 80% of
CRC tissues as compared to the corresponding noncancerous mucosa
(2,4). RNF43 expression cannot be detected in
normal human adult organs with Northern blotting. Thus, the
function of RNF43 has been associated with the proliferation of
tumor cells. Since suppression of TOMM34 by siRNA was found to
markedly reduce the growth of colon cancer cells, the gene product
is a potential therapeutic target for human CRC (3). HLA-A24-restricted epitope peptides
from RNF43 and TOMM34 for cancer vaccination for CRC patients were
recently identified (2,4).
We previously reported a phase I trial involving
vaccination with cancer peptides in combination with UFT and LV
(UZEL) for advanced CRC patients (5). UFT is an oral anticancer drug
consisting of tegafur (FT), a prodrug of 5-fluorouracil (5-FU) and
uracil, an inhibitor of 5-FU degradation. LV is an oral drug
consisting of calcium folinate which modulates 5-FU. We previously
demonstrated that the standard dose of UFT and LV did not impede
the immunological responses of advanced CRC patients to the peptide
vaccination.
To investigate the safety and immunological
responses of a peptide vaccination with RNF43 and TOMM34 in
combination with UFT and LV, we conducted a phase I clinical study
involving patients with metastatic CRC.
Materials and methods
Patients and eligibility criteria
The study protocol was approved by the Institutional
Ethics Review Boards of Kinki University (approval no. 18-15) and
was registered in the UMIN Clinical Trials Registry as
UMIN000003728 (http://www.umin.ac.jp/ctr/index.htm). Complete written
informed consent was obtained from the patients at the time of
enrollment. The patients (n=23) had histologically confirmed
metastatic CRC unsuitable for surgical resection and were
HLA-A*2402-positive. A total of 19 patients failed to respond to
prior standard chemotherapy, and the remaining 4 patients agreed to
receive this immunochemotherapy (Table
I). Patients were required to have completed prior chemotherapy
at least 4 weeks before trial enrollment and to have fully
recovered from any adverse event with a toxicity of grade 3 or
higher according to the Common Terminology Criteria for Adverse
Events (CTCAE) scale. The patients were required to have an Eastern
Cooperative Oncology Group performance status (PS) of 0 or 1, to be
older than 20 years of age and to have a life expectancy of at
least 3 months. Adequate bone marrow (white blood cell count
≥3,000/mm3, hemoglobin ≥10 g/dl and platelet count
≥75,000/mm3), renal function (serum creatinine ≤1.4
mg/dl) and liver function (bilirubin ≤1.5 mg/dl and transaminase
within 2.5 times the institution's upper limit of normal) were
required. Patients were excluded if they were pregnant or had
hepatitis B or C virus antigens or human immunodeficiency virus
(HIV).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient no. | Age | Gender | Primary cancer | Sites of
metastases | PS | Previous
treatment |
|---|
| 1 | 56 | M | R | Pelvis | 0 | UFT, CPT-11 |
| 2 | 64 | F | S | Lung | 0 | 5-FU, UFT/LV |
| 3 | 57 | F | R | Lymph nodes | 1 | 5-FU/LV, CPT-11,
S-1 |
| 4 | 42 | M | R | Pelvis | 0 | None |
| 5 | 53 | F | S | Lung | 0 | UFT/LV, vaccine |
| 6 | 54 | M | R | Lung | 0 | None |
| 7 | 74 | F | S | Lymph nodes | 0 | 5-FU, UFT/LV |
| 8 | 78 | M | R | Lung, lymph
nodes | 1 | 5-FU, UFT/LV,
CPT-11 |
| 9 | 58 | M | R | Lung | 1 | None |
| 10 | 46 | M | T | Liver, lymph
nodes | 1 | FOLFOX, FOLFIRI,
vaccine |
| 11 | 59 | M | S | Primary cancer,
liver, lymph nodes | 1 | FOLFIRI, FOLFOX |
| 12 | 66 | M | S | Lung, liver, lymph
nodes | 0 | S-1 |
| 13 | 66 | F | RS | Lung | 0 | UFT/LV |
| 14 | 49 | M | S | Lung, liver | 0 | None |
| 15 | 51 | F | S | Liver, lymph
nodes | 1 | UFT/LV, CPT-11 |
| 16 | 66 | M | R | Lung, liver, lymph
nodes | 1 | UFT/LV |
