Introduction
Colorectal cancer (CRC) is the fourth most common
malignancy worldwide (1). Nineteen
percent of CRC patients are diagnosed with metastatic disease, and
50% of those with local disease ultimately develop metastases; the
5-year survival rates are 90.8% for localized, 69.5% for regional
and 11.3% for metastatic disease (2). However, due to the proper combination
of active drugs, the survival of metastatic colorectal cancer
(mCRC) patients has been increased to over 25 months.
The combination of oxaliplatin and capecitabine
(XELOX) is considered a standard regimen for patients with mCRC
based on the results of several studies. Recently, a randomized
phase III trial designed to demonstrate the non-inferiority of
XELOX vs. FOLFOX met its primary endpoint showing no difference
between the two treatments with regard to response rate (RR),
progression-free survival (PFS) and overall survival (OS) (3). Moreover, a meta-analysis of six phase
II and III clinical trials, comparing 5FU- and capecitabine-based
regimens, showed no difference in terms of PFS and OS between the
two types of regimens, and a slight advantage in terms of RR for
the 5FU-based therapy (4).
Angiogenesis is crucial to tumor growth and
development of distant metastases (5); VEGF is a key player and a potential
target for therapy (6). Since
2005, bevacizumab, a humanized monoclonal antibody directed against
VEGF-A, has been integrated in the first-line therapy of mCRC
patients based on the results of a phase III randomized trial by
Hurwitz et al (7). The
addition of bevacizumab to 5FU-irinotecan (IFL) therapy produced a
significant increase in median OS when compared to IFL alone (20.3
vs. 15.6 months). When bevacizumab was added to first-line FOLFOX
or XELOX therapy, a significant increase in PFS (9.4 vs. 8.0
months), median OS (21.3 vs. 19.9 months) and RR (47 vs. 49%) was
noted when compared to the chemotherapy alone (8). However, the shorter duration of
therapy and the smaller number of patients receiving bevacizumab
until disease progression in the latter study were claimed to be
the main reasons for the lower strength of these results as
compared to those found by Hurwitz et al.
The EGFR pathway plays a pivotal role in the growth
and progression of the majority of human epithelial cancers, and
its overexpression is associated with a poor prognosis (9). Monoclonal antibodies targeting EGFR
have been approved to treat mCRC patients with a wild-type KRAS
tumor, either in combination with chemotherapy (10–12)
or as a monotherapy (13,14). Conversely, data are limited in
regards to the use of erlotinib in combination with
oxaliplatin-based therapy for either pre-treated (15) or chemo-naïve (16) mCRC patients. One study in which
erlotinib was administered continuously at the dose of 150 mg in
combination with capecitabine and oxaliplatin to previously treated
patients reported beneficial activity, but raised important safety
issues. In fact, the first 13 patients enrolled in this study
presented G3 diarrhea, leading to the reduction of the capecitabine
dose from 2,000 to 1,500 mg/m2 (15). A second phase Ib trial identified
the maximum tolerated dose (MTD) of erlotinib as 100 mg/day
administered continuously when combined with XELOX. In this trial,
G3 diarrhea was the most commonly noted adverse event and the
dose-limiting toxicity (DLT) (16).
Several pre-clinical studies have suggested that
EGFR and VEGF are functionally linked (17). VEGF appears to be a preferred
escape pathway to overcome the inhibitory effect of anti-EGFR
agents in colon cancer cells with acquired resistance to anti-EGFR
agents (15,16). Consequently, the resistant cancer
cells express 5- to 10-fold more VEGF-A and VEGFR-1 than the
parental cells (18).
Pre-clinical studies suggest that erlotinib may have
potential cooperative activity with fluoropyrimidines, oxaliplatin
and bevacizumab. However, few clinical data are available in
regards to the possibility of combining these agents.
Based on the above findings, we prospectively
evaluated in two trials, MO17974 and ML18511 (EudraCT
2005005548-21), the feasibility of the addition of erlotinib to
therapy with fluoropyrimidines and oxaliplatin, with or without
bevacizumab.
