Introduction
Hepatocellular carcinoma (HCC) is one of the most
serious malignancies worldwide (1), particularly in Asian countries due to
the high prevalence of the hepatitis virus (2). It is also the third most common cause
of cancer-related mortality (3,4).
Despite intensive efforts to develop novel treatment modalities for
HCC, the prognosis remains poor. Thus, there is a strong demand for
effective new approaches to HCC therapy.
Receptor tyrosine kinases (RTKs) are a family of 56
proteins each characterized by a transmembrane domain and a
tyrosine kinase motif (5). The
known RTKs consist of a ligand-binding domain at the extracellular
surface, a single transmembrane segment and a cytoplasmic part
harboring the protein kinase activity. They are divided into 21
families, including the epidermal, vascular endothelial and
fibroblast growth factor receptor families, which are characterized
by a similar structure and the potential for intrafamilial
dimerization (6). Various RTKs
have been implicated in intracellular signal transduction pathways
involved in growth, differentiation, adhesion, migration, apoptosis
and carcinogenesis (7). In regard
to the relationship between human cancers and RTKs, aberrant RTK
activity was initially found in various epithelial cancers,
including breast (8), gastric
(9), lung (10), colon (11) and esophageal cancer (12), and HCC (13,14).
It is now accepted that the activation of certain RTKs plays a key
role in the development of almost all types of cancer. Accordingly,
a number of clinical trials with various settings and designs are
currently exploring the potential of anti-RTK therapies in various
cancers (15). A recent study
reported that the receptor tyrosine kinase inhibitor sorafenib is
effective in patients with advanced HCC (16). However, the anti-cancer effects of
sorafenib under investigation for the treatment of HCC remain
unknown.
The aims of this study were two-fold: i) to use
protein array technology to determine the expression status of
various activated RTKs in HCC; and ii) upon identifying ErbB2 as
the most consistently up-regulated RTK, to investigate whether an
ErbB2-targeting drug, trastuzumab, would be effective as an
anti-cancer agent in an HCC xenograft model.
Materials and methods
Materials
The RayBio™ Human Phospho Array kit (catalog no. ARY
001) was purchased from RayBiotech Inc. (Norcross, GA, USA).
Trastuzumab (Herceptin™) was purchased from Chugai Pharmaceutical
Co., Ltd. (Tokyo, Japan).
Human tissues
Human HCC tissue samples and the adjacent hepatic
tissues were obtained during surgery from 5 patients (3 male and 2
female; mean age 69.6±10.6 years; range 57–81). A total of 3
patients were positive for hepatitis C virus RNA, and 2 patients
with chronic hepatitis were positive for the hepatitis B surface
antigen. The histology of the adjacent hepatic tissue in the
patients was F4 according to Desmet's classification (17). None of the patients had received
any chemotherapy or radiotherapy prior to surgery. The use of human
specimens was approved by the Human Subjects Committee of Kagawa
University School of Medicine.
Cell lines
Alex, HuH7, Li-7, Hep3B, HLE and HLF cells, a kind
gift from the Japanese Cancer Resource Bank (Tokyo, Japan), were
used as the HCC cell lines. These cell lines were plated at a
density of 1×105 cells/cm3 in plastic flasks
containing Dulbecco's modified minimum essential medium (DMEM)
(Gibco BRL Co., Grand Island, NY, USA) supplemented with 10%
heat-inactivated fetal calf serum, 100 μg/ml penicillin and
100 μg/ml streptomycin at 37°C in 5% CO2 in air.
The human normal hepatocyte cell line, hNHeps, was used as the
normal hepatocyte cell line.
Cell and tissue lysates
The cell lysate was prepared according to the
methods previously described (9,18,19).
The steps were performed at 4°C. The protein concentration of the
cell and tissue lysates was measured using a dye-binding protein
assay based on the Bradford method (13,14,20).
Antibody arrays of phospho-receptor
tyrosine kinases
An assay for phospho-RTK array was performed as
previously described (9,11). Briefly, phospho-RTK array membranes
were blocked with 5% BSA/TBS (0.01 M Tris HCl, pH 7.6) for 1 h. The
membranes were subsequently incubated with ∼2 ml (protein contents:
100 μg/ml) of lysate prepared from cell lines or tissues
after normalization with equal amounts of protein. To remove
unbound materials, the membranes were washed three times with TBS,
including 0.1% v/v Tween-20 for 10 min each time, and then twice
with TBS alone for 10 min each time. They were then incubated with
anti-phospho-tyrosine-HRP antibody for 2 h at room temperature. The
unbound HRP antibody was washed out with TBS, including 0.1%
Tween-20. Finally, each array membrane was exposed to X-ray film
using a chemiluminescence detection system (Amersham Life Sciences,
Tokyo, Japan).
