Introduction
Breast cancer is one of the most common neoplasms in
women and is a leading cause of cancer-related mortality, resulting
in approximately 500,000 deaths worldwide annually (1). Surgery, radiotherapy and chemotherapy
are widely used treatment methods for breast cancer. Despite
significant improvements in cancer diagnosis and therapy, breast
cancer remains a challenging disease to treat, and approximately
one quarter of breast cancer patients succumb to the disease. Thus,
further investigations into the mechanisms of this disease are
required to aid in the development of novel treatments (2).
The formation of new blood vessels by the extension
or elaboration of existing vasculature is called angiogenesis. This
mechanism plays a central role in both local tumor growth and
distant metastasis in breast cancer (3,4).
Angiogenesis is regulated by angiogenic and anti-angiogenic
factors, and the expression levels of angiogenic factors reflect
the aggressiveness of tumor cells. Since the discovery of
angiogenic inhibitors, the inhibition of tumor angiogenesis has
become a promising strategy for the treatment of cancer, and
thousands of patients have received anti-angiogenic therapy to
date. Unfortunately, despite their theoretical effects,
anti-angiogenic treatments have not proven beneficial in terms of
long-term survival. Thus, there is clear need for a new
comprehensive treatment strategy combining anti-angiogenic agents
with conventional treatments, such as chemotherapy or radiotherapy,
in the treatment of cancer (5–7).
Thalidomide (a derivative of glutamic acid that
exists as an equal mixture of its enantiomers) was introduced in
Europe for the treatment of morning sickness in pregnant women.
However, due to its teratogenicity, it was withdrawn from the
market in the late 1960s (8). Many
years later, D’Amato et al revealed that thalidomide
inhibits limb development by suppressing angiogenesis via the
inhibition of basic fibroblast growth factor (bFGF) and/or vascular
endothelial growth factor (VEGF) (8–10).
Today, thalidomide is one of the most well-known teratogens in
medical history and is clinically recognized as an efficient
therapeutic agent for the treatment of various types of cancer;
however, the anti-angiogenic mechanism of thalidomide remains
unknown.
Certain studies conducted in pre-clinical tumor
models have documented the advantages of combining cytotoxic
chemotherapeutic agents with radiation therapy. Over the last few
years, significant survival benefits for breast cancer patients
have been achieved with the use of postoperative systemic therapies
and radiotherapy (11,12). Currently, the majority of early
breast cancer patients are routinely managed with breast-conserving
surgery followed by radiation therapy and adjuvant systemic
therapies, including chemotherapy and hormone therapy. Despite the
extensive use of radiotherapy and systemic treatments, the optimal
strategy for their use in combination remains unclear, and their
mechanisms are unkown (13,14).
Recently, it was confirmed that neuroimmune
mechanisms also play a role in the defense against cancer, as well
as in its progression. The involvement of the nervous system in the
modulation of cancer development and its progression is indicated
by clinical and experimental data from various studies. Several
retrospective studies of patients who have undergone vagotomy
suggest that the loss of various sensory nerve mediators, such as
substance P (SP), leads to an increased risk of cancer development
(15).
The angiogenesis-related peptide SP is a member of
the tachykinin family encoded by the preprotachykinin A (PPT-A) l
gene (16). SP is generally
accepted to be the major neuropeptide involved in neurogenic
inflammation, and is the most important neuropeptide in cancer. The
PPT-A gene is expressed in many other cell types, such as
monocytes, human fibroblasts, keratinocytes, lymphocytes, platelets
and tumor cells. SP also induces angiogenesis and local
inflammatory responses, which may increase cancer progression and
metastases (17). SP seems to have
a bidirectional effect on inflammation, tumor growth and
carcinogenesis. These bidirectional effects on inflammation and
carcinogenesis may be due to the counter-balancing effects of SP
fragments and the intact peptide, since the intact peptide is
tumorigenic and induces inflammation, whereas fragments produced by
peptidases are anti-tumorigenic and anti-angiogenic (18).
To the best of our knowledge, the effect of
thalidomide on SP has yet to be investigated. The close interaction
between immune system involvement in the development and
progression of cancer and the importance of combined therapy
requires further research. Thus, whether thalidomide, either alone
or in combination with radiotherapy, is capable of altering SP
levels in breast cancer cells warrants investigation. Therefore,
the present study aimed first to determine the cytotoxic effects of
thalidomide, radiotherapy and their combination on the 4T1 cell
line and its metastatic derivative line, 4THMpc, and second, to
determine the changes in SP expression induced by the
treatments.