Patients and methods
Patient eligibility
Patients with histologically confirmed mCRC,
previously untreated for metastatic disease, ≥18 years of age, an
ECOG performance status (PS) 0 to 1, with a life expectancy of ≥12
weeks and at least one measurable lesion according to RECIST
criteria (19) were eligible. All
patients had normal organ function defined as: neutrophils ≥1,500
and platelets ≥100,000; total bilirubin ≤1.5 × UNL, ASAT and ALAT
≤2.5 × UNL (≤5 in case of liver metastases), ALP ≤2.5 × UNL (<5
in case of liver metastases; ≤10 in case of bone metastases);
creatinine ≤1.5 × UNL or creatinine clearance >50 ml/min; and
urine dip-stick proteinuria <2+.
Main exclusion criteria included: radiotherapy
administered within 4 weeks before the start of the study, the
presence of brain metastases, prior adjuvant therapy containing
oxaliplatin, history of inflammatory bowel disease and or acute
occlusion/sub-occlusion, presence of non-healing ulcers or
coagulopathies, uncontrolled hypertension or clinically active
cardiovascular disease, current and ongoing treatment with
anticoagulant drugs, receiving other investigational drugs within
30 days before the study entry, or subjected to major surgical
procedures within 28 days before the study entry.
Prior informed consent was obtained from all
patients before enrollment in the study. The trials were conducted
in accordance with the Declaration of Helsinki and the latest
version of the ICH Harmonized Tripartite Guideline: Guideline for
Good Clinical Practice and local laws and regulations. The
protocols were approved by the Ethics Committee of the University
Federico II of Naples. Both trials were designed, implemented and
analyzed independently by the investigators.
Treatment plan
A classical 3+3 dose escalation design was used for
both trials.
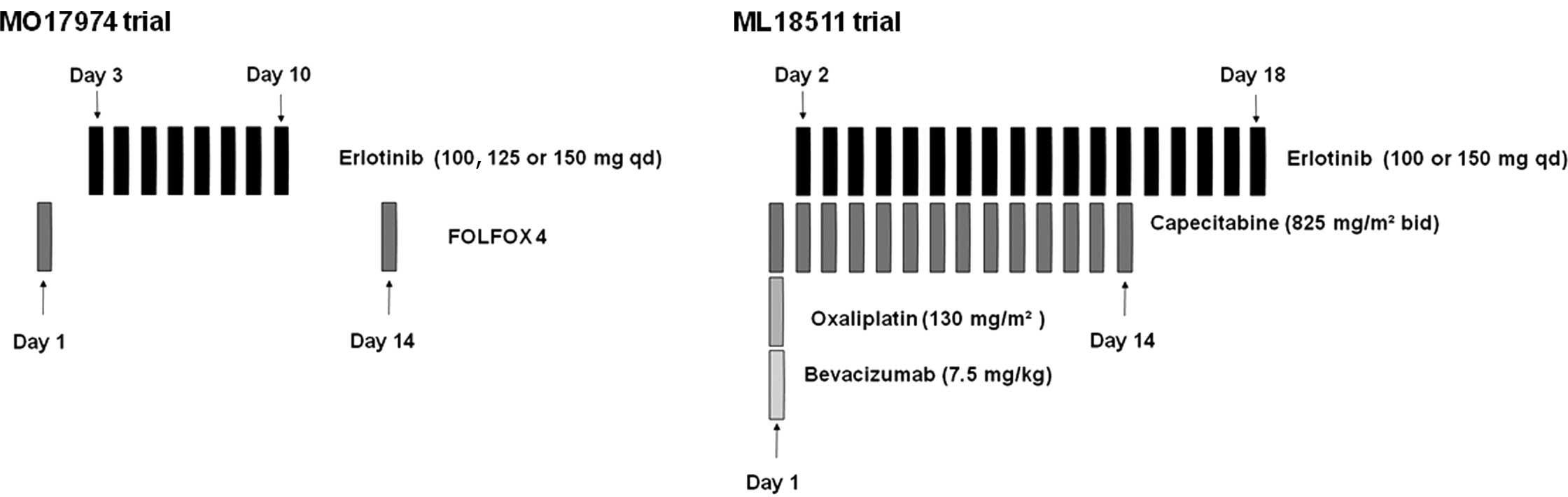
The first trial (MO17974) evaluated the combination
of fixed doses of FOLFOX4 with escalating doses of erlotinib (100,
125 and 150 mg/day) administered orally from day 3 to day 10 of
each 14-day cycle (Fig. 1).