| 17 | 61 | F | C | Liver, lymph
nodes | 1 | FOLFOX+Bev,
FOLFIRI+Bev |
| 18 | 54 | M | S | Primary cancer,
liver, lymph nodes | 0 | FOLFOX+Bev,
UFT/LV |
| 19 | 83 | M | S | Lung | 0 | UFT |
| 20 | 66 | M | R | Lung, pelvis,
bone | 0 | FOLFOX+Bev,
FOLFIRI+Bev |
| 21 | 61 | M | R | Lung, pelvis | 1 | FOLFOX+Bev, FOLFIRI,
CPT-11+Cet |
| 22 | 73 | M | R | Lung, pelvis, lymph
nodes | 0 | FOLFOX+Bev, FOLFIRI,
CPT-11+Cet |
| 23 | 65 | M | R | Lung, pelvis | 0 | FOLFOX+Bev,
FOLFIRI+Bev, IRIS |
Peptides
The RNF43-721 (NSQPVWLCL) and TOMM34-299
(KLRQEVKQNL) peptides were synthesized by American Peptide Company
Inc. (Sunnyvale, CA, USA) according to a standard solid-phase
synthesis method and purified by reverse-phase high performance
liquid chromatography (HPLC) (4,6). The
purity (>95%) and the identity of the peptides were determined
by analytical HPLC and mass spectrometry analysis, respectively.
RNF43-721, TOMM34-299 and the epitope peptide derived from the
human immunodeficiency virus-envelope (HIV-Env) protein restricted
with HLA-A*2402 (RYLRDQQLL) were used to measure the
cytotoxic T lymphocyte (CTL) response.
Clinical protocol
The present open-label phase I study involved a
vaccine consisting of two peptides (1 mg of each peptide) derived
from RNF43 and TOMM34 mixed with incomplete Freund's adjuvant (IFA)
and Montanide ISA 51 (Seppic) administered to patients with locally
advanced, recurrent, or metastatic colorectal cancer. The patients
received a subcutaneous injection of vaccine into the thigh or back
once a week for 5 weeks. Simultaneously, patients received orally
administered UFT (300 mg/m2/day) and UZEL®
(75 mg/day) for 4 weeks, followed by 1 week of rest (one cycle).
The immunological responses to the inoculated peptides and clinical
responses were examined after every five vaccinations. The protocol
consisted of two cycles. After the second cycle, vaccinations were
given biweekly or monthly (depending on patient condition), while
UFT/UZEL administration was continued for 4 weeks followed by a
1-week rest period during the entire treatment period. A complete
blood count and results of serum chemistry tests were obtained
every 2 weeks. Clinical responses were evaluated at the end of
every cycle by examining computed tomography (CT) scans and tumor
markers. The vaccinated patients (n=21) were assessed for
immunological and clinical responses according to the Response
Evaluation Criteria in Solid Tumors (RECIST). Signs of toxicity
were assessed according to CTCAE version 3.0. Overall survival
rates were analyzed by the Kaplan-Meier method, and survival was
measured in days from the first vaccination to succumbing to the
disease. p-values were assessed using a log-rank test.
Cells
TISI cells and HLA-A*2402-positive B-lymphoblastoid
cell lines were purchased from the IHWG Cell and Gene Bank (IHW no.
9042; Seattle, WA, USA) in November 2008 and stored at −80°C.
Within 2 months of purchase, the cells were resuscitated and
maintained in RPMI supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin in a humidified 5% CO2 incubator
at 37°C. The peripheral blood was periodically collected from the
enrolled patients. Peripheral blood mononuclear cells (PBMCs) were
isolated using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden)
and density gradient centrifugation and were frozen immediately
after isolation. PBMCs from each patient were simultaneously thawed
and used to measure the CTL response.
Enzyme-linked immunospot assay
For detecting antigen-specific immune responses,
enzyme-linked immunospot (ELISPOT) assays were performed with the
human γ-interferon (IFN-γ) ELISPOT kit (Mabtech, Nacka Strand,
Sweden). Plates with 96 wells and nitrocellulose membranes
(Millipore, Molshelm, France) were precoated with primary
anti-IFN-γ antibody (1-D1K) at 4°C overnight.