In vivo anti-tumor effects of trastuzumab
on hepatocellular carcinoma
Athymic 8-week-old male BALB/c-nu/nu mice, weighing
20–22 g, were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan)
and kept under specific pathogen-free conditions at 24±2°C. The
animal experiments were performed with approved protocols and in
accordance with the institutional recommendations for the proper
care and use of laboratory animals. HuH7 and HLF HCC cells were
suspended in PBS at a concentration of 5×107 cells/ml,
respectively, and 100 μl inoculum volumes were injected
subcutaneously into the flank regions of the mice. When the tumors
became palpable in the treated group (n=10), 500 μl of PBS
containing 750 mg/0.5 ml trastuzumab (Herceptin®,
directed against the ErbB2 receptor, also known as the Her2/Neu
oncogene) was administered intraperitoneally three times a week for
3 weeks. Only PBS was administered to the control group (n=10).
After initiation of the trastuzumab administration, the tumor
growth was monitored by the same investigators (I.G. and T.M.), and
the tumor diameters were measured every week using a graduated
caliper. Tumor growth was assessed weekly by measuring the two
greatest perpendicular tumor dimensions. Tumor volume was
calculated as follows: tumor volume (mm3) = [tumor
length (mm) × tumor width (mm)2]/2 (21). The animals were sacrificed on day
16 after treatment. The animals remained alive throughout the
observation.
Statistical analysis
The results are expressed as the means ± SD. The
analysis was performed using the computer-assisted StatView program
(SAS Institute, Cary, NC, USA). Paired analysis between two groups