In the second trial (ML18511), escalating doses of
erlotinib (100 and 150 mg/day, on days 2–18) were combined with
fixed doses of capecitabine (1,650 mg/m2, on days 1–14)
and oxaliplatin (130 mg/m2, day 1) (XELOX) plus
bevacizumab (7.5 mg/ kg, day 1). Treatment was repeated every 21
days (Fig. 1). The initial dose of
erlotinib in both trials was lower than the MTD reported in the
literature to minimize potential side effects.
Patients received up to 6 months of combined
treatment (12 cycles in the FOLFOX trial or 9 cycles in the XELOX
trial). Thereafter, they continued treatment with erlotinib or with
erlotinib plus bevacizumab until disease progression or intolerable
toxicity.
Dose escalation, dose-limiting toxicities
and definitions of the maximum tolerated dose
Three patients were sequentially treated at each
dose level (DL). DL was expanded to six when a DLT was observed
during the first cycle. Dose escalation was continued when <33%
of patients experienced a DLT. In the event that ≥2/3 or ≥2/6 of
patients experienced a DLT, the MTD was considered to be
exceeded.
DLT was reached when any of the following events
occurred in the first cycle of treatment: i) febrile neutropenia
for >3 days, ii) G4 neutropenia lasting >7 days, iii) G4
thrombocytopenia, or iv) any drug-related non-hematological
toxicity ≥G3 (apart from alopecia). The MTD was defined as the dose
below that which caused DLT in >33% of patients treated at a
DL.
Patient monitoring and response
assessment
Pre-treatment assessment within 2 weeks of the start
of treatment included receipt of informed consent, evaluation of
demographic information, procurement of a full medical history,
general physical examination, ECG, laboratory tests (including
blood chemistry and urine analysis) and total body CT-scan. Chest
X-ray was required when a CT-scan of the chest was unavailable.
A clinical examination was performed at each cycle
of treatment. Laboratory analyses were performed weekly. The
severity of all the adverse events and abnormal laboratory results
were graded according to the CTCAE v3.0.
Reassessment imaging was performed every 4 cycles
for FOLFOX and 3 cycles for XELOX, or as required when progressive
disease (PD) was suspect. Responses were evaluated according to the
RECIST guidelines (19).
Materials
Roche kindly supplied erlotinib for both the trials
(MO17974 and ML18511) and bevacizumab for the ML18511 trial.