Measurement of the cytotoxic T lymphocyte
response
The IFN-γ ELISPOT assay was performed to measure the
specific CTL response against the peptide. PBMCs were obtained from
patients and frozen prior to vaccination and at the end of each
treatment course. The frozen PBMCs were thawed and in vitro
sensitization was performed. In brief, PBMCs were stimulated with
10 μg/ml of each peptide and 20 IU/ml of interleukin (IL)-2
at 37°C, in 5% CO2 for 2 weeks. Peptides were added on
day 0 and 7. Following incubation, the harvested cells were used as
responder cells, and RNF43-721 or TOMM34-299 peptide-pulsed TISI
cells were used as stimulator cells (105 cells per
well). The HLA-A*2402-restricted epitope peptide derived from the
HIV-Env protein was used as a control peptide. The IFN-γ ELISPOT
kit and the AEC substrate set (BD Biosciences Pharmingen, San
Diego, CA, USA) were used to measure the CTL response. Spots were
captured and analyzed using an automated ELISPOT reader, ImmunoSPOT
4S (CTL Ltd., Cleveland, OH, USA). The ELISPOT assays were
performed in triplicate wells. The number of peptide-specific spots
was calculated by subtracting the number of spots when stimulated
with the HIV-Env peptide from the number of spots when stimulated
with the RNF43-721 or TOMM34-299 peptide. The percentage of
specific spots was calculated by dividing the number of
peptide-specific spots by the number of spots when stimulated with
the RNF43-721 or TOMM34-299 peptide. CTL induction was defined as
positive when more than 10 specific spots were detected or the
percentage of specific spots was greater than 5%. The number of
peptide-specific spots was detected as the responder/stimulator
ratio-dependency.
Statistical analysis
Overall survival rates were analyzed by the
Kaplan-Meier method, and survival was calculated in days from the
first vaccination to succumbing to the disease. The statistical
analyses were performed with SPSS statistics 17.0 (SPSS, Chicago,
IL, USA).
Results
Characteristics of the patients and
vaccinations
Between January 2007 and June 2009, 23
HLA-A*2402-positive patients with metastatic colorectal cancer were
enrolled in the present trial. All the patients had one or more
metastatic foci that were unsuitable for surgical resection. A
total of 19 patients had not responded to prior standard
chemotherapy, and the remaining 4 patients agreed to receive this
immunochemotherapy (Table I). A
total of 2 patients (nos. 10 and 17) were disqualified as they did
not meet the inclusion criteria. The final subject group thus
consisted of 21 patients (15 men and 6 women) with a median age of
61 years (range 42–83). A total of 727 vaccinations were
administered with a median of 31 vaccinations per patient (range
7–69). The vaccination with chemotherapy protocol was well
tolerated by all patients.
Toxicities
The overall toxicities are shown in Table II. The most frequent adverse events
were vaccination-site reactions (n=15), anemia (n=5), anorexia
(n=5), malaise (n=3) and elevation of serum transaminase (n=3).
With the exception of one incident of grade 3 acute renal
dysfunction (no. 20) due to hydronephrosis, all of the adverse
events were grade 1. A double-J catheter was placed by a urologist
into the patient who experienced acute renal dysfunction, which led
to the disappearance of the hydronephrosis and the resumption of
therapy. This patient had a large area of tumor recurrence in the
pelvis prior to therapy; therefore, the renal dysfunction due to
ureteral obstruction was considered to be caused by the metastasis
and not related to the therapy.
 | Table II.Adverse events. |
Table II.
Adverse events.