Materials and methods
Thalidomide
Water-soluble thalidomide (Thal) was purchased from
A.G. Scientific (cat. no. T 1020). Thal (100 μg) was dissolved in
sterile distilled water and aliquoted into standard Eppendorf tubes
at quantities of 500 μl for daily assay. These aliquots were stored
at −70˚C until use.
Cell lines and in vitro cell culture
conditions
4T1 breast cancer cells and 4THM (4T1 Heart
Metastases Post Capsaicin) cells, a cell line obtained from
orthotopically transplanted 4T1 breast cancer cells, were used in
this study. The cell lines were a kind gift from Dr Nuray Erin
(Akdeniz University, Faculty of Medicine, Antalya, Turkey). Cells
were grown as monolayer adherent cultures in plastic cell culture
petri dishes (BD, Bedford, MA, USA) in DMEM-F12 (Biochrom, Germany)
supplemented with 5% fetal bovine serum (FBS), 2 mM L-glutamine, 1
mM sodium pyruvate and 0.02 mM non-essential amino acids. The cell
lines were maintained at 37˚C in a humidified atmosphere of 5%
CO2. All cell lines used in this study were tested and
shown to be free of mycoplasma contamination.
Thalidomide treatment
To examine the effects of Thal, radiation therapy
(RT) and the combination therapy (Thal + RT) on cell growth in
vitro, initial experiments were performed to determine the
optimal cell treatment conditions.
To determine the optimal cell number, 4T1 and 4THMp
cells were plated at a density of 1,000 to 20,000 cells/wells.
Thirty-six hours after plating, the cells were treated with Thal at
concentrations of 2.5, 5, 10, 20 and 40 μg/ml. Four hours after
Thal treatment, the cells were irradiated with a single dose of
ionizing radiation. After irradiation, the cells were incubated for
24, 48 or 72 h, following which cell growth was determined. The
optimal cell density was found to be 5,000 cells/well.
Radiation therapy
To determine the appropriate dose of RT, initial
experiments were performed using various doses of ionizing
radiation. Cells were seeded at 5,000 cells/well in a 96-well
plate. After 36 h, the medium was replaced with fresh medium
containing 1% serum, and RT was applied at doses of 5 to 45 Gy.
Each cell plate (2 cm thick) was irradiated in a Co-60 teletherapy
unit at a distance of 100 cm. In order to achieve a homogeneous
dose (+2.5%) at the cell plate, the plate was embedded in water
equivalent bolus material and a 0.5-cm-thick bolus material was
additionally placed on the cover. Cell viability was measured 24,
48 or 72 h after RT. The optimal dose of irradiation was found to
be 45 Gy at 1.5 cm (in the middle of the plate), carried out at a
dose rate of ∼145 cGy/min.
Determination of cell viability
Cell growth was determined after 72 h of incubation
in three sets of experiments: cells treated with Thal alone at a
concentration of 40 μg/ml, cells treated with RT at a dose of 45
Gy, and cells treated with a combination therapy of 40 μg/ml Thal +
45 Gy ionizing radiation, applied 4 h after Thal treatment. As a
negative control for all assays, 4T1 and 4THMp cells were treated
with conditioned medium containing 1% serum.
The proliferation of the 4T1 and 4THMp cells was
first determined using the tetrazolium compound
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium,
inner salt (MTS) according to the manufacturer’s instructions (Cell
Titer 96 Aqueous One Solution Cell Proliferation Assay; Promega
Corp., Madison, WI, USA). Formasan formation was quantified at an
optical density (OD) of 490 nm and compared between groups to
determine cell growth and viability. To calculate the percentage of
growth inhibition, the following formula was used: growth
inhibition (%) = [(mean OD value of the control group - mean OD
value of the treatment group)/mean OD value of the control group] ×
100%.
To verify the results of the MTS assay, the
Live/Dead viability/cytotoxicity kit for mammalian cells
(Invitrogen, Eugene, OR, USA) was used. According to the
manufacturer’s protocol, the 4T1 and 4THMp cells were dually
stained with two fluorescently labeled probes that enable the
simultaneous determination of live and dead cells in a sample:
Calcein AM, which stains live cells since it fluoresces only when
cleaved by intracellular esterases, and EthD-1, which identifies
dead/dying cells since it exclusively enters cells with disrupted
membranes. Fluorescence intensity was measured on a Victor™ X2
Multilabel Plate Reader (PerkinElmer Inc., Waltham, MA, USA) at
excitation wavelengths of 560 and 535 nm and emission wavelengths
of 645 and 610 nm, respective to each reagent dye. Experiments were
repeated five times in four replicative wells. Data from four
highly reproducible independent experiments were pooled and used
for statistical comparisons using the Student’s t-test.