Results
Patient characteristics and
treatment
MO17974 trial. From June 2004 to July 2005,
12 patients were enrolled in the trial (Table I). Seventy-five percent of the
patients had ≥2 sites of metastatic disease, most of them involving
the liver. Overall, 100 cycles of chemotherapy were administered,
with a median of 9.5 cycles/patient (range 2–12). Three of the 12
enrolled patients discontinued treatment due to disease progression
(after cycle 6, 8 and 9); 4 patients discontinued chemotherapy due
to toxicity (1 unacceptable toxicity after cycle 1; 1 after cycle 3
due to G2 hyperbilirubinemia; 1 after cycle 7 for prolonged G3
thrombocytopenia and 1 after cycle 11 due to G3 peripheral
neuropathy and mucositis). Five patients, after the completion of
chemotherapy, continued with erlotinib treatment alone for a median
of 3.7 months (range 1–8.8) until disease progression.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| MO17974 trial
(n=12) | ML18511 trial
(n=9) |
|---|
| Age (years) | | |
| 18–64 | 8 | 5 |
| ≥65 | 4 | 4 |
| Gender | | |
| Male | 8 | 6 |
| Female | 4 | 3 |
| Baseline PS
(WHO) | | |
| 0 | 11 | 6 |
| 1 | 1 | 3 |
| 2 | - | - |
| Site of
metastasis | | |
| Liver | 10 | 8 |
| Lung | 1 | 4 |
| Node | 7 | 7 |
| Other | 2 | 1 |
| (peritoneum) | (serratus
muscle) |
ML18511 trial. From June 2006 to June 2007, 9
patients were enrolled in the trial (Table I). All patients completed at least
1 cycle of therapy. A total number of 51 cycles of therapy was
delivered with a median of 3 per patient (range 1–19). One patient
at DL 1 and one at DL 2 received further cycles (3 and 10 cycles,
respectively) of erlotinib and bevacizumab after the completion of
the first 9 cycles of therapy. Three patients at DL 1 withdrew from
treatment due to PD after 12, 3 and 5 cycles of therapy,
respectively. Five patients withdrew due to toxicity: 3 at DL 1 (1
patient due to rectal bleeding at cycle 5, and 2 patients due to G4
diarrhea at cycle 2 and 3, respectively) and 2 at DL 2 (due to G4
diarrhea experienced at cycle 1 and 2). One patient withdrew on a
voluntary basis after 19 cycles, although she experienced only mild
toxicity, consisting of G2 rectal bleeding.
Toxicity
MO17974 trial. At DL 1 (erlotinib 100 mg) and
2 (erlotinib 125 mg), no unacceptable toxicity was noted during the
first cycle of treatment. At DL 3 (erlotinib 150 mg), 1/6 of the
enrolled patients experienced unacceptable toxicity at the first
cycle of treatment, consisting of G3 diarrhea and G3 neutropenia.
Thus, the MTD was not reached.
The most severe side effects experienced by the 12
enrolled patients throughout treatment are listed in Tables II and III. Non-hematological toxicity was mild.
In addition to the episodes of unacceptable toxicity reported above
(G3 diarrhea), only 1 patient experienced ≥G3 gastrointestinal
toxicity (mucositis); ≥G2 peripheral neuropathy occurred in 2
patients and was related to the cumulative administered dose of
oxaliplatin, as it appeared after the eighth cycle of chemotherapy.
As expected with the FOLFOX regimen, hematological toxicity was
frequent: 50% of patients experienced G3–4 neutropenia and 2
patients presented with G3 thrombocytopenia.
 | Table II.Adverse events per dose cohort at
cycle 1. |
Table II.
Adverse events per dose cohort at
cycle 1.
| Erlotinib dose | MO17974 trial (n=12)
| ML18511 trial (n=9)
|
|---|
| 100 mg (n=3) | 125 mg (n=3) | 150 mg (n=6) | 100 mg (n=3) | 125 mg (n=3) | 150 mg (n=6) | 100 mg (n=6) | 150 mg (n=3) | 100 mg (n=6) | 150 mg (n=3) |
|---|
|
|
|
|
|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
|---|
| Mucositis | 1 | - | - | - | - | - | | | | |
| Diarrhea | 1 | - | 1 | - | - | 1 | 4 | 1 | - | 1 |
| Asthenia | - | 1 | - | - | - | - | | | | |
| Nausea | 1 | 1 | 3 | - | - | - | | | | |
| Vomiting | | | | | | | 1 | 1 | - | - |
| Anemia | - | - | 1 | - | - | - | 1 | 1 | - | - |
| Neutropenia | - | - | 1 | - | - | 1 | | | | |
| Constipation | - | - | 1 | - | - | - | | | | |
| Neurotoxicity | - | - | 1 | - | - | - | - | 2 | - | - |
| Skin rash | - | - | 1 | - | - | - | 3 | 1 | - | - |
| Rectal
bleeding | | | | | | | - | 1 | - | - |
| Proctitis | | | | | | | 1 | - | - | - |
 | Table III.Adverse events per dose cohort
(cycles other than 1). |
Table III.