was performed using the Student's t-test. Values of p<0.05 were
considered to indicate a significant difference between groups.
Results
Activity level of activated receptor
tyrosine kinases is associated with hepatocellular carcinoma
A phospho-RTK array system was used to identify the
‘key RTKs’ that are associated with HCC. Using the antibody array,
the expression of 42 different activated RTKs were simultaneously
screened (Fig. 1). Compared to the
hNHeps cell line, ErbB2, ErbB3, ErbB4, FGFR2α, FGFR3, insulin R,
Mer, PDGFRβ, c-Ret, ROR2, Tie, TrkA, VEGFR3, EphA1 and EphA4 were
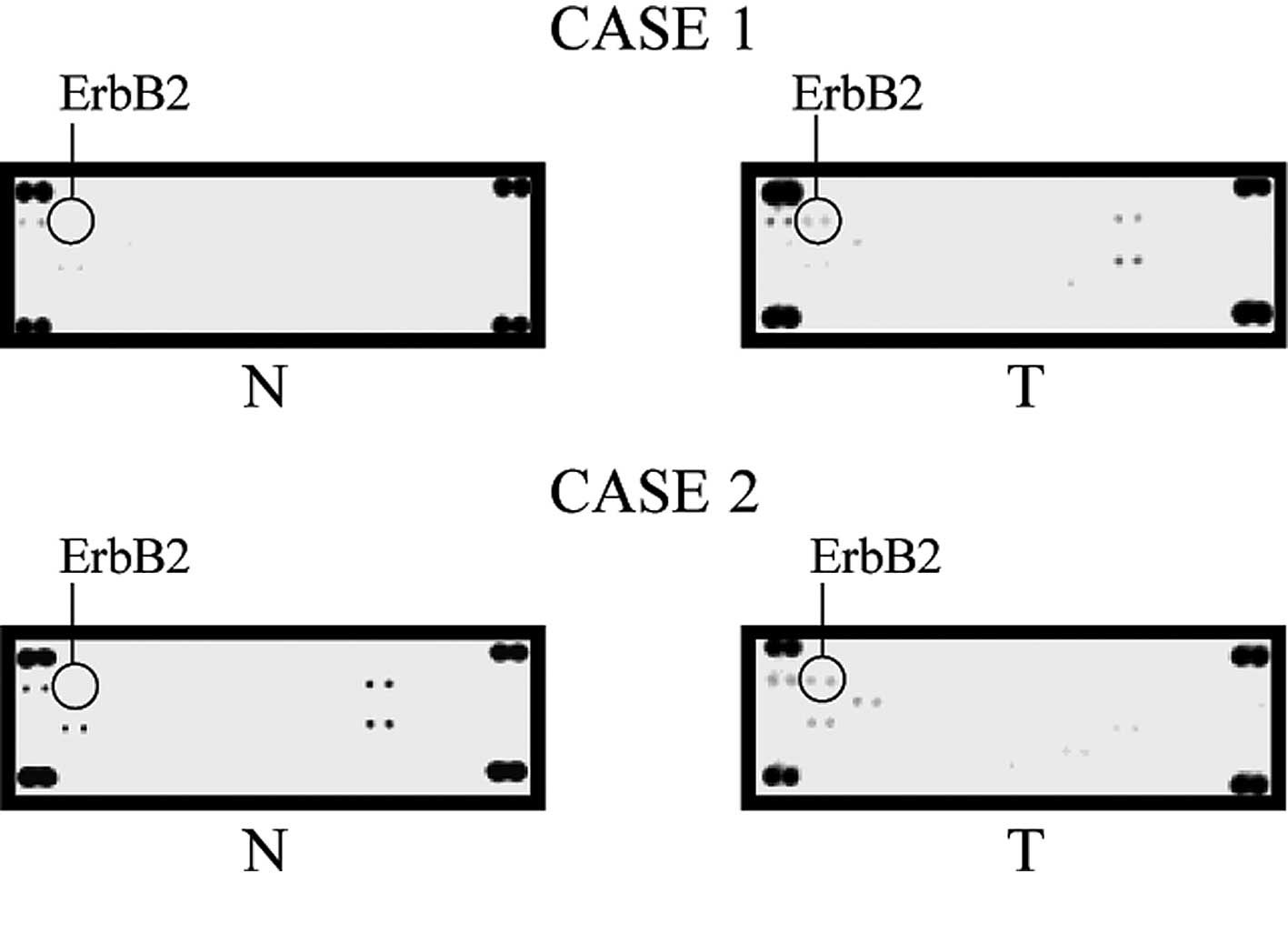
up-regulated in some of cancer cell lines studied (Fig. 2). One of these molecules, ErbB2
(•), was up-regulated in all of the HCC cell lines examined in this
study, while it was not detected in the hNHeps cell line. Also, in
the cancerous tissue, ErbB2 was the only RTK up-regulated in all
five tissue samples (Fig. 3).
These results suggest that an ErbB2-targeting drug is a useful
agent for the treatment of HCC.
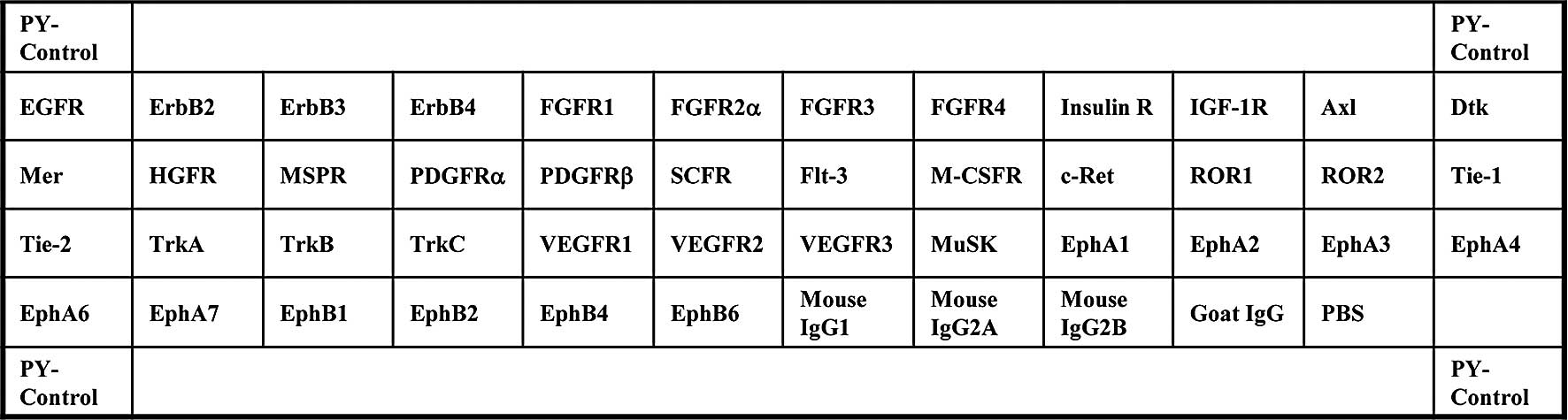 | Figure 1.Template showing the location of a
tyrosine kinase antibody spotted onto a RayBio™ Human phospho
array. PY-Control, phospho-tyrosine positive control; EGFR,
epidermal growth factor receptor; ErbB2, v-erb-b2 erythroblastic
leukemia viral oncogene homolog 2; ErbB3, v-erb-b2 erythroblastic
leukemia viral oncogene homolog 3; ErbB4, v-erb-a erythroblastic
leukemia viral oncogene homolog 4; FGFR, fibroblast growth factor
receptor; Insulin R, insulin receptor; IGF-1R, insulin-like growth
factor I receptor; Axl, Axl receptor tyrosine kinase; Dtk,
developmental receptor tyrosine kinase; Mer, tyrosine-protein