The number of dead cells as well as the total cell
number in the 4T1 and 4THMp cell lines were further examined using
the trypan blue (0.4% trypan blue in HBSS) exclusion assay. Images
were captured under a phase contrast microscope. The percentage of
decrease in cell survival was calculated according to the results
of four independent highly reproducible experiments. The following
formulas were used: for RT, 100 - [no. of live cells in treated (45
Gy RT) RT group/no. of live cells in the untreated control group] ×
100; for Thal, 100 -[no. of live cells in the treated (40 μg/ml
Thal) group/no.of live cells in untreated-control group] × 100; for
the combination therapy, 100 - (no of live cells in the treated (40
μg/ml Thal and 45 Gy RT) RT group/no. of live cells in the
untreated RT group] × 100.
Determination of SP levels
Cells were seeded in 6-well plates at a density of
200,000 cells/well then, 36 h after plating, the medium was
replaced with 40 μg/ml Thal in medium containing 1% serum. After 4
h, one group of the plates was irradiated in serum-free medium.
Conditioned medium was collected 24 h after irradiation, and SP was
extracted using the Oasis Extraction Column (Waters Corp., Milford,
MA, USA). SP extractions from the cell lysates were examined as
previously described without the use of column extraction (19). Briefly, 2×106 purified
4T1 and 4THMpc cell pellets were lysed by incubation in 2%
(vol/vol) glacial acetic acid at 95˚C for 45 min. Supernatants were
dried in a speed vacuum and re-suspended in the sample buffer
provided in the SP EIA kit. The SP concentration in both the
conditioned medium and cell lysates was measured in duplicate using
a sensitive (20 pg/ml detection limit) competitive EIA kit
according to the manufacturer’s instructions (Substance P Enzyme
Immunoassay kit; cat. no. 583751; Cayman, Ann Arbor, MI, USA).
Absorbances were read at 420 nm with a microplate reader (Model
450; Bio-Rad, Richmond, CA, USA).
Immunoprecipitation and Western
blotting
To ascertain whether alterations in the SP levels of
4T1 and 4THMpc cells were due to changes in SP content,
immunoprecipitation and Western blotting were performed as
previously described (20).
Protein concentrations were determined using the BCA Protein Assay
(Pierce, Rockford, IL, USA). The conditioned media were
concentrated with an Oasis Extraction Column (Waters Corp.) for
immunoprecipitation. Briefly, equivalent protein from each sample
was incubated at 4˚C overnight with anti-SP (1:10,000) Control
samples were incubated with non-immune, species-specific IgG.
Immune complexes were selected by incubation at 4˚C for 6 h with
protein A-Sepharose CL 4B (Sigma). Subsequently, protein
A-Sepharose was centrifuged at 4˚C for 30 min at 10,000 × g.
Pellets were washed with PBS, resuspended in sample buffer, and
loaded onto a sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) gel, and then transferred to a
polyvinylidene difluoride membrane (Hybond-P; Amersham Pharmacia
Biotech, Piscataway, NJ, USA) using a semi-dry transfer apparatus.
Membranes were blocked for 2 h in 5% skimmed milk in PBS containing
0.05% Tween-20, and were then incubated overnight at 4˚C with an SP
goat polyclonal antibody (1:200, sc-14104; Santa Cruz). The blots
were then incubated with an HRP-conjugated secondary antibody
(1:5,000 sc-2020; Santa Cruz) and detected using ECL Western
blotting detection reagent (ECL Plus kit; Amersham Biosciences).
The Ultra Low Range molecular weight marker (M3546; Sigma Chemical
Co., St. Louis, MO, USA) was used to determine the molecular
weights of the visualized bands. Positive control lanes contained
SP (Sigma; cat. no., 6883).
Statistical analysis
All data are presented as the mean ± standard
deviation (SD). Analysis was performed using professional
statistics software (Graph Pad Software, San Diego, CA, USA). ANOVA
with Dunnett’s Multiple Comparison post-test and t-tests were used
for intergroup comparisons. Statistical analyses of SP levels were
performed using either ANOVA followed by the Tukey-Kramer multiple
comparison test or the Student’s paired t-test for the percentage
of alteration values. Graphs were drawn using Sigma Plot version
10.0 (SPSS Inc., USA) and CorelDRAW version X4 (Corel, Co.,
Minneapolis, MN, USA) software. p<0.05 was considered
statistically significant.