Adverse events per dose cohort
(cycles other than 1).
| Erlotinib dose | MO17974 trial
(n=12)
| Ml18511 trial (n=9)
|
|---|
| 100 mg (n=3) | 125 mg (n=3) | 150 mg (n=6) | 100 mg (n=3) | 125 mg (n=3) | 150 mg (n=6) | 100 mg (n=6) | 150 mg (n=3) | 100 mg (n=6) | 150 mg (n=3) |
|---|
|
|
|
|
|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
|---|
| Mucositis | 1 | 1 | 2 | 1 | - | - | | | | |
| Diarrhea | 3 | - | 2 | 1 | - | 1 | 4 | 2 | 1 | 1 |
| Asthenia | 2 | 2 | - | - | - | - | 2 | - | 1 | |
| Nausea | 2 | 2 | 4 | - | - | - | 1 | - | 2 | - |
| Vomiting | | | | | | | - | - | 1 | - |
| Anemia | - | - | 1 | 1 | - | - | - | - | 1 | - |
| Neutropenia | - | - | 1 | 2 | 2 | 2 | 1 | 1 | - | - |
| Constipation | 2 | 1 | 1 | - | - | - | | | | |
| Neurotoxicity | 1 | 2 | 2 | 1 | 1 | - | 1 | 1 | - | - |
| Urinary
infection | - | 2 | - | - | - | - | | | | |
| Numbness | 1 | - | - | - | - | - | | | | |
| Abdominal pain | - | - | 1 | - | - | - | | | | |
| Fever | - | - | - | - | - | 1 | | | | |
| Transaminitis | - | 1 | 2 | - | - | - | | | | |
|
Thrombocytopenia | 1 | - | 1 | 1 | 1 | - | | | | |
| Rectal
bleeding | 1 | - | - | - | - | - | - | 1 | 1 | - |
| Skin rash | 3 | 2 | 1 | - | - | - | 4 | 1 | - | - |
| Anorexia | | | | | | | 3 | - | - | - |
| Epistaxis | - | - | - | - | - | | 1 | - | - | - |
G1–2 skin reactions as acneiform dermatitis, very
likely related to EGFR inhibition by erlotinib, were observed in
50% of the treated patients, but in no cases were delay or
discontinuation of erlotinib administration required.
ML18511 trial. No DLT was observed at DL 1,
while at DL 2, 1 patient experienced a DLT consisting of G4
diarrhea. Most common toxicities occurring during the first cycle
consisted of diarrhea, nausea and vomiting, skin rash, paresthesia
and rectal bleeding (Table II).
Their entity was moderate and did not require a treatment delay.
Table III summarizes the toxicity
observed at cycles other than 1. The most common adverse event was
diarrhea. In 2 cases, 1 at DL 1 and 1 at DL 2, diarrhea was severe
and required medical therapy. The incidence of nausea and vomiting
was lower than expected and was severe in 1 patient at DL 1. One
patient at DL 1 experienced hypersensitivity during bevacizumab
administration, consisting in a spasm of the larynx and requiring
medical treatment. Two patients, 1 at DL 1 and 1 at DL 2,
experienced rectal bleeding, which was complicated by G3 anemia in
the patient at DL 1. Only 1 patient at DL 1 and 1 at DL 2
experienced G2 neutropenia, after cycle 6 and 3 of therapy,
respectively. Three patients at DL 1 experienced a mild increase in
liver enzymes.