kinase Mer; HGFR, hepatocyte growth factor receptor; MSPR,
macrophage stimulatory protein receptor; PDGFR, platelet-derived
growth factor receptor; SCFR, stem-cell factor receptor; Flt-3,
Fms-like tyrosine kinase 3; M-CSFR, macrophage colony-stimulating
factor receptor; c-Ret, receptor tyrosine kinase c-ret; ROR,
receptor tyrosine kinase-like orphan receptor; Tie, tyrosine kinase
with immunoglobulin-like and EGF-like domains; TrkA, neurotrophic
tyrosine kinase, receptor, type 1; TrkB, neurotrophic tyrosine
kinase, receptor, type 2; TrkC, neurotrophic tyrosine kinase,
receptor, type 3; VEGFR, vascular endothelial growth factor
receptor; MuSK, muscle, skeletal, receptor tyrosine kinase; Eph,
Eph receptor; PBS, phosphate-buffered saline. |
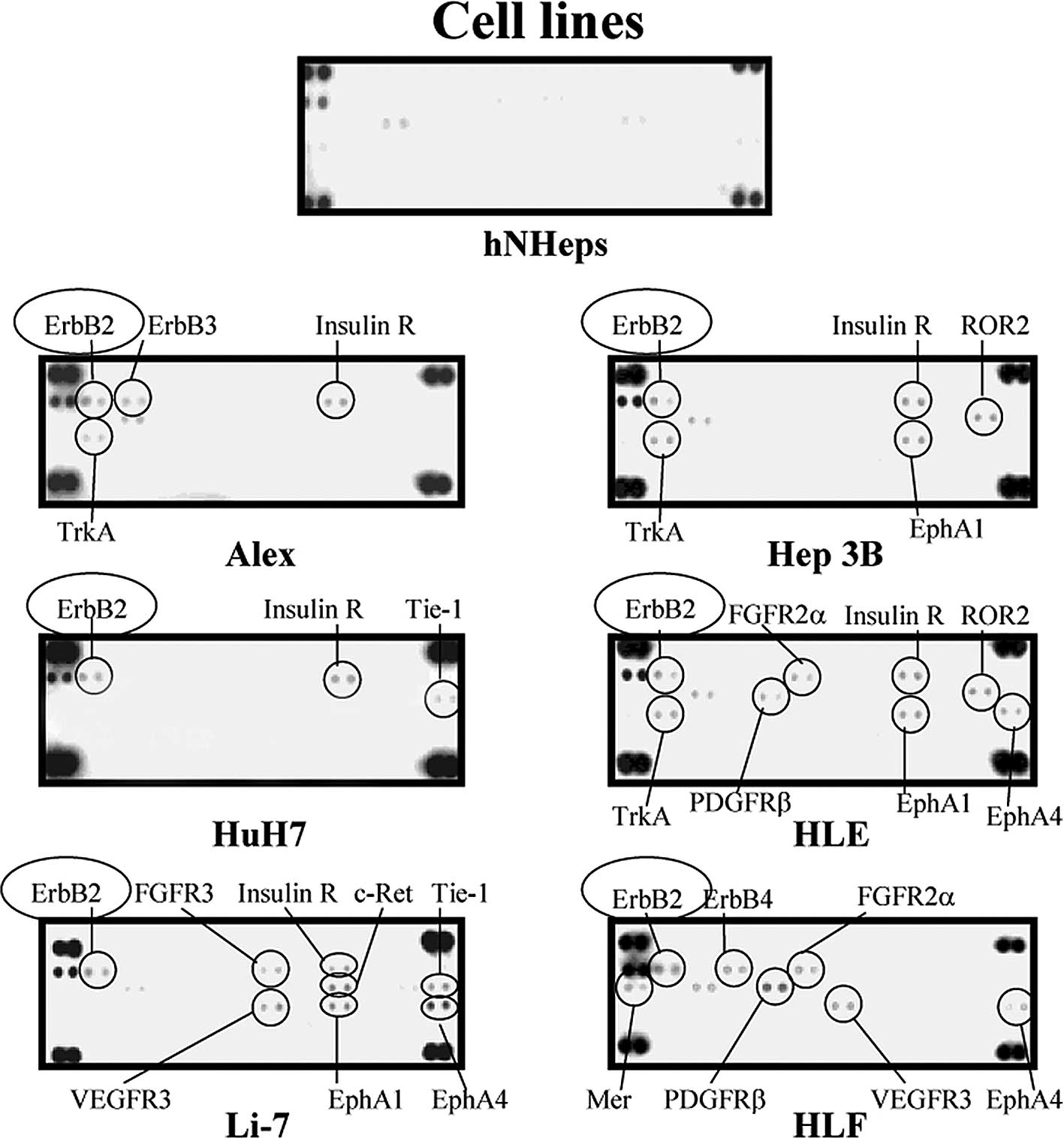 | Figure 2.Representative expression in the
hNHeps and HCC cell lines of various tyrosine kinases, including
Alex, HuH7, Li-7, Hep3B, HLE and HLF. As compared to the hNHeps
cell line, ErbB2, ErbB3, ErbB4, insulin R, ROR2, TrkA, EphA1,
Tie-1, FGFR2α, FGFR3, PDGFRβ, EphA4, c-Ret, Mer and VEGFR3 were
up-regulated in some of the cancer cell lines studied. The
up-regulation of ErbB2 (•) was detected in all of the HCC cell
lines examined in this study, while it was not detected in the
hNHeps cell line. |
In vivo anti-tumor effects of an
ErbB2-targeting drug, trastuzumab
Athymic 8-week-old male BALB/c-nu/nu mice were
implanted subcutaneously with HuH7 and HLF cells. When the animals
developed palpable tumors, they were treated intraperitoneally with
trastuzumab three times a week for 3 weeks. Animals in the control
group received intraperitoneal administration of the vehicle
(PBS).