Results
In vitro cytotoxic effects of Thal, RT
and their combination on 4T1 and 4THMpc cell lines
To determine the effects of Thal, RT and the
combination therapy (Thal + RT) on cell growth in vitro,
initial experiments were conducted to determine the optimal cell
density and irradiation dose (data not shown). The optimal cell
density was found to be 5,000 cells/ well. At this density, none of
the tested irradiation doses up to 45 Gy were found to inhibit cell
proliferation or to induce cell death. However, the 45-Gy dose of
irradiation was determined to have a cytotoxic effect on the
cells.
The cytotoxic effects of RT alone, (Fig. 1A) Thal alone (Fig. 1B) and of the combination therapy
(Fig. 1C) were evaluated and
compared (Fig. 1D) using the MTS
assay. The results indicated that after 72 h of treatment, RT at 45
Gy caused a 19.2 and 23.31 % inhibition of growth of the 4T1 and
4THMpc cells, respectively, suggesting that the 4THMpc cells were
more resistant to RT than the 4T1 cells. The number of untreated
control cells indicated that the 4THMpc cells proliferated much
faster than the 4T1 cells. These results were confirmed by the
Live/Dead cytotoxicity assay.
To further verify these results, a trypan blue
exclusion assay was performed on the 4T1 and 4THMpc cell lines. In
images captured under a phase contrast microscope 72 h after
treatment (Fig. 2), cell death was
evident, with an abundance of seemingly condensed apoptotic cells
and cell fragments in the Thal- and RT-treated groups.
The results regarding the percentage of decrease in
cell survival calculated for each experiment using the Live/Dead
cell viability assay and trypan blue exclusion test are summarized
in Tables I and II.
 | Table I.Percentage of decrease in cell
survival according to the Live-Dead cell viability assay. |
Table I.
Percentage of decrease in cell
survival according to the Live-Dead cell viability assay.
| RT | Thal | Combination
therapy |
|---|
|
|
|
|---|
| LC (%) | DC (%) | LC (%) | DC (%) | LC (%) | DC (%) |
|---|
| 4T1 | 71.50±10.02 | 25.34±11.12 | 43.25±7.71 | 51.63±10.34 | 37.25±11.06 | 52.75±9.56 |
| 4THMpc | 63.01±11.22 | 45.47±10.73 | 45.72±7.91 | 50.07±9.35 | 34.92±10.66 | 61.27±11.02 |
 | Table II.Percentage of decrease in cell
survival according to the trypan blue exclusion assay. |
Table II.
Percentage of decrease in cell
survival according to the trypan blue exclusion assay.
| RT | Thal | Combination
therapy |
|---|
| 4T1 | 19.20±3.61 | 34.1±8.73 | 47.9±8.95 |
| 4THMpc | 23.31±7.49 | 52.6±10.31 | 62.03±11.57 |
SP levels in the media and cell
lysates
SP levels were examined in the media and cell
lysates from 4T1 and 4THMpc cells 72 h after treatment with Thal
alone, RT alone or the combination therapy, at multiple stages.
First, time-dependent amounts of basal SP levels were determined in
the control cells and also in 2% acetic acid-administered cells,
since SP was extracted by an acetic acid extraction method. Second,
each sample was divided in two equal amounts; one sample was both
acid and column extracted, whereas the other was only acid
extracted. Third, a two-step extraction was used in order to
measure SP levels. Finally, an experiment was performed in which
the extraction step with either Oasis cartridges or acetic acid was
omitted. The supernatants obtained by following any of the steps
above were evaporated in a vacuum for SP ELISA. It was not possible
to detect SP levels in any repeated experiments using Oasis
cartridges or the two-step acetic acid extraction method in order
to extract SP from the cell lysates. It is likely that both of
these extraction procedures are responsible for the loss of
measurable SP in the cell lysates, but not in the conditioned
media. Thus, the cartridges and the two-step acetic acid extraction
steps were used only in order to extract SP in the conditioned
media.