Tumor response
MO17479 trial. All patients were assessable
for tumor response: at DL 1, 2 patients obtained a partial response
(PR) and 1 stable disease (SD); at DL 2, 1 patient experienced a PR
and 2 SD. Five SD and 1 PD cases were noted at DL 3 (Table IV). Ten patients received further
chemotherapy after disease progression (mostly an
irinotecan-containing regimen with or without cetuximab); 5
patients received ≥2 lines of additional chemotherapy.
 | Table IV.Clinical outcome. |
Table IV.
Clinical outcome.
| MO17974 trial
| ML18511 trial
|
|---|
Erlotinib dose (mg)
|
|---|
| Response | 100 | 125 | 150 | 100 | 150a |
|---|
| PR | 2 | 1 | - | 3 | 1 |
| SD | 1 | 2 | 5 | 1 | 1 |
| PD | - | - | 1 | 2 | - |
ML18511 trial. Six patients at DL 1 and 2
patients at DL 2 were evaluable for tumor response. Three patients
at DL 1 and 1 patient at DL 2 experienced a PR; 2 SD cases were
noted at DL 1 and 2, respectively. Two patients at DL 1 experienced
PD as the best response (Table
IV). All patients received at least a second-line chemotherapy
regimen. One patient at DL 2 underwent liver metastasectomy after
second-line chemotherapy. Seven patients received ≥2 lines of
therapy after the trial.
Discussion
Oxaliplatin-based therapy is at present considered a
standard first-line treatment for mCRC patients. Since the addition
of cetuximab to FOLFOX therapy has demonstrated substantial
efficacy in the treatment of mCRC patients (12,20)
and erlotinib has showed beneficial activity as a monotherapy in
first-line mCRC therapy (21), a
combination of erlotinib and FOLFOX in a first-line setting may
represent an appealing treatment option.
In both the present trials, MTD was not reached. Our
first trial, MO174974, found that FOLFOX-erlotinib is a feasible
treatment for first-line mCRC, since the combination demonstrated a
good safety profile, with no synergism of toxicity between the
drugs and an acceptable median number of cycles delivered (9
cycles). The results are consistent with the encouraging results of
two recently published phase I studies, which demonstrated a
satisfactory toxicity profile and beneficial activity for erlotinib
combined with oxaliplatin and fluoropyrimidines (16,22).
In a study by Hanauske et al, (22) the MTD of erlotinib was found to be
150 mg when administered continuously with FOLFOX in 32 patients
with advanced solid tumors (including 23 with mCRC). The most
common adverse events included diarrhea, nausea, stomatitis and
rash. Among the mCRC patients, 1 obtained a complete response (CR)
and 7 a PR. In another trial (16), the MTD of erlotinib was determined
to be 100 mg when administered continuously with XELOX. The most
common adverse events were diarrhea, rash, nausea and
neurotoxicity, with an expected dose-dependent trend. The rate of
rash and diarrhea prevented any further escalation to higher doses
of erlotinib and capecitabine.
In our second trial, ML18511, the most common
toxicity was diarrhea, thus confirming the finding of previous
studies (23,24). Although the treatment regimen
resulted in only 1 DLT at the first cycle, in subsequent cycles
almost all patients experienced a moderate/severe toxicity that
variably hampered daily living activities and forced patient
withdrawal from treatment. The median number of administered cycles
of therapy was very low (3 cycles) considering the first-line
setting.
Previous studies have also explored this combination
as a first-line therapy of mCRC patients. In a phase II trial by
Spigel et al (23) the
combination of erlotinib (150 mg) administered continuously with
FOLFOX-4 and bevacizumab every 14 days caused G3 diarrhea in 31% of
patients and G3 nausea in 14% of patients. Moreover, there were
four deaths possibly related to treatment. For these reasons, the
study was discontinued.