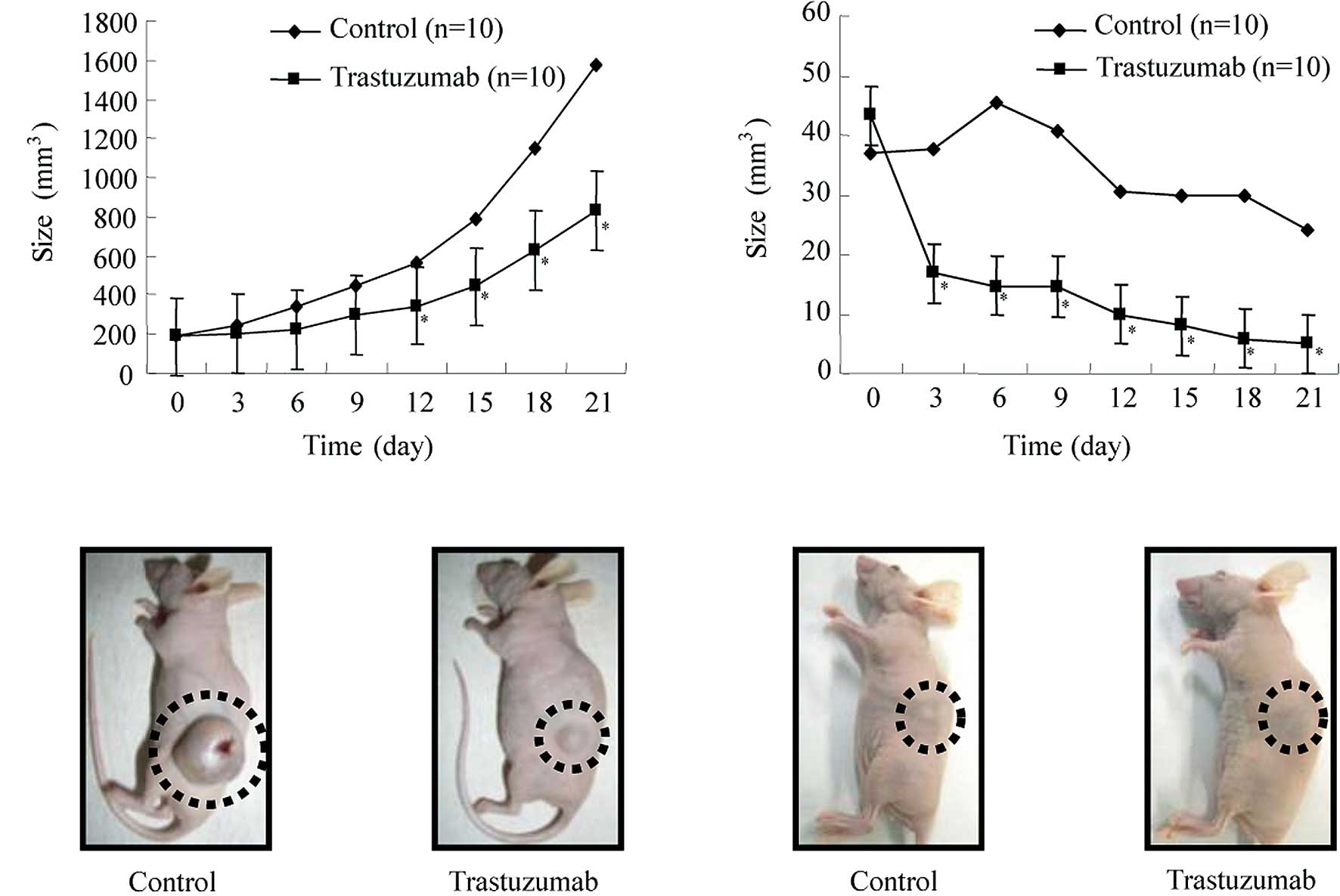
As shown in Fig.
4A, animals in the control group that were implanted
subcutaneously with HuH7 cells developed rapidly growing
subcutaneous HCC while animals in the trastuzumab group exhibited
significantly retarded tumor development compared to the animals in
the control group (Fig. 4A).