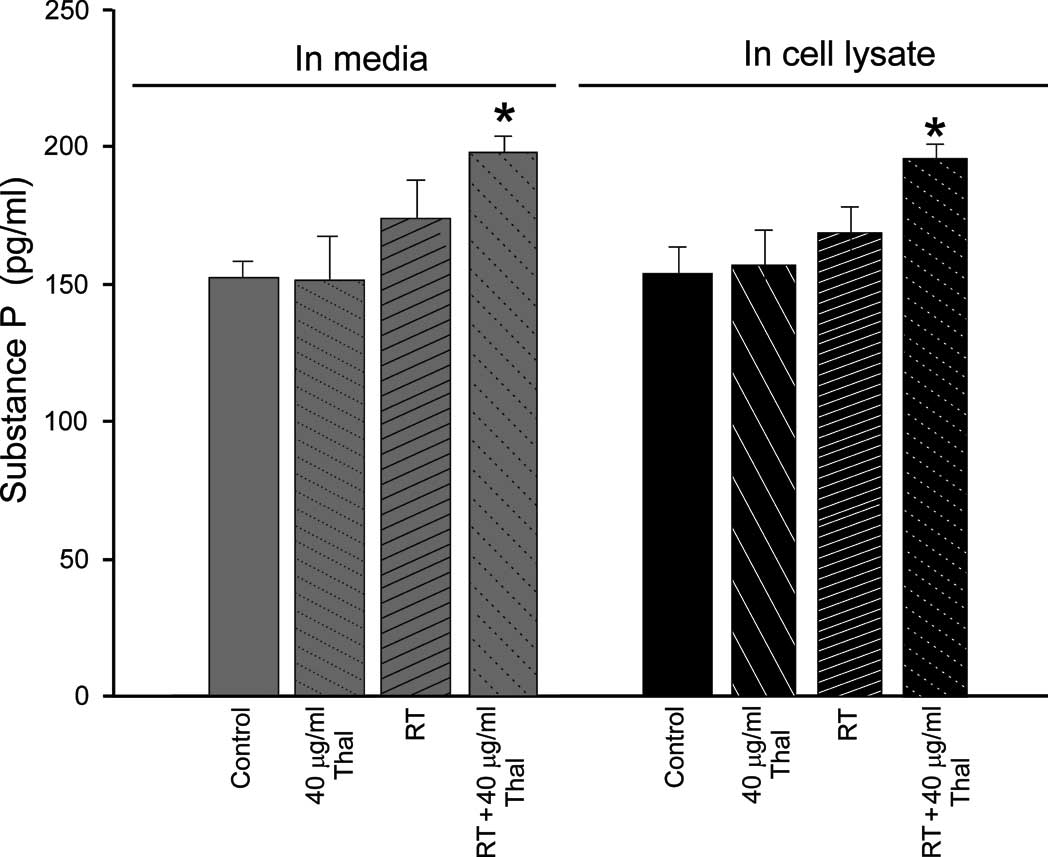
In the 4T1 cell line, no significant differences
were noted between the basal SP levels in the cell lysate
(150.28±10.2 pg/ ml) or the conditioned media (151.12±11.8 pg/ml).
When the cells were treated with 40 μg/ml Thal, no significant
differences were noted in the cell lysates (150.23±12.9 pg/ml) or
in the conditioned media (149.02±13.1 pg/ml) (p<0.05 compared to
the control). By contrast, when 45 Gy RT was applied alone, the
amount of SP in the 4T1 cells was increased in the 4T1 cell lysates
(170.12±10.1 pg/ml) and in the media (60.33±11.8 pg/ ml) (p<0.01
compared to the control). Notably, the combined therapy (40 μg/ml
Thal + 45 Gy RT) significantly increased SP concentrations in both
the conditioned media (190.17±9.9 pg/ml) and in the cell lysates
(195.28±10.48 pg/ml) (p<0.001 compared to the control). The
effects of the treatments on SP levels in the 4T1 cells are
summarized in Fig. 3A.
When the results obtained in the 4T1 cells were
compared with those obtained in the 4THMpc cells, the 4THMpc cells
were found to exhibit a significant increase in SP levels in both
the conditioned media and the cell lysates. The only distinct
finding noted in the experiments using the 4THMpc cells was that
the level of SP in the media decreased in only the Thal-treated
cells. According to the results, Thal alone had no effect on the
level of SP in the media and cell lysates, as compared to the
control. However, the SP level in the media and cell lysates was
significantly increased by the combination therapy. The effects of
the treatments on SP levels in the 4THMpc cells are summarized in
Fig. 3B. The results clearly
indicate that the combination treatment enhanced the effects of
Thal on SP levels in both the media and cell lysates.