Meyerhardt et al published the results of a
phase II trial combining mFOLFOX-6 plus bevacizumab with continuous
administration of erlotinib (150 mg) (24). The primary endpoint of the trial,
PFS, was not reached, partly due to the toxicity observed. In fact,
7 patients discontinued the trial due to toxicity before the first
restaging: 4 of them voluntarily withdrew due to uncomfortable
moderate toxicity, and 3 due to severe toxicity. In total, 77% of
the patients exited the study due to toxic effects or withdrawal of
informed consent. The toxicity with the most impact was diarrhea
(83% of patients). Fifty-four percent of the patients required at
least one dose reduction of erlotinib, and 4 patients requiring two
dose reductions (final erlotinib dose, 50 mg daily).
Although treatment activity was beyond the scope of
our trial, all patients were followed up for evaluation of tumor
response. Four patients (50%) obtained a PR, 3 at the first DL and
1 at the second DL, and only 2 patients had PD. These data are
comparable to those of the above-mentioned two phase II studies, in
which PR was obtained in 43% (23)
and 34% of the patients (24),
respectively.
Current evidence shows that severe or uncomfortable
non-acute toxicity is the main concern regarding the combination of
bevacizumab and erlotinib with fluoropyrimidine and oxaliplatin
chemotherapy. Even though these early data show promising activity
for this combination, recently published phase III trials (25,26)
have raised the question of whether the double blockade of EGFR and
VEGF by two monoclonal antibodies is an effective treatment for
mCRC patients. In fact, in the CAIRO-2 study, the combination of
cetuximab, bevacizumab and CAPOX resulted in a significant decrease
in PFS, the primary endpoint of the trial (9.7 vs. 10.4 months,
p=0.001), when compared to that of CAPOX plus bevacizumab (26). In the PACCE study, the addition of
panitumumab to bevacizumab plus oxaliplatin or irinotecan-based
chemotherapy resulted in a worse PFS vs. the same therapy without
panitumumab (25). A possible
explanation for the disappointing results achieved in these
studies, as compared to pre-clinical findings, is the decreased
amount of therapy administered to the patients receiving the
triple-combination therapy, due to toxicity.
It has recently been pointed out that only KRAS
wild-type patients are able to benefit from anti-EGFR therapies.
However, the KRAS or EGFR mutational status of our patients was
unknown. Thus, we could not draw any conclusions regarding the
degree of activity in this subgroup of patients who are the best
candidates for anti-EGFR therapy.
Therefore, based on the safety profile observed in
our and previous studies investigating the combination of erlotinib
with fluoropyrimidine-based chemotherapy, and on the concerns
raised by studies which combined anti-EGFR antibodies with
bevacizumab plus chemotherapy, we conclude that combining an
anti-EGFR TKI, such as erlotinib, with chemotherapy and bevacizumab
may be unsuitable for the treatment of mCRC patients.
Acknowledgements
This study was supported in part by a
grant from the Associazione Italiana Ricerca sul Cancro (AIRC) and
Regione Campania.
References
|
1.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer Statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2.
|
Horner MJ, Ries LAG, Krapcho M, Neyman N,
Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A,
Miller BA, Lewis DR, Eisner MP, Stinchcomb DG and Edwards BK: SEER
Cancer Statistics Review. 1975–2006, National Cancer Institute;
Bethesda, MD: http://seer.cancer.gov/csr/1975_2006/uri,
based on November 2008 SEER data submission, posted to the SEER web
site, 2009.
|
|
3.
|
Cassidy J, Clarke S, Diaz-Rubio E, et al:
Randomized phase III study of capecitabine plus oxaliplatin
compared with fluorouracil/folinic acid plus oxaliplatin as
first-line therapy for metastatic colorectal cancer. J Clin Oncol.
26:2006–2012. 2008. View Article : Google Scholar
|
|
4.
|
Arkenau HT, Arnold D, Cassidy J, et al:
Efficacy of oxaliplatin plus capecitabine or infusional
fluorouracil/leucovorin in patients with metastatic colorectal
cancer: a pooled analysis of randomized trials. J Clin Oncol.
26:5910–5917. 2008. View Article : Google Scholar
|
|
5.