Fig. 4B shows representative
images of gross HuH7 tumors from nude mice treated with either
trastuzumab or vehicle.
As shown in Fig.
4C, the tumor size increased until the sixth day after
implantation in the control animals implanted subcutaneously with
HLF cells, but decreased gradually thereafter. By contrast, the
animals in the trastuzumab group exhibited significantly retarded
tumor development, and the tumors in 4/5 animals disappeared.
Fig. 4D shows representative
images of gross HLF tumors from the nude mice treated with either
trastuzumab or the vehicle. The tumors in the trastuzumab group
disappeared completely while the tumors in the control group did
not disappear.
Animals in the trastuzumab group implanted with the
strains HuH7 or HLE did not show substantial changes, while those
in the control group showed disheveled fur and decreased body
weight. The animals remained alive throughout the experiment.
Discussion
The human epidermal growth factor receptor 2 (HER2)
gene, also known as ErbB2, encodes a 185-kDa transmembrane
glycoprotein receptor. This receptor belongs to the ErbB family of
growth factor receptors with intrinsic tyrosine kinase activity,
the membranes of which exist in homodimer and heterodimer forms
when activated (22).
ErbB2 is activated by ligand-binding in its
extracellular region and subsequently the intracellular tyrosine
kinase region is phosphorylated and sends signals to the cell to
regulate numerous crucial processes (23), including growth, differentiation
and carcinogenesis.
Overexpression of ErbB2 is frequently observed in a
variety of tumor types (24–29),
including colon, gastric, non-small cell lung, epithelial ovarian
(30), endometrial carcinoma
(31,32), prostate (33), urinary bladder (34) and uterine papillary serous cancers
(35,36). Although overexpression of ErbB2 is
rarely observed in HCC, mutation of ErbB2 is found in 11% of cases
(37). Therefore, ErbB2 is
targeted using antibodies directed against the extracellular domain
in various types of human cancers, including HCC. These strategies
have been successful in the area of breast cancer (25). However, there is currently no
evidence supporting a potential role for trastuzumab in HCC
comparable to its role in breast cancer. Thus, in the present study
we examined the possibility of an antitumor effect of trastuzumab
in HCC.
In the present study, we first identified the ‘key
RTKs’ associated with HCC by studying 42 activated phospho-RTKs,
using the phospho-RTK array system (Fig. 1). As a result, ErbB2 was found to
be activated in all six of the HCC cell lines examined (Fig. 2), and in all cancerous samples
(Fig. 3). Next, we determined that
the inhibition of ErbB2 by trastuzumab retarded the tumor
development of HCC cells (HuH7 and HLF). These data suggest that an
ErbB2-targeting drug will aid in the treatment of HCC.
Our studies demonstrated that ErbB2, ErbB3, ErbB4,
insulin R, ROR2, TrkA, EphA1, Tie-1, FGFR2α, FGFR3, PDGFRβ, EphA4,
c-Ret, VEGFR3 and Mer were up-regulated in some of the cancer cell
lines studied. Overexpression of ErbB2, ErbB3, TrkA, EphA1, Tie-1,
FGFR2, FGFR3, PDGFR, Ret and VEGFR3 was previously reported in HCC
(38–46). These previous reports support our
results on the various RTKs activated in HCC derived from the
protein array in this study. In summary, our results suggest that
protein arrays aid in studying the expression of activated RTKs in
various tissues, including malignant tissues. Furthermore, these
results suggest that the immunological inhibition of ErbB3, ErbB4,
insulin R, ROR2, TrkA, EphA1, Tie-1, FGFR2α, FGFR3, PDGFRβ, EphA4,
c-Ret, VEGFR3 and Mer in addition to ErbB2 also have an anti-tumor
effect for certain cases of HCC.
In conclusion, the ErbB2-targeting drug trastuzumab
may aid in the treatment of HCC. In addition, the present results
suggest that protein arrays are useful for detecting the expression
of activated RTKs and developing efficient RTK-targeted therapies
for HCC.
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Poon D, Anderson BO, Chen LT, et al:
Management of hepatocellular carcinoma in Asia: consensus statement
from the Asian Oncology Summit 2009. Lancet Oncol. 10:1111–1118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Robinson DR, Wu YM and Lin SF: The protein
tyrosine kinase family of the human genome. Oncogene. 19:5548–5557.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
5.
|
Becker JC, Muller-Tidow C, Serve H,
Domschke W and Pohle T: Role of receptor tyrosine kinases in
gastric cancer: new targets for a selective therapy. World J
Gastroenterol. 12:3297–3305. 2006.PubMed/NCBI
|
|
6.
|
Hubbard SR and Till JH: Protein tyrosine
kinase structure and function. Annu Rev Biochem. 69:373–398. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Tanner M, Hollmen M, Junttila TT, et al:
Amplification of HER-2 in gastric carcinoma: association with
Topoisomerase IIalpha gene amplification, intestinal type, poor
prognosis and sensitivity to trastuzumab. Ann Oncol. 16:273–278.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Svensson S, Jirström K, Rydén L, Roos G,
Emdin S, Ostrowski MC and Landberg G: ERK phosphorylation is linked
to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and
is associated with tamoxifen treatment resistance and small tumours
with good prognosis. Oncogene. 24:4370–4379. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gong J, Morishita A, Kurokohchi K, et al:
Use of protein array to investigate receptor tyrosine kinases
activated in gastric cancer. Int J Oncol. 36:101–106.