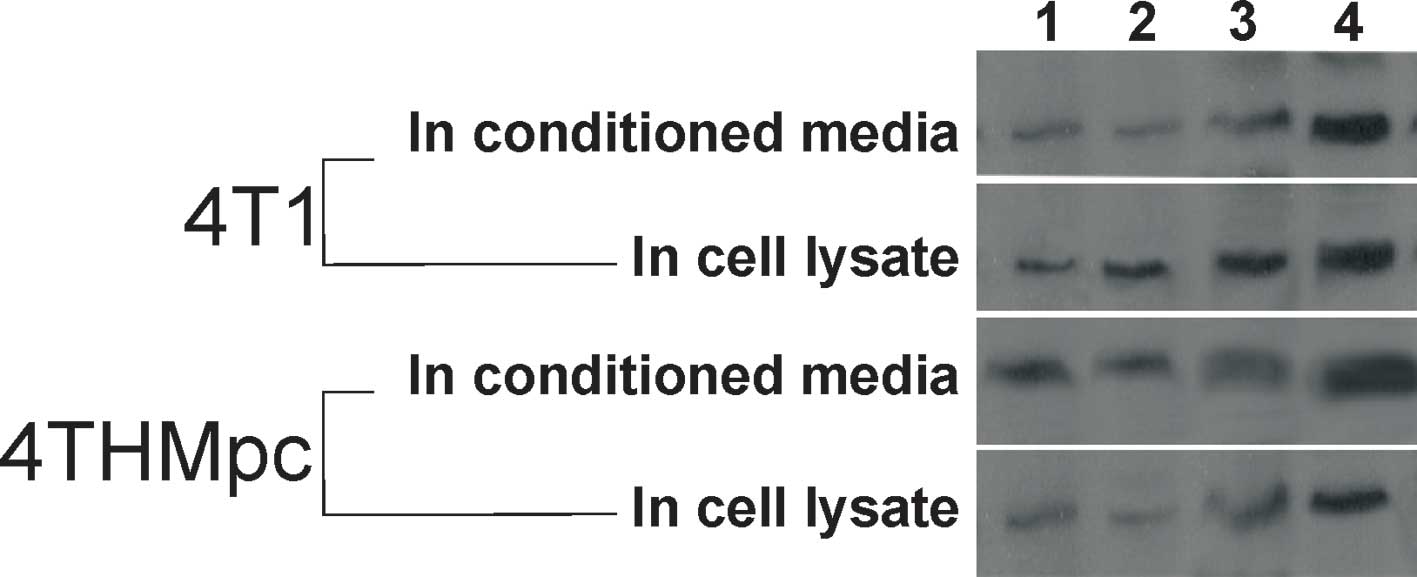
To verify the results of SP ELISA, changes in SP
content were determined by standard Western blotting. As shown in
Fig. 4, the thickness or thinness
of the bands depending on the amount of SP correlated with the SP
ELISA findings.
Discussion
After noting that thalidomide had a teratogenic
effect in pregnant women, it was discovered that the drug destroys
blood vessels in the fetus. Based on this property, thalidomide was
included in the category of anti-angiogenic drugs, and its
potential anti-angiogenic and anti-tumoral properties have been
demonstrated in animal models. Today, thalidomide is successfully
used in the treatment of multiple myeloma, prostate and kidney
cancer, and research on the potential use of thalidomide in other
types of cancer is currently being carried out (21,22).
Breast cancer is one of the most important global
health concerns, with over 1,500,000 new cases diagnosed and over
400,000 deaths occurring annually. Thalidomide alone is not
effective in the treatment of metastasic breast cancer, and must be
combined with another cytotoxic drug or an alternative therapy
(23). While thalidomide is found
to be cytotoxic in many cancer cell lines, the growth inhibition
effect of thalidomide depends not only on the doses of the drug,
but also on the cell type (24).
We first ascertained the cytotoxic dose of
thalidomide in 4T1 and 4THMpc mouse breast cancer cell lines. The
cytotoxic effects of different doses of thalidomide were evaluated
and, according to our test results, thalidomide alone at 40 μg/ ml
was found to have cytotoxic effects on 4T1 and 4THMpc mouse breast
cancer cell lines in vitro. In the same experiment, it was
found that 4THMpc, a more aggresive form of the cancer, exhibited a
more rapid growth than 4T1 cells compared to the control groups.
According to their growth rate, thalidomide alone at 40 μg/ml was
more effective in 4THMpc cells. At the end of the 72-h incubation
period, thalidomide alone, at its cytotoxic dose caused a 34.1±8.73
and 52.6±10.31% inhibition in cell growth in the 4T1 and 4THMpc
cells, respectively.
Combining a cytotoxic agent with radiotherapy is the
focus of continuing interest to oncologists, and radiotherapy is
the most popular therapy, particularly in the treatment of solid
tumors. Over the last few years, positive results in breast cancer
outcome have been demonstrated with the use of postoperative
systemic therapies and radiotherapy. Although these two modalities
have been extensively used, the exact mechanisms of their effect
remains unknown.