| Toxicity | Total n (%) | Grade 1 | Grade 2 | Grade 3 |
|---|
| Anemia | 5 (23.8) | 5 | 0 | 0 |
| Transaminase
elevation | 3 (14.3) | 3 | 0 | 0 |
|
Hyperbilirubinemia | 2 (9.5) | 2 | 0 | 0 |
| Anorexia | 5 (23.8) | 5 | 0 | 0 |
| Nausea | 2 (9.5) | 2 | 0 | 0 |
| Malaise | 3 (14.3) | 3 | 0 | 0 |
| Vaccination site
reaction | 15 (71.4) | 15 | 0 | 0 |
| Renal
dysfunctiona | 1a (4.8) | 0 | 0 | 1a |
Immunological monitoring
Peripheral blood lymphocytes obtained before,
during, and after the vaccination periods were cultured in rIL-2
without any antigen stimulation for 14 days and subjected to the
ELISPOT assay to detect the antigen-specific T-cell response
induced by the vaccination. The CTL response was considered to be
positive when more than 10 specific spots were detected or the
percentage of specific spots was greater than 5%. In addition, the
number of peptide-specific spots was detected as the
responder/stimulator ratio-dependency. Representative CTL-positive
data from ELISPOT assays against the TOMM34 antigen are shown for
patient no. 5 (Fig. 1). Among the
21 patients, 8 patients had positive CTL responses against RNF43
and TOMM34, 12 patients had a positive response against one of the
antigens, and the remaining patient had a negative response
(Table III). The magnitude of the
CTL response varied depending on the timing of the vaccinations.
However, there was a clear separation between positive and negative
CTL responses.
 | Table III.Immunological and clinical
responses. |
Table III.
Immunological and clinical
responses.
| Patient no. | No. of
vaccinations | Vaccination site
reaction | CTL response | Clinical
response | TTP (days) | OS (days) |
|---|
| 1 | 69 | Ind, red | RNF, TOMM | SD | 252 | 1226 (alive) |
| 2 | 7 | (-) | RNF, TOMM | - | 38 | 1026 |
| 3 | 17 | Ind, red | TOMM | SD | 169 | 448 |
| 4 | 16 | (-) | RNF, TOMM | SD | 211 | 741 |
| 5 | 69 | Ind, red | RNF, TOMM | SD | 365 | 1086 (alive) |
| 6 | 31 | Ind | RNF, TOMM | SD | 428 | 1054 (alive) |
| 7 | 37 | Ind, red | RNF, TOMM | PD | 49 | 1012 |
| 8 | 8 | (-) | RNF | - | 36 | 80 |
| 9 | 69 | Ind, red | TOMM | SD | 694 | 904 (alive) |
| 11 | 11 | (-) | RNF | PD | 36 | 183 |
| 12 | 29 | Ind | RNF | SD | 219 | 387 |
| 13 | 37 | Ind | TOMM | SD | 219 | 521 |
| 14 | 54 | Ind | RNF, TOMM | SD | 260 | 512 (alive) |
| 15 | 22 | Ind | RNF | SD | 107 | 197 (alive) |
| 16 | 16 | Ind, red | TOMM | SD | 73 | 132 |
| 18 | 41 | Ind | TOMM | PD | 70 | 414 (alive) |
| 19 | 52 | Ind, red | RNF, TOMM | SD | 309 | 414 (alive) |
| 20 | 46 | Ind | RNF | SD | 218 | 330 |
| 21 | 50 | Red | TOMM | SD | 246 | 365 (alive) |
| 22 | 15 | (-) | TOMM | SD | 69 | 151 |
| 23 | 31 | (-) | (-) | SD | 176 | 288 |
Clinical response and overall
survival
Among the 21 patients, 19 patients were assessed for
clinical response at the end of the 10th vaccination (2nd cycle)
according to the RECIST criteria (Table III). The clinical responses of the
remaining 2 patients were not assessed as they received fewer than
10 vaccinations (6 and 8, respectively). None of the patients
showed a complete response or a partial response. A total of 16
patients had stable disease and 3 patients had progressive disease.