2010.PubMed/NCBI
|
|
10.
|
Cao C, Albert JM, Geng L, Ivy PS, Sandler
A, Johnson DH and Lu B: Vascular endothelial growth factor tyrosine
kinase inhibitor AZD2171 and fractionated radiotherapy in mouse
models of lung cancer. Cancer Res. 66:11409–11415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Morishita A, Gong J, Nomura T, et al: The
use of protein array to identify targetable receptor tyrosine
kinases for treatment of human colon cancer. Int J Oncol.
37:829–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhang G, Zhang Q, Zhang Q, et al:
Expression of nucleostemin, epidermal growth factor and epidermal
growth factor receptor in human esophageal squamous cell carcinoma
tissues. J Cancer Res Clin Oncol. 136:587–594. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Masaki T, Tokuda M, Yoshida S, et al:
Comparison study of the expression of myristoylated alanine-rich C
kinase substrate in hepatocellular carcinoma, liver cirrhosis,
chronic hepatitis, and normal liver. Int J Oncol. 26:661–671.
2005.
|
|
14.
|
Yoshida S, Masaki T, Feng H, et al:
Enhanced expression of adaptor molecule p46 Shc in nuclei of
hepatocellular carcinoma cells: study of LEC rats. Int J Oncol.
25:1089–1096. 2004.PubMed/NCBI
|
|
15.
|
Bennasroune A, Gardin A, Aunis D, Crémel G
and Hubert P: Tyrosine kinase receptors as attractive targets of
cancer therapy. Crit Rev Oncol Hematol. 50:23–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Llovet JM, Ricci S, Mazzaferro V, et al:
Sorafenib in advanced hepatocellular carcinoma. N Engl J Med.
359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Desmet VJ, Gerber M, Hoofnagle JH, Manns M
and Scheuer PJ: Classification of chronic hepatitis: diagnosis,
grading and staging. Hepatology. 19:1513–1520. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Yukimasa S, Masaki T, Yoshida S, et al:
Enhanced expression of p46 Shc in the nucleus and p52 Shc in the
cytoplasm of human gastric cancer. Int J Oncol. 26:905–911.
2005.PubMed/NCBI
|
|
19.
|
Mohammad HS, Kurokohchi K, Yoneyama H, et
al: Annexin A2 expression and phosphorylation are up-regulated in
hepatocellular carcinoma. Int J Oncol. 33:1157–1163.
2008.PubMed/NCBI
|
|
20.
|
Nonomura T, Masaki T, Morishita A, et al:
Identification of c-Yes expression in the nuclei of hepatocellular
carcinoma cells: involvement in the early stages of
hepatocarcinogenesis. Int J Oncol. 30:105–111. 2007.PubMed/NCBI
|
|
21.
|
D'Incalci M, Colombo T, Ubezio P, et al:
The combination of yondelis and cisplatin is synergistic against
human tumor xenografts. Eur J Cancer. 39:1920–1926. 2003.PubMed/NCBI
|
|
22.
|
Bazley LA and Gullick WJ: The epidermal
growth factor receptor family. Endocr Relat Cancer. 12:17–27. 2005.
View Article : Google Scholar
|
|
23.
|
Schlessinger J: Ligand-induced,
receptor-mediated dimerization and activation of EGF receptor.
Cell. 110:669–672. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Hermanova M, Lukas Z, Nenutil R, et al:
Amplification and overexpression of HER-2/neu in invasive ductal
carcinomas of the pancreas and pancreatic intraepithelial neoplasms
and the relationship to the expression of p21 (WAF1/CIP1).
Neoplasma. 51:77–83. 2004.PubMed/NCBI
|
|
25.
|
Friess T, Scheuer W and Hasmann M:
Erlotinib antitumor activity in non-small cell lung cancer models
is independent of HER1 and HER2 overexpression. Anticancer Res.
26:3505–3512. 2006.PubMed/NCBI
|
|
26.
|
Hatake K, Tokudome N and Ito Y:
Tanstuzumab treatment for breast cancer. Intern Med. 46:149–150.
2007. View Article : Google Scholar
|
|
27.
|
Larbouret C, Robert B, Navarro-Teulon I,
et al: In vivo therapeutic synergism of anti-epidermal growth
factor receptor and anti-HER2 monoclonal antibodies against
pancreatic carcinomas. Clin Cancer Res. 13:3356–3362. 2007.