Thus, in the second part of our study, we determined
the effects of combination therapy (Thal + RT) on the growth of 4T1
and 4THMpc cells, with the aim of ascertaining the effective
irradiation dose on these cell lines. The cells were treated with
various doses of irradiation (5, 10 and 20 Gy) alone, and 45 Gy
radiation was found to be effective. Mouse breast cancer cells were
resistant to both low and conceivable doses of RT. The growth of
the 4T1 and 4THMpc cells was not significantly inhibited by low RT
doses of 5, 10 and 20 Gy. Thus, we used a relatively high dose of
radiation (45 Gy). This high dose 45-Gy radiotherapy alone caused a
19.2±3.61 and 23.31±7.49% inhibition of the growth of 4T1 and
4THMpc cells, respectively. There are no studies in the literature
on the effects of irradiation on these cell lines, therefore this
was an initial study showing that irradiation has an inhibitory
effect on mouse breast cancer cell lines.
To determine the time factor in combination therapy,
another set of experiments was designed. Cells were divided into
two groups. One group was first treated with Thal followed by RT
administration and the other was first treated with RT followed by
Thal administration at different time points. The significant
results from the different independent trials indicated that 4T1
and 4THMpc cells should be irradiated after Thal treatment, not
before nor immediately after. According to our results, 4 h were
sufficient for 4T1 and 4THMpc cells to potentiate the efficacy of
each treatment.
After determining the effective cytotoxic doses of
both Thal and RT alone, and also the suitable treatment times,
another set of experiments was designed to evaluate the effects of
combined therapy on 4T1 and 4THMpc cells. According to our results,
Thal (40 μg/ml) and RT (45 Gy) combination therapy most effectively
inhibited the growth of these cells.
It is known that irradiation treatment increases the
expression of angiogenic factors (24,25).
Chan et al revealed that, when combined with anti-angiogenic
molecules, the potential effects of radiation increased, as
anti-angiogenic therapy removes pro-angiogenic molecules (26). Thus, to analyze the mechanism of
the increased anti-proliferative effects of Thal and RT, we
evaluated the changes in the level of the pro-angiogenic peptite,
SP. SP is a mitogenic peptide found to induce angiogenesis and
tumor cell proliferation (27).
Aalto et al reported that RT induces SP expression in human
breast cancer cell lines (28).
Our results confirm this finding: 45 Gy RT alone caused a 13.3 and
6.6% increase in the amount of SP in the media and cell lysate of
4T1 cells, respectively. The specific increase in the levels of SP
in response to RT may indicate its importance in the growth of
breast cancer cells, and may also explain their metastatic
potential after RT. The amount of SP was significantly increased
when RT was combined with Thal. Combination therapy caused a 30 and
26.6% increase in the amount of SP in the media and cell lysate of
the 4T1 cells, respectively. Similar results were obtained in the
4THMpc cell line. According to our results, 45 Gy RT alone caused a
16.6 and 15.8% increase in the amount of SP in the media and cell
lysate of the 4THMpc cells, respectively. Combination therapy
caused a 27.27 and 18.75% increase in the amount of SP in the media
and cell lysate of the 4THMpc cells, respectively. In addition,
Thal alone had no effect on the level of SP in the media, and
caused only a 3.3% increase in SP in the cell lysates of 4T1 cells.
In the 4THMpc cells, Thal caused an 18.18 and 21.87% decrease in SP
in the media and the cell lysates, respectively. The present study
demonstrates that high dose irradiation (45 Gy) has systemic side
effects, such as an alteration in the amount of neuropeptide (SP)
content in breast cancer cells. We suggest that the combination of
Thal and RT increases the level of SP, which may potentiate the
tumor growth of metastatic breast cancer cells.
In conclusion, this study indicates that thalidomide
exhibits anti-proliferative effects against breast cancer cells
in vitro and potentiates the anti-tumoral effects of
radiotherapy. The level of SP observed after radiation therapy
alone was increased by the combination of Thal and RT, and this may
limit the use of this combination therapy in metastatic breast
cancer patients. These data may be helpful in the design of future
clinical trials to potentiate the use of anti-angiogenic treatments
in combination with other treatment modalities.
Acknowledgements
This study was supported by The
Scientific and Technological Research Council of Turkey
(TÜBİTAK-project no. 104 T 204). We are indebted to all the
employees of the Akdeniz University Scientific Research Project
Unit.
References
|
1.
|
Polyak K: On the birth of breast cancer.
Biochim Biophys Acta. 1552:1–13. 2001.PubMed/NCBI
|
|
2.
|
Goss PE: Breast cancer prevention –
clinical trials strategies involving aromatase inhibitors. J
Steroid Biochem Mol Biol. 86:487–493. 2003.
|
|
3.
|
Dong X, Han ZC and Yang R: Angiogenesis
and antiangiogenic therapy in hematologic malignancies. Crit Rev
Oncol Hematol. 62:105–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schneider BP and Miller KD: Angiogenesis
of breast cancer. J Clin Oncol. 23:1782–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Nishida N, Yaho H, Nishida T, Kamura T and
Kojiro M: Angiogenesis in cancer. Vasc Health Risk Manag.