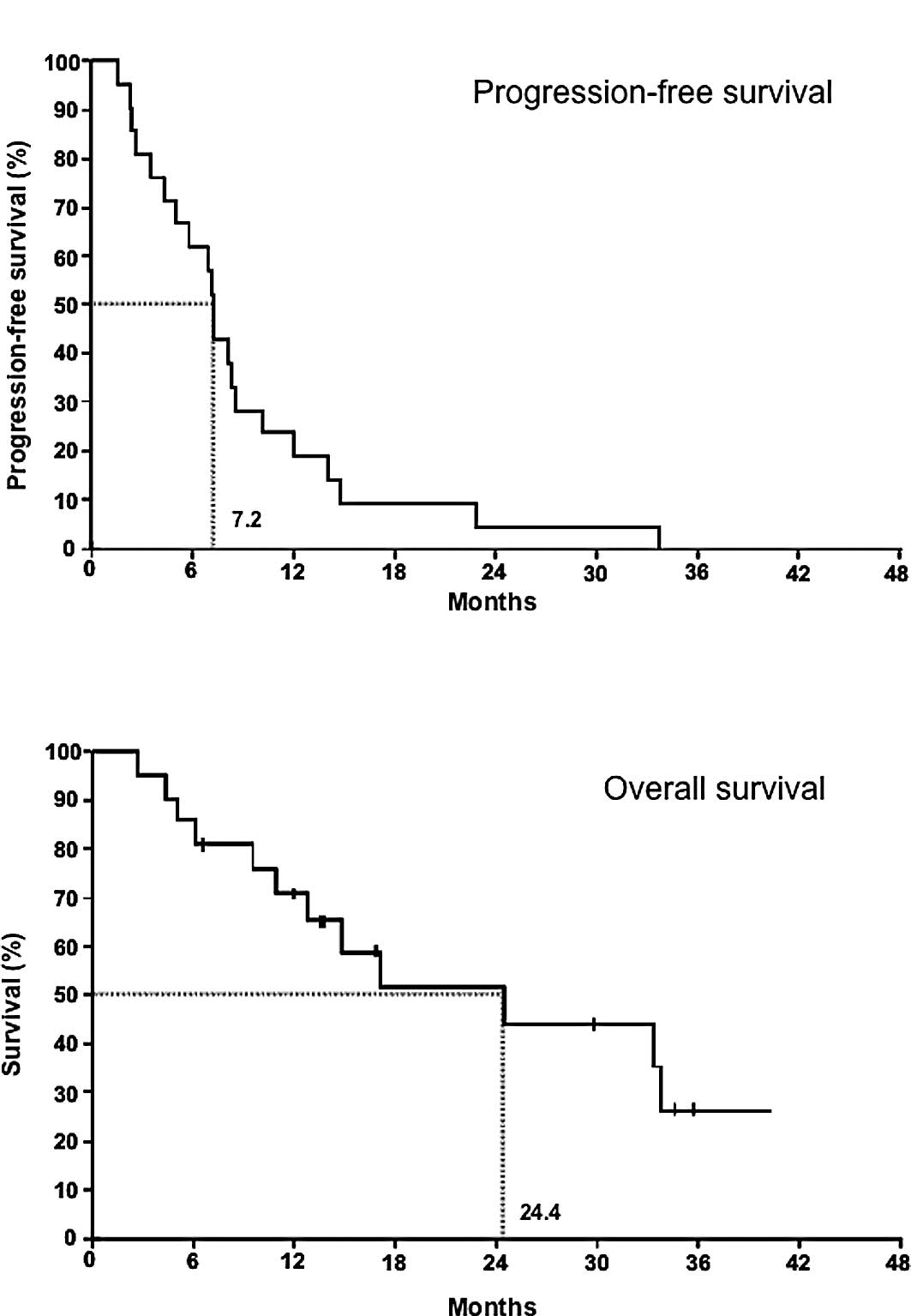
The median time of progression-free survival was 7.2 months
(Fig. 2A), and the mean survival
time was 24.4 months (Fig.
2B).