View Article : Google Scholar
|
|
28.
|
Lara PN Jr, Chee KG, Longmate J, et al:
Trastuzumab plus docetaxel in HER-2/neu-positive prostate
carcinoma: final results from the California Cancer Consortium
Screening and Phase II Trial. Cancer. 100:2125–2131. 2004.
View Article : Google Scholar
|
|
29.
|
Shun CT, Wu MS, Lin JT, et al:
Relationship of p53 and c-erbB-2 expression to histopathological
features, Helicobacter pylori infection and prognosis in
gastric cancer. Hepatogastroenterology. 44:604–609. 1997.PubMed/NCBI
|
|
30.
|
Berchuck A, Kamel A, Whitaker R, et al:
Overexpression of her2/neu is associated with poor survival in
advanced epithelial ovarian cancer. Cancer Res. 50:4087–4091.
1990.PubMed/NCBI
|
|
31.
|
Grushko TA, Filiaci VL, Mundt AJ,
Ridderstråle K, Olopade OI and Fleming GF; Gynecologic Oncology
Group: An exploratory analysis of HER-2 amplification and
overexpression in advanced endometrial carcinoma: a Gynecologic
Oncology Group study. Gynecol Oncol. 108:3–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Saffari B, Jones LA, el-Naggar A, Felix
JC, George J and Press MF: Amplification and overexpression of
HER-2/neu (c-erbB2) in endometrial cancers: correlation with
overall survival. Cancer Res. 55:5693–5698. 1995.PubMed/NCBI
|
|
33.
|
Ross JS, Sheehan C, Hayner-Buchan AM, et
al: HER-2/neu gene amplification status in prostate cancer by
fluorescence in situ hybridization. Hum Pathol. 28:827–833. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Sato K, Moriyama M, Mori S, et al: An
immunohistologic evaluation of C-erbB-2 gene product in patients
with urinary bladder carcinoma. Cancer. 70:2493–2498. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Slomovitz BM, Broaddus RR, Burke TW, et
al: Her-2/neu overexpression and amplification in uterine papillary
serous carcinoma. J Clin Oncol. 22:3126–3132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Villella JA, Cohen S, Smith DH, Hibshoosh
H and Hershman D: HER-2/neu overexpression in uterine papillary
serous cancers and its possible therapeutic implications. Int J
Gynecol Cancer. 16:1897–1902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Bekaii-Saab T, Williams N, Plass C, Calero
MV and Eng C: A novel mutation in the tyrosine kinase domain of
ERBB2 in hepatocellular carcinoma. BMC Cancer. 6:2782006.
View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Fuchs BC, Fujii T, Dorfman JD, et al:
Epithelial-to-mesenchymal transition and integrin-linked kinase
mediate sensitivity to epidermal growth factor receptor inhibition
in human hepatoma cells. Cancer Res. 68:2391–2399. 2008. View Article : Google Scholar
|
|
39.
|
Neo SY, Leow CK, Vega VB, et al:
Identification of discriminators of hepatoma by gene expression
profiling using a minimal dataset approach. Hepatology. 39:944–953.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Rasi G, Serafino A, Bellis L, et al: Nerve
growth factor involvement in liver cirrhosis and hepatocellular
carcinoma. World J Gastroenterol. 13:4986–4995. 2007.PubMed/NCBI
|
|
41.
|
Chen G, Wang Y, Zhou M, Shi H, Yu Z, Zhu Y
and Yu F: EphA1 receptor silencing by small interfering RNA has
antiangiogenic and antitumor efficacy in hepatocellular carcinoma.
Oncol Rep. 23:563–570. 2010.PubMed/NCBI
|
|
42.
|
Dhar DK, Naora H, Yamanoi A, Ono T, Kohno
H, Otani H and Nagasue N: Requisite role of VEGF receptors in
angiogenesis of hepatocellular carcinoma: a comparison with
angiopoietin/Tie pathway. Anticancer Res. 22:379–386.
2002.PubMed/NCBI
|
|
43.
|
Harimoto N, Taguchi K, Shirabe K, et al:
The significance of fibroblast growth factor receptor 2 expression
in differentiation of hepatocellular carcinoma. Oncology.
78:361–368. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Qiu WH, Zhou BS, Chu PG, et al:
Over-expression of fibroblast growth factor receptor 3 in human
hepatocellular carcinoma. World J Gastroenterol. 11:5266–5272.
2005.PubMed/NCBI
|
|
45.
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Musholt PB, Imkamp F, von Wasielewski R,
Schmid KW and Musholt TJ: RET rearrangements in archival oxyphilic
thyroid tumors: new insights in tumorigenesis and classification of
Hürthle cell carcinomas? Surgery. 134:881–889. 2003.PubMed/NCBI
|