2:213–219. 2006. View Article : Google Scholar
|
|
6.
|
Pang R and Ronnie TP: Angiogenesis and
antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett.
242:151–167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Eichhorn ME, Kleespies A, Angele MK, et
al: Angiogenesis in cancer: molecular mechanisms clinical impact.
Langenbecks Arch Surg. 392:371–379. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
D’Amato RJ, Loughnan MS, Flynn E, et al:
Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci
USA. 91:4082–4085. 1994.
|
|
9.
|
Kenyon BM, Browne F and D’Amato RJ:
Effects of thalidomide and related metabolites in a mouse corneal
model of neovascularization. Exp Eye Res. 64:971–978. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Bauer KS, Dixon SC and Figg WD: Inhibition
of angiogenesis by thalidomide requires metabolic activation, which
is speciesdependent. Biochem Pharmacol. 55:1827–1834. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gasparini G, Longo R, Fanelli M, et al:
Combination of antiangiogenic therapy with other anticancer
therapies: results, challenges, and open questions. J Clin Oncol.
23:1295–1311. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Adamowicz K, Marczewska M and Jassem J:
Combining systemic therapies with radiation in breast cancer.
Cancer Treat Rev. 35:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nieder C, Wiedenmann N, Andratschke N, et
al: Current status of angiogenesis inhibitors combined with
radiation therapy. Cancer Treat Rev. 32:348–364. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Harrison L and Blackwell K: Hypoxia and
anemia: factors in decreased sensitivity to radiation therapy and
chemotherapy? Oncologist. 9:31–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Mravec B, Gidron Y and Hulin I:
Neurobiology of cancer: interactions between nervous, endocrine and
immune systems as a base for monitoring and modulating the
tumorigenesis by the brain. Sem Cancer Biol. 18:150–163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Sumner SC, Gallagher KS, Davis DG, et al:
Conformational analysis of the tachykinins in solution: substance P
and physalaemin. J Biomol Struct Dyn. 8:687–707. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Carter MS and Krause JE: Structure,
expression and some regulatory mechanisms of the rat
preprotachykinin gene encoding substance P, neurokinin A,
neuropeptide K and neuropeptide gamma. J Neurosci. 10:2203–2214.
1990.
|
|
18.
|
Erin N and Ulusoy O: Differentiation of
neuronal from nonneuronal Substance P. Regul Pept. 152:108–113.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Erin N and Clawson GA: Parameters
affecting substance P measurement in heart, lung, and skin.
Biotechniques. 37:232–239. 2004.PubMed/NCBI
|
|
20.
|
Singh D, Joshi DD, Hameed M, et al:
Increased expression of preprotachykinin-I and neurokinin receptors
in human breast cancer cells: implications for bone marrow
metastasis. Proc Natl Acad Sci USA. 97:388–393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Verheul HM, Pangigrahy D, Yuan J, et al:
Combination oral antiangiogenic therapy with thalidomide and
sulindac inhibits tumor growth in rabbits. Br J Cancer. 79:114–118.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kotoh T, Dhar DK, Masunaga R, et al:
Antiangiogenic therapy of human esophageal cancers with thalidomide
in nude mice. Surgery. 125:536–544. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
McMeekin DS, Sill MW, Benbrook D, et al: A
phase II trial of thalidomide in patients with refractory
endometrial cancer and correlation with angiogenesis biomarkers: a
Gynecologic Oncology Group study. Gynecol Oncol. 105:508–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Itasaka S, Komaki R, Herbst RS, et al:
Endostatin improves radioresponse and blocks tumor
revascularization after radiation therapy for A431 xenografts in
mice. Int J Radiat Oncol Biol Phys. 67:870–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Vala IS, Martins LR, Imaizumi N, et al:
Low doses of ionizing radiation promote tumor growth and metastasis
by enhancing angiogenesis. PLoS One. 5:e112222010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Chan LW and Camphausen K: Angiogenic tumor
markers, anti-angiogenic agents and radiation therapy. Expert Rev
Anticancer Ther. 3:357–366. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Esteban F, Gonzalez-Moles MA, Castro D, et
al: Expression of substance P and neurokinin-1-receptor in
laryngeal cancer: linking chronic inflammation to cancer promotion
and progression. Histopathology. 54:258–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Aalto Y, Forsgren S, Kjörell U, Bergh J,
Franzén L and Henriksson R: Enhanced expression of neuropeptides in
human breast cancer cell lines following irradiation. Peptides.
19(2): 231–239. 1998. View Article : Google Scholar : PubMed/NCBI
|