Effect of a cytotoxic T lymphocyte
response against RNF43 and TOMM34 on overall survival
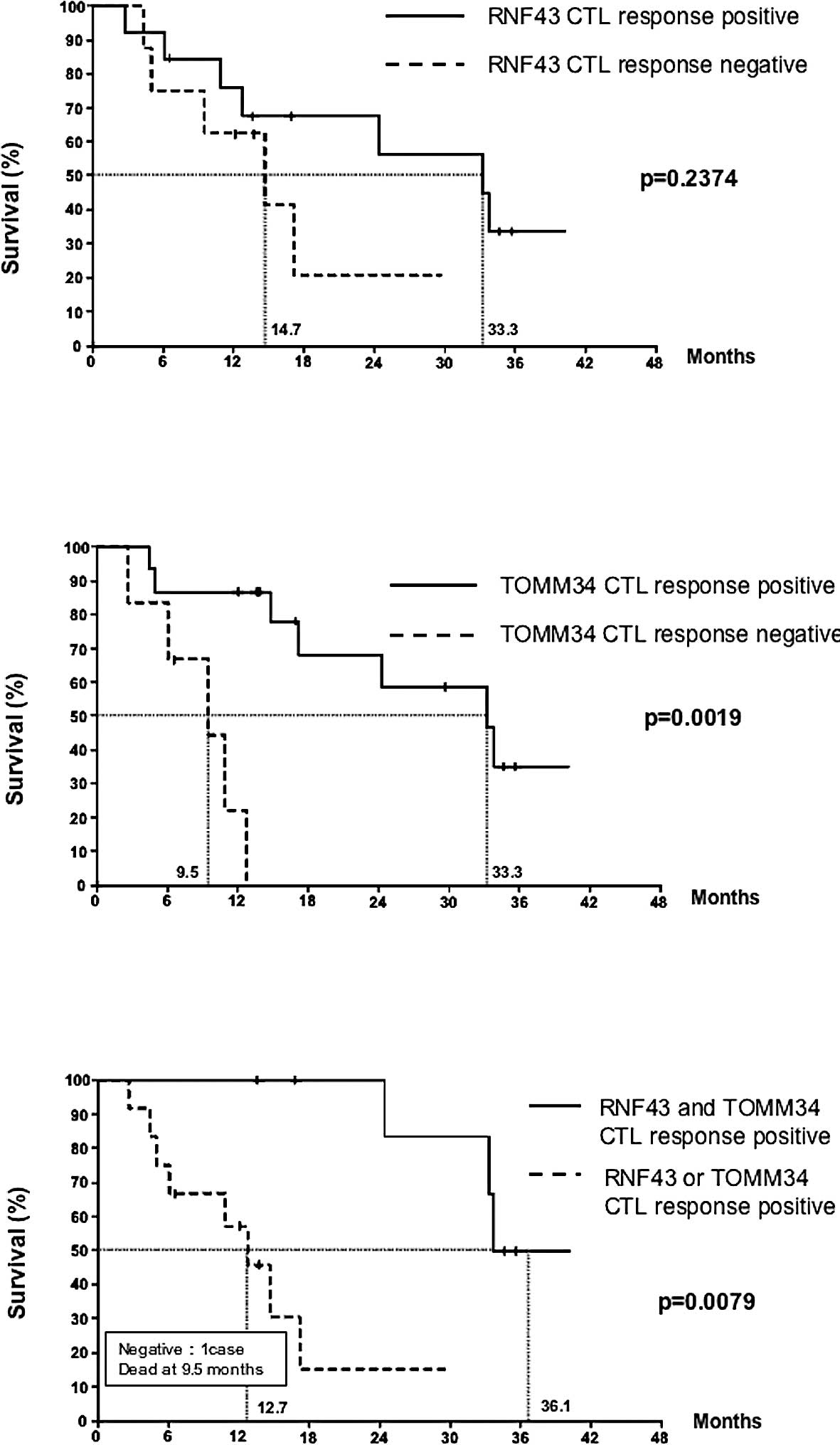
The effect of a positive CTL response to RNF43 or
TOMM34 on overall survival was analyzed. The Kaplan-Meier estimates
for the overall survival of patients with detected CTL responses as
compared to patients with no response are shown in Fig. 3. No statistical difference was
found between the two groups with or without a response to RNF43
(p=0.2374) (Fig. 3A). However,
there was a statistical difference between the two groups based on
the TOMM34 response (p=0.0019) (Fig.
3B). Furthermore, we investigated the relationship between CTL
response to both antigens and overall survival. The best long-term
survival was observed in the group with CTL responses against both
antigens, followed by the group showing CTL responses against only
RNF43 or TOMM34 (p=0.0079). The patient with no response had the
lowest survival (Fig. 3C).