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Saltz LB, Clarke S, Diaz-Rubio E, et al:
Bevacizumab in combination with oxaliplatin-based chemotherapy as
first-line therapy in metastatic colorectal cancer: a randomized
phase III study. J Clin Oncol. 26:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Van Cutsem E, Kohne CH, Hitre E, et al:
Cetuximab and chemotherapy as initial treatment for metastatic
colorectal cancer. N Engl J Med. 360:1408–1417. 2009.PubMed/NCBI
|
|
11.
|
Cunningham D, Humblet Y, Siena S, et al:
Cetuximab mono-therapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Bokemeyer C, Bondarenko I, Makhson A, et
al: Fluorouracil, leucovorin, and oxaliplatin with and without
cetuximab in the first-line treatment of metastatic colorectal
cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jonker DJ, O'Callaghan CJ, Karapetis CS,
et al: Cetuximab for the treatment of colorectal cancer. N Engl J
Med. 357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Van Cutsem E, Peeters M, Siena S, et al:
Open-label phase III trial of panitumumab plus best supportive care
compared with best supportive care alone in patients with
chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol.
25:1658–1664. 2007.
|
|
15.
|
Meyerhardt JA, Zhu AX, Enzinger PC, et al:
Phase II study of capecitabine, oxaliplatin, and erlotinib in
previously treated patients with metastastic colorectal cancer. J
Clin Oncol. 24:1892–1897. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Van Cutsem E, Verslype C, Beale P, et al:
A phase Ib dose-escalation study of erlotinib, capecitabine and
oxaliplatin in metastatic colorectal cancer patients. Ann Oncol.
19:332–339. 2008.PubMed/NCBI
|
|
17.
|
Bianco R, Rosa R, Damiano V, et al:
Vascular endothelial growth factor receptor-1 contributes to
resistance to anti-epidermal growth factor receptor drugs in human
cancer cells. Clin Cancer Res. 14:5069–5080. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Ciardiello F, Bianco R, Caputo R, et al:
Antitumor activity of ZD6474, a vascular endothelial growth factor
receptor tyrosine kinase inhibitor, in human cancer cells with
acquired resistance to antiepidermal growth factor receptor
therapy. Clin Cancer Res. 10:784–793. 2004. View Article : Google Scholar
|
|
19.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
20.
|
Tabernero J, van Cutsem E, Diaz-Rubio E,
et al: Phase II trial of cetuximab in combination with
fluorouracil, leucovorin, and oxaliplatin in the first-line
treatment of metastatic colorectal cancer. J Clin Oncol.
25:5225–5232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Townsley CA, Major P, Siu LL, et al: Phase
II study of erlotinib (OSI-774) in patients with metastatic
colorectal cancer. Br J Cancer. 94:1136–1143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hanauske AR, Cassidy J, Sastre J, et al:
Phase 1b dose escalation study of erlotinib in combination with
infusional 5-fluorouracil, leucovorin, and oxaliplatin in patients
with advanced solid tumors. Clin Cancer Res. 13:523–531. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Spigel DR, Hainsworth JD, Burris HA, et
al: Phase II of FOLFOX4, bevacizumab and erlotinib as first-line
therapy in patients with advanced colorectal cancer. In: ASCO
Gastrointestinal Cancer Symposium: abs. 238, 2006.
|
|
24.
|
Meyerhardt JA, Stuart K, Fuchs CS, et al:
Phase II study of FOLFOX, bevacizumab and erlotinib as first-line
therapy for patients with metastatic colorectal cancer. Ann Oncol.
18:1185–1189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hecht JR, Mitchell E, Chidiac T, et al: A
randomized phase IIIB trial of chemotherapy, bevacizumab, and
panitumumab compared with chemotherapy and bevacizumab alone for
metastatic colorectal cancer. J Clin Oncol. 27:672–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tol J, Koopman M, Cats A, et al:
Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal
cancer. N Engl J Med. 360:563–572. 2009. View Article : Google Scholar : PubMed/NCBI
|