Discussion
In this clinical trial, cancer vaccination with two
peptides in combination with oral UFT/LV chemotherapy was well
tolerated without any severe side effects in metastatic CRC
patients. Common adverse events included vaccination site reaction,
anemia, anorexia, malaise and elevation of transaminase. With the
exception of the skin reaction, the rates of other adverse events
did not exceed those of the UFT/LV chemotherapy (7). Therefore, addition of the peptide
vaccination did not increase the adverse events (beyond mild
vaccination site reactions) in this combination therapy. The design
of this clinical trial was based on the results of two previous
phase I trials. These previous trials found that vaccination with
multiple peptides derived from novel cancer-testis antigens in
advanced cancer was feasible and that antigen-specific T-cell
responses were induced with objective clinical responses (8). These trials also showed that the
peptide vaccination combined with oral UFT/LV chemotherapy was well
tolerated in the metastatic CRC patients and induced
peptide-specific IgG responses that correlated well with overall
survival (5).
The combined chemo-immunotherapy approach has been
criticized on the grounds that chemotherapy is immunosuppressive.
This opinion is based on the fact that most cytotoxic drugs kill
granulocyte precursors in bone marrow and thus induce leucopenia,
which is associated with the occurrence of bacterial and mycotic
infection. However, there is no evidence that cytotoxic
chemotherapy affects the antigen-specific CTL response. Recently,
Correale et al (9) reported
that the antigen-specific killing ability of human CTL lines in
vitro is not affected by 5-FU or oxaliplatin when exposure to
these drugs does not occur during the stimulation phase. Moreover,
they found that chemotherapy i) up-regulated tumor-associated
antigen expression including CEA or other target molecules such as
TS; ii) down-regulated tumor cell resistance to the death signals
induced by tumor antigen-specific CTL; iii) reduced the percentage
of PBMCs containing immune-suppressive regulatory T cells
(CD4+CD25+T reg) and the number of cells
expressing the FAS receptor (CD95); and iv) induced the complete
restoration of the CD4/CD8 T-cell ratio, which is often reduced in
advanced cancer patients resulting in a progressively deteriorating
immune response (10). Based on
these considerations, we believe that the rationale for
chemoimmunotherapy in advanced cancer patients will be
accepted.
The two cancer-specific peptides, RNF43 and TOMM34,
used in the present study are novel cancer-testis antigens specific
for CRC. More than 80% of colorectal cancers express these
antigens, and these antigens can induce potent CTLs against colon
cancer cell lines (4,6). RNF43 and TOMM34 are defined as
oncoantigens. They are highly expressed in cancer cells, are
involved in the critical functions of cancer cells (i.e.,
proliferation) and can induce potent CTL responses. In this
context, it is of note that common antigens, such as MUC-1 or CEA,
in colorectal cancer, are not critical for tumor cell survival;
therefore, they can be lost under the selective pressure of a
vaccine-induced antigen-specific immune response without
significantly damaging tumor development (11–14).
Using the two crucial cancer-testis antigen-derived
peptides, CTL responses were observed in 95% of the study patients
(20 of 21 patients). Potent CTL responses against both antigens
were induced in 8 patients (38%), and a CTL response against one
peptide occurred in 12 patients (57%). Therefore, the use of two
peptides allowed CTL responses to occur in almost all patients who
received the vaccinations.
Overall survival was well correlated with the
response to TOMM34. The patients exhibiting a response to RNF43
also experienced longer survival, although the correlation was not
statistically significant. Notably, the patients exhibiting CTL
responses to both peptides (n=8) had the longest survival, followed
by the patients who showed a CTL response to one peptide (n=12).
The patient exhibiting no response had the lowest survival (n=1)
(Fig. 2). We do not have evidence
to prove that the induced CTLs interacted directly with the cancer
lesions in the patients with metastatic CRC to control the cancer
lesions and thus contribute to the longer survival. However, we can
conclude that the CTL response is a useful biomarker for patients
receiving peptide vaccination therapy.
In conclusion, this study suggests that vaccination
with two colorectal cancer-specific peptides in combination with
UFT/LV is well tolerated and can induce potent and specific CTL
responses to at least one peptide antigen in 95% of patients.
Furthermore, the patients who developed potent CTL responses
against both antigens showed the longest survival. This treatment
approach warrants further clinical study.
Acknowledgements
The authors would like to thank
Professor Yusuke Nakamura and Dr Takuya Tsunoda, Laboratory of
Molecular Medicine, Human Genome Center, Institute of Medical
Science, University of Tokyo, for their excellent advice and
cooperation. This study was supported in part by a Grant-in-Aid for
Department of the New Energy and Industrial Technology Development
Organization (NEDO).
References
|
1.
|
Lin YM, Furukawa Y, Tsunoda T, Yue CT,
Yang KC and Nakamura Y: Molecular diagnosis of colorectal tumors by
expression profiles of 50 genes expressed differentially in
adenomas and carcinomas. Oncogene. 21:4120–4128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Yagyu R, Furukawa Y, Lin YM, Shimokawa T,
Yamamura T and Nakamura Y: A novel oncoprotein RNF43 functions in
autocrine manner in colorectal cancer. Int J Oncol. 25:1343–1348.
2004.PubMed/NCBI
|
|
3.
|
Chewawiwat N, Yano M, Terada M, Hoogenraad
NJ and Mori M: Characterization of the novel mitochondrial protein
import component, Tom34, in mammalian cells. J Biochem.
125:721–727. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Shimokawa T, Matsushima S, Tsunoda T,
Tahara H, Nakamura Y and Furukawa Y: Identification of TOMM34,
which shows elevated expression in the majority of human colon
cancers, as a novel drug target. Int J Oncol. 29:381–386.
2006.PubMed/NCBI
|
|
5.
|
Hattori T, Mine T, Komatsu N, et al:
Immunological evaluation of personalized peptide vaccination in
combination with UFT and UZEL for metastatic colorectal carcinoma
patients. Cancer Immunol Immunother. 58:1845–1854. 2009. View Article : Google Scholar
|
|
6.
|
Uchida N, Tsunoda T, Wada S, Furukawa Y,
Nakamura Y and Tahara H: Ring finger protein 43 as a new target for
cancer immunotherapy. Clin Cancer Res. 10:8577–8586. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shirao K, Hoff PM, Ohtsu A, et al:
Comparison of the efficacy, toxicity, and pharmacokinetics of a
uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between
Japanese and American patients with advanced colorectal cancer:
joint United States and Japan study of UFT/LV. J Clin Oncol.
22:3466–3474. 2004. View Article : Google Scholar
|
|
8.
|
Kono K, Mizukami Y, Daigo Y, et al:
Vaccination with multiple peptides derived from novel cancer-testis
antigens can induce specific T-cell responses and clinical
responses in advanced esophageal cancer. Cancer Sci. 100:1502–1509.
2009. View Article : Google Scholar
|
|
9.
|
Correale P, Del Vecchio MT, Genova GD, et
al: 5-Fluorouracil-based chemotherapy enhances the antitumor
activity of a thymidylate synthase-directed polyepitopic peptide
vaccine. J Natl Cancer Inst. 97:1437–1445. 2005. View Article : Google Scholar
|
|
10.
|
Correale P, Cusi MG, Tsang KY, et al:
Chemo-immunotherapy of metastatic colorectal carcinoma with
gemcitabine plus FOLFOX 4 followed by subcutaneous granulocyte
macrophage colony-stimulating factor and interleukin-2 induces
strong immunologic and antitumor activity in metastatic colon
cancer patients. J Clin Oncol. 23:8950–8958. 2005.
|
|
11.
|
Nagorsen D and Thiel E: HLA typing demands
for peptide-based anti-cancer vaccine. Cancer Immunol Immunother.
57:1903–1910. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Liu K, Wang C, Chen L, et al: Generation
of carcinoembryonic antigen (CEA)-specific T-cell responses in
HLA-A0201 and HLA-A2402 late-stage colorectal cancer patients after
vaccination with dendritic cells loaded with CEA peptides. Clin
Cancer Res. 10:2645–2651. 2004. View Article : Google Scholar
|
|
13.
|
Weihrauch MR, Ansen S, Jurkiewicz E, et
al: Phase I/II combined chemoimmunotherapy with carcinoembryonic
antigen-derived HLA-A2-restricted CAP-1 peptide and irinotecan,
5-fluorouracil, and leucovorin in patients with primary metastatic
colorectal cancer. Clin Cancer Res. 11:5993–6001. 2005. View Article : Google Scholar
|
|
14.
|
Dittmann J, Keller-Matschke K, Weinschenk
T, et al: CD8+ T-cell response against MUC-1-derived
peptides in gastrointestinal cancer survivors. Cancer Immunol
Immunother. 54:750–758. 2005.
|